Collective Molecular Activities of the Plant: Kopsia Pauciflora
Overview of Ingredients
17 All known Ingredients in Total
Unique ingredients have been isolated from this plant.Plant-Ingredients Associations were manually curated from publications or collected from other databases.
12 Ingredients with Acceptable Bioavailablity
Unique ingredients exhibit acceptable human oral bioavailablity, according to the criteria of SwissADME [PMID: 28256516] and HobPre [PMID: 34991690]. The criteria details:SwissADME: six descriptors are used by SwissADME to evaluate the oral bioavailability of a natural product:
☑ LIPO(Lipophility): -0.7 < XLOGP3 < +5.0
☑ SIZE: 150g/mol < MW < 500g/mol
☑ POLAR(Polarity): 20Ų < TPSA < 130Ų
☑ INSOLU(Insolubility): -6 < Log S (ESOL) < 0
☑ INSATU(Insaturation): 0.25 < Fraction Csp3 < 1
☑ FLEX(Flexibility): 0 < Num. rotatable bonds < 9
If 6 descriptors of a natural plant satisfy the above rules, it will be labeled high HOB.
HobPre: A natural plant ingredient with HobPre score >0.5 is labeled high human oral availability (HOB)
15 Ingredients with experimental-derived Activity
Unique ingredients have activity data available.Ingredient Structrual Cards
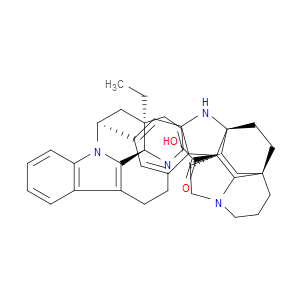
Ingredient ID: NPC483483
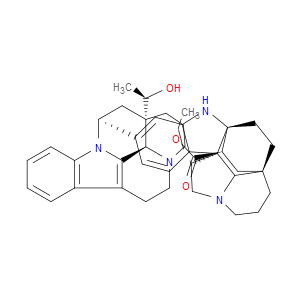
Ingredient ID: NPC483482
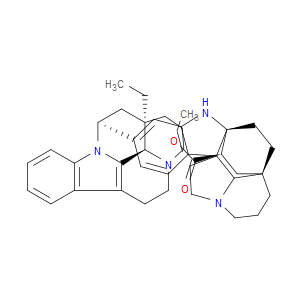
Ingredient ID: NPC483481
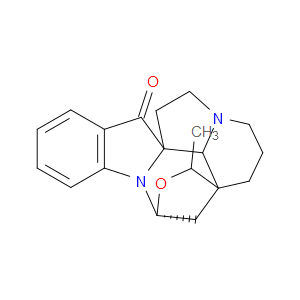
Ingredient ID: NPC483480
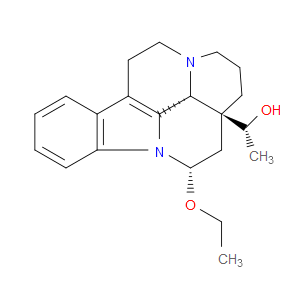
Ingredient ID: NPC483479
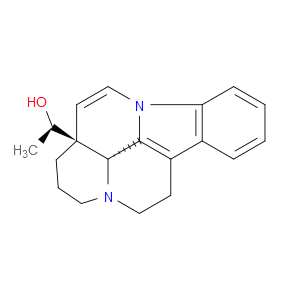
Ingredient ID: NPC483478

Ingredient ID: NPC483477
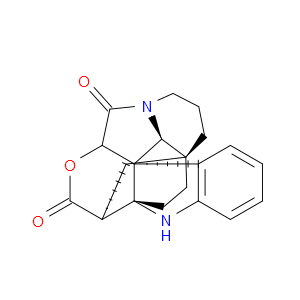
Ingredient ID: NPC483475
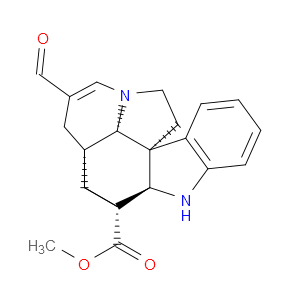
Ingredient ID: NPC483474
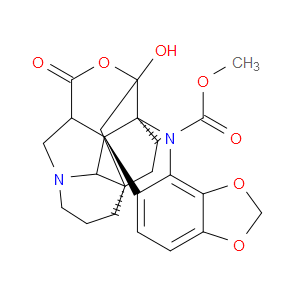
Ingredient ID: NPC483473
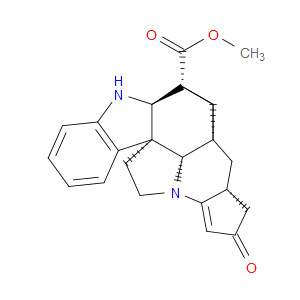
Ingredient ID: NPC483472
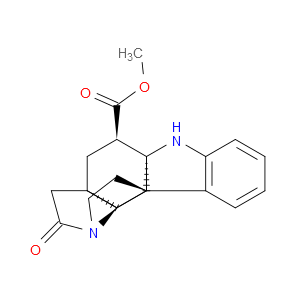
Ingredient ID: NPC483471
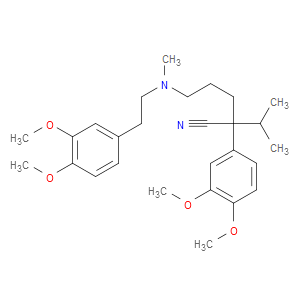
Ingredient ID: NPC42793
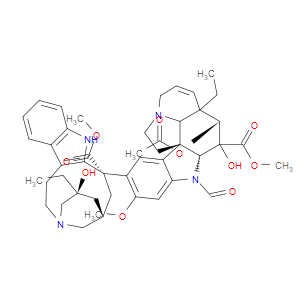
Ingredient ID: NPC260909
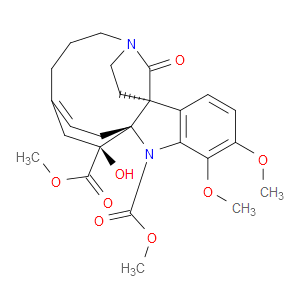
Ingredient ID: NPC170109

Ingredient ID: NPC157892
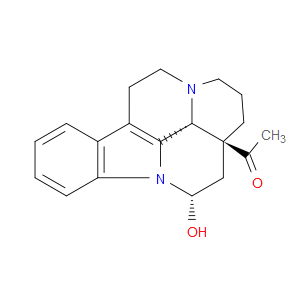
Ingredient ID: NPC125963
Classification of Human Proteins Collectively Targeted by the Plant
Detailed Information of Target Proteins
| Target Type | Protein Class | Gene ID | Protein Name | Uniprot ID | Target ChEMBL ID |
|---|---|---|---|---|---|
| Drug Transporter | Potassium channels | KCNH2 | HERG | Q12809 | CHEMBL240 |
| Drug Transporter | SLC superfamily of solute carriers | SLC6A4 | Serotonin transporter | P31645 | CHEMBL228 |
| Therapeutic Target | Small molecule receptor (family A GPCR) | HTR2B | Serotonin 2b (5-HT2b) receptor | P41595 | CHEMBL1833 |
| Therapeutic Target | Small molecule receptor (family A GPCR) | ADRA2A | Alpha-2a adrenergic receptor | P08913 | CHEMBL1867 |
| Therapeutic Target | Small molecule receptor (family A GPCR) | HTR2C | Serotonin 2c (5-HT2c) receptor | P28335 | CHEMBL225 |
| Therapeutic Target | Small molecule receptor (family A GPCR) | HTR2A | Serotonin 2a (5-HT2a) receptor | P28223 | CHEMBL224 |
| Therapeutic Target | Small molecule receptor (family A GPCR) | DRD3 | Dopamine D3 receptor | P35462 | CHEMBL234 |
| Therapeutic Target | Structural protein | LMNA | Prelamin-A/C | P02545 | CHEMBL1293235 |
| Therapeutic Target | Unclassified protein | HTT | Huntingtin | P42858 | CHEMBL5514 |
Clinical trials associated with plant from natural product (NP) & plant level:
| Clinical trials type | Number of clinical trials | |
|---|---|---|
| 401 | ||
| NCT ID | Title | Condition | Form in clinical use | Associated by plant or compound |
|---|---|---|---|---|
| NCT03504644 | Venetoclax and Vincristine Liposomal in Treating Patients With Relapsed or Refractory T-cell or B-cell Acute Lymphoblastic Leukemia | childhood acute lymphoblastic leukemia;T-cell acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02427620 | Ibrutinib, Rituximab, and Consolidation Chemotherapy in Treating Young Patients With Newly Diagnosed Mantle Cell Lymphoma | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT00052936 | Combination Chemotherapy With or Without Rituximab in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00225173 | Combination Chemotherapy +/- Radiation in High Risk Hodgkin's Disease | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT02845882 | LBL-2016 for Children or Adolescents in China | lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT03589326 | A Study of Ponatinib Versus Imatinib in Adults With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01055496 | Study Evaluating Chemotherapy in Combination With Inotuzumab Ozogamicin In Subjects With Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01704716 | High Risk Neuroblastoma Study 1.8 of SIOP-Europe (SIOPEN) | neuroblastoma | Vincristine (NPC260909) | |
| NCT00850512 | Study to Evaluate the Efficacy and Safety of Subsequent Treatment With the Zevalin (Ibritumomab Tiuxetan) in Elderly (More Than 60 Years) Patients With Diffuse Large B Cell Lymphoma After 4 Cycles of CHOP21-Rituximab (CHOP21-R) Therapy | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01186328 | EZN-3042 Administered With Re-induction Chemotherapy in Children With Relapsed Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00774826 | Multicentric Study, Three Randomized Arms (R-CVP vs R-CHOP vs R-FM),for Patients With Stage II-IV Follicular Lymphoma | follicular lymphoma | Vincristine (NPC260909) | |
| NCT01261286 | Drug-Disease Interaction in Crohn's Disease | Crohn's disease | Verapamil (NPC42793) | |
| NCT03017326 | Paediatric Hepatic International Tumour Trial | Hepatoblastoma | Vincristine (NPC260909) | |
| NCT03817320 | PO Ixazomib in Combination With Chemotherapy for Childhood Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma | acute lymphoblastic leukemia;lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT00264953 | HD11 for Intermediate Stages | lymphoma | Vincristine (NPC260909) | |
| NCT03206671 | Treatment Protocol of the NHL-BFM and the NOPHO Study Groups for Mature Aggressive B-cell Lymphoma and Leukemia in Children and Adolescents | neoplasm of mature B-cells | Vincristine (NPC260909) | |
| NCT04388839 | Evolutionary Therapy for Rhabdomyosarcoma | rhabdomyosarcoma | Vincristine (NPC260909) | |
| NCT00451178 | A Study of Participants With Lymphoma Who Take R-CHOP and Enzastaurin Compared to Participants Who Take R-CHOP Only | lymphoma | Vincristine (NPC260909) | |
| NCT02889523 | Study of Tazemetostat in Newly Diagnosed Diffuse Large B Cell and Follicular Lymphoma Patients Treated by Chemiotherapy | diffuse large B-cell lymphoma;follicular lymphoma | Vincristine (NPC260909) | |
| NCT04025593 | Biomarker Guided Treatment in DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01096368 | Maintenance Chemotherapy or Observation Following Induction Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Ependymoma | anaplastic ependymoma | Vincristine (NPC260909) | |
| NCT03359005 | Irinotecan and Temozolomide for Ewing Sarcoma | Ewing sarcoma | Vincristine (NPC260909) | |
| NCT05304585 | Chemotherapy for the Treatment of Patients With Newly Diagnosed Very Low-Risk and Low Risk Fusion Negative Rhabdomyosarcoma | alveolar rhabdomyosarcoma;embryonal rhabdomyosarcoma | Vincristine (NPC260909) | |
| NCT02372253 | Verapamil for Beta Cell Survival Therapy in Type 1 Diabetes | type 1 diabetes mellitus | Verapamil (NPC42793) | |
| NCT00593463 | Drug Discrimination in Methadone-Maintained Humans Study 1 | drug dependence | Verapamil (NPC42793) | |
| NCT00575406 | Multicentre Study to Determine the Cardiotoxicity of R-CHOP Compared to R-COMP in Patients With Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01399372 | Rituximab, Methotrexate, Vincristine Sulfate, Procarbazine Hydrochloride, and Cytarabine With or Without Radiation Therapy in Treating Patients With Primary Central Nervous System Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01783535 | Protocol for the Study and Treatment of Participants With Intraocular Retinoblastoma | retinoblastoma | Vincristine (NPC260909) | |
| NCT03786783 | Dinutuximab, Sargramostim, and Combination Chemotherapy in Treating Patients With Newly Diagnosed High-Risk Neuroblastoma | ganglioneuroblastoma;neuroblastoma | Vincristine (NPC260909) | |
| NCT00186888 | Study of Treatment for Patients With Cancer of the Eye -Retinoblastoma | retinoblastoma | Vincristine (NPC260909) | |
| NCT00290498 | Study of Rituximab-HCVAD Alternating With Rituximab-Methotrexate-Cytarabine Versus Standard Rituximab-CHOP Every 21 Days for Patients With Newly Diagnosed High Risk Aggressive B-Cell Non-Hodgkin's Lymphomas in Patients 60 Years Old or Younger | lymphoma | Vincristine (NPC260909) | |
| NCT04351763 | Amiodarone or Verapamil in COVID-19 Hospitalized Patients With Symptoms | COVID-19 | Verapamil (NPC42793) | |
| NCT01004991 | Phase I/II Trial of R-CHOP + Azacytidine in Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00774202 | Higher Dose of Rituxan Versus Standard Doses of Rituxan With Cyclophosphamide, Vincristine, and Prednisone in Subjects With Chronic ITP | autoimmune thrombocytopenic purpura | Vincristine (NPC260909) | |
| NCT02137928 | Carboplatin Periocular Injection for Retinoblastoma | retinoblastoma | Vincristine (NPC260909) | |
| NCT00361621 | Ph II CHOP+Velcade in Mediastinal LBCL | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT02809573 | Clinical Trial of Chidamide Combined With CHOP in Peripheral T-cell Lymphoma Patients | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT00335140 | Rituximab and Combination Chemotherapy in Treating Patients With Primary Central Nervous System Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT02881086 | Optimization of Therapy in Adult Patients With Newly Diagnosed Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma by Individualised, Targeted and Intensified Treatment | acute lymphoblastic leukemia;lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT01906814 | Adjuvant Chemotherapy for High-risk Retinoblastoma After Enucleation | retinoblastoma | Vincristine (NPC260909) | |
| NCT05303792 | Testing the Combination of Inotuzumab Ozogamicin and Lower Dose Chemotherapy Compared to Usual Chemotherapy for Adults With B-Cell Acute Lymphoblastic Leukemia or B-Cell Lymphoblastic Lymphoma | acute lymphoblastic leukemia;lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT00804895 | Cluster Headache Cortivazol Injection (CHCI) | pain | Verapamil (NPC42793) | |
| NCT04322318 | A Study of Combination Chemotherapy for Patients With Newly Diagnosed DAWT and Relapsed FHWT | kidney Wilms tumor | Vincristine (NPC260909) | |
| NCT01009970 | Study With Rituximab, Cyclophosphamide, Doxorubicin Liposomal (Myocet®), Vincristine, Prednisone, (R-COMP) to Treat Non-Hodgkin's Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00562965 | Study Comparing Inotuzumab Ozogamicin In Combination With Rituximab Versus Defined Investigator's Choice In Follicular Non-Hodgkin's Lymphoma (NHL) | follicular lymphoma | Vincristine (NPC260909) | |
| NCT02823106 | Superselective Citicoline and Verapamil for Ischemic Neuroprotection and Greater Effective Response | Ischemic stroke | Verapamil (NPC42793) | |
| NCT00006721 | S0016 Combination Chemotherapy With Monoclonal Antibody Therapy in Newly Diagnosed Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01528046 | Metformin in Children With Relapsed or Refractory Solid Tumors | brain neoplasm | Vincristine (NPC260909) | |
| NCT00890656 | Study of Augmented Hyper-CVAD in Acute Lymphoblastic Leukemia Salvage | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00118209 | Rituximab and Combination Chemotherapy in Treating Patients With Diffuse Large B-Cell Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00031590 | Low-Dose Radiation and Combination Chemotherapy Following Surgery in Children With Newly Diagnosed Medulloblastoma | medulloblastoma | Vincristine (NPC260909) | |
| NCT05278715 | Modified CV Regimen in Optic Pathway Glioma | optic nerve glioblastoma | Vincristine (NPC260909) | |
| NCT03467373 | A Study of Glofitamab in Combination With Rituximab or Obinutuzumab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP), or Polatuzumab Vedotin Plus Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (CHP) in Participants With Non-Hodgkin Lymphomas or With DLBCL | neoplasm of mature B-cells;non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT01430013 | Trial of Endostar Combined With CHOPT for T Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00577993 | Fludarabine, Mitoxantrone, and Dexamethasone (FND) Plus Rituximab for Lymphoma Patients | lymphoma | Vincristine (NPC260909) | |
| NCT01004497 | First-line Dasatinib Plus Conventional Chemotherapy in Adults With Newly Diagnosed Ph-Positive ALL | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00589303 | AV Node Ablation and Pacemaker Therapy Compared to Drug Therapy for Atrial Fibrillation - Pilot Study | atrial fibrillation;heart failure | Verapamil (NPC42793) | |
| NCT00313079 | Monoclonal Antibody (mAb) 216 With Chemotherapy in Adult Relapsed or Refractory B-Lineage Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00787527 | SAHA + CHOP in Untreated T-cell Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01777152 | ECHELON-2: A Comparison of Brentuximab Vedotin and CHP With Standard-of-care CHOP in the Treatment of Patients With CD30-positive Mature T-cell Lymphomas | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00051311 | Modified Stem Cell Transplant Procedure to Treat Patients With Blood and Immune System Cancers | hematopoietic and lymphoid cell neoplasm | Vincristine (NPC260909) | |
| NCT00145002 | A Study for Aggressive Adult T-cell Leukemia-lymphoma (ATLL) | adult T-cell leukemia/lymphoma | Vincristine (NPC260909) | |
| NCT02523976 | Dasatinib Combined With Chemotherapy in Philadelphia Chromosome-positive Acute Lymphoblastic Leukemia | lymphoid leukemia | Vincristine (NPC260909) | |
| NCT00285389 | Treatment of Mantle Cell Lymphoma at Diagnosis for Patients Under 65 Years | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT03677141 | A Phase Ib/II Study Investigating the Safety, Tolerability, Pharmacokinetics, and Efficacy of Mosunetuzumab (BTCT4465A) in Combination With CHOP or CHP-Polatuzumab Vedotin in Participants With B-Cell Non-Hodgkin Lymphoma | neoplasm of mature B-cells | Vincristine (NPC260909) | |
| NCT05384821 | Metronomic Chemotherapy in Wilms Tumor (MetroWilms-1906) | Wilms tumor | Vincristine (NPC260909) | |
| NCT05371093 | Study of Axicabtagene Ciloleucel Versus Standard of Care Therapy in Participants With Relapsed/Refractory Follicular Lymphoma | follicular lymphoma | Vincristine (NPC260909) | |
| NCT00450801 | R-MACLO-IVAM and Thalidomide in Untreated Mantle Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT02531308 | Metformin in Combination With Standard Induction Therapy for Large B-cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00072007 | Cladribine and Rituximab as Remission Induction Therapy Followed By Rituximab and Stem Cell Mobilization in Treating Patients With CLL | leukemia | Vincristine (NPC260909) | |
| NCT04884035 | Study of Safety and Efficacy of Iberdomide (CC-220) and CC-99282 Combined With R-CHOP to Treat Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00131027 | High-Dose Methotrexate (MTX) for Adult Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT04625907 | FaR-RMS: An Overarching Study for Children and Adults With Frontline and Relapsed RhabdoMyoSarcoma | rhabdomyosarcoma | Vincristine (NPC260909) | |
| NCT03991884 | Inotuzumab Ozogamicin and Chemotherapy in Treating Patients With Recurrent or Refractory B-cell Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia;lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT05192889 | Trial Treating Relapsed Acute Lymphoblastic Leukemia With Venetoclax and Navitoclax | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00440726 | Bortezomib With Chemotherapy for Relapsed Pediatric Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00003595 | Combination Chemotherapy With or Without Monoclonal Antibody Therapy in Treating Patients With Previously Untreated HIV-Associated Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00274924 | Rituximab and Combination Chemotherapy in Treating Patients With Stage II, Stage III, or Stage IV Diffuse Large B-Cell Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00477412 | Bortezomib, Rituximab and Combination Chemotherapy in Treating Participants With Mantle Cell Lymphoma | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT00003578 | High Dose Chemotherapy With or Without Bone Marrow Transplantation in Treating Patients With Intermediate- or High-Grade Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT02828358 | Azacitidine and Combination Chemotherapy in Treating Infants With Acute Lymphoblastic Leukemia and KMT2A Gene Rearrangement | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT04139304 | A Study of Daratumumab and Dose-Adjusted EPOCH in Plasmablastic Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01463852 | A Study of the Effect of Vinca Alkaloids on c-Jun N-terminal Kinase (JNK) Phosphorylation in Patients With Chronic Lymphocytic Leukemia (CLL) | chronic lymphocytic leukemia | Vincristine (NPC260909) | |
| NCT03758989 | A Study of PET Adapted Therapy and Non-invasive Monitoring for Previously Untreated Limited Stage Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT02162771 | To Demonstrate Equivalence of Pharmacokinetics and Noninferiority of Efficacy for CT-P10 in Comparison With Rituxan | follicular lymphoma | Vincristine (NPC260909) | |
| NCT00719472 | A Study of Rituximab Alternative Dosing Rate in Patients With Previously Untreated Diffuse Large B-cell or Follicular Non-Hodgkin's Lymphoma (RATE) | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT04307576 | A Treatment Study Protocol for Participants 0-45 Years With Acute Lymphoblastic Leukaemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02870907 | Adjuvant Treatment in Extensive Unilateral Retinoblastoma Primary Enucleated (RB SFCE 2009) | retinoblastoma | Vincristine (NPC260909) | |
| NCT03023358 | Compared the Efficacy and Safety of CDOP Combined With Chidamide and CDOP in de Novo Peripheral T Cell Lymphoma Patients | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT02359162 | Efficacy and Safety Study of P-Gemox vs.EPOCH as First-line Chemotherapy to Treat NK/T-cell Lymphoma With Early Stage | lymphoma | Vincristine (NPC260909) | |
| NCT01848132 | Efficacy/Safety Study of R-CHOP vs Bortezomib-R-CAP for Young Patients With Diffuse Large B-cell Lymphoma With Poor IPI. | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00632827 | Treatment of PTCL With Aggressive Induction Therapy Followed by Autologous SCT Using Denileukin Diftitox (Ontak) | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT00004031 | SWOG-9704 Chemoradiotherapy and Peripheral Stem Cell Transplantation Compared With Combination Chemotherapy in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00554164 | Positron Emission Tomography Guided Therapy of Aggressive Non-Hodgkin's Lymphomas | lymphoma | Vincristine (NPC260909) | |
| NCT00841945 | Treatment of Aggressive Localized Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00770224 | S0801 Iodine I 131 Tositumomab, Rituximab, and Combination Chemotherapy in Previously Untreated Stage II, Stage III, or Stage IV Follicular Non-Hodgkin Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00825149 | A Study of Obinutuzumab in Combination With Chemotherapy in Participants With CD20+ B-Cell Follicular Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00201318 | A Randomized Study in Non-Hodgkin's Lymphoma Patients Carrying Hepatitis B Surface Antigen | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT01309789 | A Phase 1 Study of Brentuximab Vedotin Given Sequentially and Combined With Multi-Agent Chemotherapy for CD30-Positive Mature T-Cell and NK-Cell Neoplasms | anaplastic large cell lymphoma | Vincristine (NPC260909) | |
| NCT02724579 | Reduced Craniospinal Radiation Therapy and Chemotherapy in Treating Younger Patients With Newly Diagnosed WNT-Driven Medulloblastoma | medulloblastoma | Vincristine (NPC260909) | |
| NCT02661503 | HD21 for Advanced Stages | classic Hodgkin lymphoma | Vincristine (NPC260909) | |
| NCT03536039 | RCHOP Chemoimmunotherapy Preceded BY BBB Permeabilization by t-NGR Necrosis Factor | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT04231448 | Phase III Study of Tucidinostat in Combination With R-CHOP in Patients With Newly Diagnosed Double-Expressor DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00130195 | Study of Imatinib-Combined Chemotherapy for BCR-ABL-Positive Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02918747 | PEG-ASP+Gemoxd vs. PEG-ASP+CHOP as First-line Chemotherapy to Treatment NK/T-cell Lymphoma With Early Stage | lymphoma | Vincristine (NPC260909) | |
| NCT02776605 | Ponatinib With Chemotherapy for Young Adults Ph Positive Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02063022 | Efficacy of Dose Intensification in Patients With Non-metastatic Ewing Sarcoma | Ewing sarcoma | Vincristine (NPC260909) | |
| NCT00669877 | Rituximab and Hyper-CVAD (Cyclophosphamide, Vincristine, Adriamycin, and Dexamethasone) for Burkitt's and Burkitt's -Like Leukemia/Lymphoma | Burkitts lymphoma | Vincristine (NPC260909) | |
| NCT01030900 | Phase II Trial of Alemtuzumab (Campath) and Dose-Adjusted EPOCH-Rituximab (DA-EPOCH-R) in Relapsed or Refractory Diffuse Large B-Cell and Hodgkin Lymphomas | diffuse large B-cell lymphoma;Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00169156 | A Phase II Study of Rituximab Combined With CHOP in T-cell Angio-immunoblastic Lymphoma | angioimmunoblastic T-cell lymphoma | Vincristine (NPC260909) | |
| NCT02228772 | Phase I Study of MLN 9708 in Addition to Chemotherapy for the Treatment of Acute Lymphoblastic Leukemia in Older Adults | B-cell acute lymphoblastic leukemia;lymphoblastic lymphoma;T-cell acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00500890 | Treatment of Tumors of the Choroid Plexus Epithelium | choroid plexus neoplasm | Vincristine (NPC260909) | |
| NCT04043494 | International Cooperative Treatment Protocol for Children and Adolescents With Lymphoblastic Lymphoma | lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT00431561 | Phase IIb Clinical Trial With TGF-β2 Antisense Compound AP 12009 for Recurrent or Refractory High-grade Glioma | anaplastic astrocytoma;glioblastoma multiforme | Vincristine (NPC260909) | |
| NCT03553238 | Precision Diagnosis Directing HDACi Chidamide Target Therapy for Adult ETP-ALL | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00736320 | HD16 for Early Stage Hodgkin Lymphoma | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT03117751 | Total Therapy XVII for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia and Lymphoma | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01371630 | Inotuzumab Ozogamicin and Combination Chemotherapy in Treating Patients With Acute Lymphoblastic Leukemia | Burkitts lymphoma;acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01355445 | Vincristine and Irinotecan With or Without Temozolomide in Children and Adults With Refractory/Relapsed Rhabdomyosarcoma | rhabdomyosarcoma | Vincristine (NPC260909) | |
| NCT02677116 | A Study of Olaratumab Alone and in Combination With Standard Chemotherapies in Children With Cancer | metastasis | Vincristine (NPC260909) | |
| NCT01857934 | Therapy for Children With Advanced Stage Neuroblastoma | neuroblastoma | Vincristine (NPC260909) | |
| NCT00003541 | Combination Chemotherapy, Radiation Therapy, and Peripheral Stem Cell Transplantation in Treating Patients With Stage III or Stage IV Mantle Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00001209 | A Pilot Study for the Treatment of Patients With Metastatic and High Risk Sarcomas and Primitive Neuroectodermal Tumors | osteosarcoma;Ewing sarcoma | Vincristine (NPC260909) | |
| NCT00905034 | Methotrexate, Vincristine, Pegylated L-Asparaginase and Dexamethasone (MOAD) in Acute Lymphoblastic Leukemia (ALL) Salvage | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT03225924 | Study of Entospletinib (ENTO) in Newly Diagnosed DLBCL Patients With aaIPI>=1 Treated by Chemiotherapy | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT05351346 | Genotype-guided Treatment in DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT02420717 | Ruxolitinib Phosphate or Dasatinib With Chemotherapy in Treating Patients With Relapsed or Refractory Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00114738 | EPOCH-R Chemotherapy Plus Bortezomib to Treat Mantle Cell Lymphoma | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT04974996 | A Study to Evaluate the Tolerability, Safety, Pharmacokinetics, and Antitumor Activity of Loncastuximab Tesirine in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Participants With Previously Untreated Diffuse Large B-cell Lymphoma (LOTIS-8) | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00109837 | S0333 Combination Chemotherapy in Treating Patients With Newly Diagnosed Acute Lymphoblastic Leukemia | leukemia | Vincristine (NPC260909) | |
| NCT03943901 | Split-Dose R-CHOP for Older Adults With DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00887146 | Radiation Therapy With Concomitant and Adjuvant Temozolomide Versus Radiation Therapy With Adjuvant PCV Chemotherapy in Patients With Anaplastic Glioma or Low Grade Glioma | Central Nervous System Neoplasm | Vincristine (NPC260909) | |
| NCT00901069 | Azacitidine With Rituximab, Vincristine, and Cyclophosphamide in Refractory Lymphoma | Hodgkins lymphoma;non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT04660799 | A Study on Pharmacokinetics (PK), Efficacy and Safety of Subcutaneous (SC) Versus Intravenous (IV) Rituximab, in Combination With CHOP (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone) in Previously Untreated Participants With CD20 Positive Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00854568 | Comparison Study of Doxorubicin Versus Epirubicin-induced Cardiotoxicity in Patients With DLBCL | lymphoma | Vincristine (NPC260909) | |
| NCT02055820 | A Study Evaluating the Safety, Efficacy and Pharmacokinetics of Venetoclax Combined With Chemotherapy in Participants With B-Cell Non-Hodgkin's Lymphoma (NHL) and DLBCL | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT02553460 | Total Therapy for Infants With Acute Lymphoblastic Leukemia (ALL) I | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01451515 | NHL16: Study For Newly Diagnosed Patients With Acute Lymphoblastic Lymphoma | lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT02003222 | Combination Chemotherapy With or Without Blinatumomab in Treating Patients With Newly Diagnosed BCR-ABL-Negative B Lineage Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00187083 | A Study of Children With Refractory or Relapsed ALL | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00038142 | Vincristine, Doxorubicin, Cyclophosphamide and Dexrazoxane (VACdxr) in High Risk Ewing's Sarcoma Patients | Ewing sarcoma | Vincristine (NPC260909) | |
| NCT00866749 | Augmented Berlin-Frankfurt-Munster (BFM) Therapy for Adolescent/Young Adults With Acute Lymphoblastic Leukemia or Acute Lymphoblastic Lymphoma | lymphoid leukemia;lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT04199026 | Implantable Microdevice for the Delivery of Drugs and Their Effect on Tumors in Patients With Metastatic or Recurrent Sarcoma | sarcoma | Vincristine (NPC260909) | |
| NCT01251107 | Study Comparing ABVD vs BEACOPP in Advanced Hodgkin's Lymphoma | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT05359237 | Vincristine Pharmacokinetics in Infants | hematopoietic and lymphoid cell neoplasm | Vincristine (NPC260909) | |
| NCT00186979 | Study of ZD1839 Combined With Irinotecan and Vincristine in Pediatric Patients With Refractory Solid Tumors | neoplasm | Vincristine (NPC260909) | |
| NCT00135499 | R-ACVBP Versus R-CHOP in Patients Aged 60-65 With Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT05290090 | ZR2 Followed by Immunochemotherapy in Elderly Patients With Newly-diagnosed DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT05058404 | Shortened vs Standard Chemotherapy Combined With Immunotherapy for the Initial Treatment of Patients With High Tumor Burden Follicular Lymphoma | follicular lymphoma | Vincristine (NPC260909) | |
| NCT04747912 | Study of Chemotherapy-Free Induction Regimen for Ph+ Acute Lymphoblastic Leukemia With Inotuzumab Ozogamicin (InO) | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00001339 | A Study of Combination Chemotherapy and Surgical Resection in the Treatment of Adrenocortical Carcinoma: Continuous Infusion Doxorubicin, Vincristine and Etoposide With Daily Mitotane Before and After Surgical Resection | carcinoma | Vincristine (NPC260909) | |
| NCT01607073 | Verapamil as Therapy for Children and Young Adults With Dravet Syndrome | Dravet syndrome | Verapamil (NPC42793) | |
| NCT02101853 | Blinatumomab in Treating Younger Patients With Relapsed B-cell Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02235558 | Superselective Administration of VErapamil During Recanalization in Acute Ischemic Stroke | Ischemic stroke | Verapamil (NPC42793) | |
| NCT04681417 | Ocular Conservative Treatment for Retinoblastoma : Efficacy of the New Management Strategies and Visual Outcome | retinoblastoma | Vincristine (NPC260909) | |
| NCT04478292 | A Multi-institutional Study for Treatment of Children With Newly Diagnosed Hepatoblastoma Using a Modified PHITT Strategy | Hepatoblastoma | Vincristine (NPC260909) | |
| NCT01285765 | Evaluate a Treatment Adapted to the PET Response Compared to a Standard Treatment, for Low Risk DLBCL CD 20+ Patients | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00176462 | CINJALL: Treatment for Children With Acute Lymphocytic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT04668365 | Zanubrutinib Combined With Standard Chemotherapy in the Treatment for Patients With Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00126191 | Intensive Chemotherapy and Rituximab in the Treatment of Burkitt Lymphoma | Burkitts lymphoma | Vincristine (NPC260909) | |
| NCT00352027 | Chemotherapy With Low-Dose Radiation for Pediatric Hodgkin Lymphoma | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT01864109 | Irinotecan and Temozolomide in Combination With Existing High Dose Alkylator Based Chemotherapy for Treatment of Patients With Newly Diagnosed Ewing Sarcoma | Ewing sarcoma | Vincristine (NPC260909) | |
| NCT03347786 | Verapamil for Neuroprotection in Stroke | Ischemic stroke | Verapamil (NPC42793) | |
| NCT00324831 | GM-CSF With or Without Vaccine Therapy After Combination Chemotherapy and Rituximab as First-Line Therapy in Treating Patients With Stage II, Stage III, or Stage IV Diffuse Large B-Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00344422 | Vincristine, DOXIL (Doxorubicin HCl Liposome Injection) and Dexamethasone vs. Vincristine, Doxorubicin, and Dexamethasone in Patients With Newly Diagnosed Multiple Myeloma | multiple myeloma | Vincristine (NPC260909) | |
| NCT05270057 | Loncastuximab Tesirine in Combination With DA-EPOCH-R in Patients With Previously Untreated Aggressive B-cell Lymphoid Malignancies | Burkitts lymphoma | Vincristine (NPC260909) | |
| NCT02535806 | Four Drug Reinduction With Bortezomib for Relapsed or Refractory ALL or LL in Children and Young Adults | acute lymphoblastic leukemia;lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT04980222 | A Study to Evaluate the Safety and Efficacy of Glofitamab in Combination With Rituximab (R) Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) in Circulating Tumor (ct)DNA High-Risk Patients With Untreated Diffuse Large B-Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT03018626 | R-ACVBP and DA-EPOCH-R in Patients With Non-GCB DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01370694 | Study of MK-8808 for Participants With Follicular Lymphoma (MK-8808-001) | follicular lymphoma | Vincristine (NPC260909) | |
| NCT02660762 | Modified MRCUKALLⅫ/ECOGE2993 Regimen for ALL | leukemia | Vincristine (NPC260909) | |
| NCT02981628 | Inotuzumab Ozogamicin in Treating Younger Patients With B-Lymphoblastic Lymphoma or Relapsed or Refractory CD22 Positive B Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia;lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT03349281 | Pevonedistat With VXLD Chemotherapy for Adolescent/Young Adults With Relapsed/Refractory ALL or Lymphoblastic NHL | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01000285 | EPOCH Chemotherapy and Bortezomib for Associated T-Cell Leukemia Lymphoma | T-cell acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT04402073 | Personalized Risk-Adapted Therapy in Post-Pubertal Patients With Newly-Diagnosed Medulloblastoma | medulloblastoma | Vincristine (NPC260909) | |
| NCT04529772 | A Combination of Acalabrutinib With R-CHOP in Subjects With Previously Untreated Non-GCB DLBCL (ACE-LY-312) | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00069238 | Campath-1H and EPOCH to Treat Non-Hodgkin's T- and NK-Cell Lymphomas | lymphoma | Vincristine (NPC260909) | |
| NCT00217425 | Bevacizumab and Combination Chemotherapy in Treating Patients With Peripheral T-Cell Lymphoma or Natural Killer Cell Neoplasms | lymphoma | Vincristine (NPC260909) | |
| NCT01014767 | Intercontinental Multidisciplinary Registry and Treatment Optimization Study for Choroid Plexus Tumors | brain cancer;choroid plexus neoplasm | Vincristine (NPC260909) | |
| NCT01390584 | Chemotherapy Based on PET Scan in Treating Patients With Stage I or Stage II Hodgkin Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01332968 | A Study of Obinutuzumab (RO5072759) Plus Chemotherapy in Comparison With Rituximab Plus Chemotherapy Followed by Obinutuzumab or Rituximab Maintenance in Patients With Untreated Advanced Indolent Non-Hodgkin's Lymphoma (GALLIUM) | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00791947 | A Nordic Phase II Study of PTCL Based on Dose-intensive Induction and High-dose Consolidation With ASCT | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT00968253 | RAD001 Study in Treatment of Relapsed or Refractory Acute Lymphocytic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01899066 | Efficacy Study of Lucentis in the Treatment of Retinoblastoma | retinoblastoma | Vincristine (NPC260909) | |
| NCT02529852 | A Phase I/II Study of Lenalidomide and Obinutuzumab With CHOP for Diffuse Large B Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT03283202 | Study of Safety and Efficacy of Avadomide (CC-122) Combined With RCHOP for Newly-diagnosed DLBCL With Poor Risk Factors | neoplasm of mature B-cells | Vincristine (NPC260909) | |
| NCT04356196 | Comparative Study Between the Efficacy of Verapamil and Bisoprolol on Reduction of Bleeding During Endoscopic Sinus Surgery Under General Anaesthesia. | hemorrhage | Verapamil (NPC42793) | |
| NCT04233034 | Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes | type 1 diabetes mellitus | Verapamil (NPC42793) | |
| NCT00085202 | Treatment of Patients With Newly Diagnosed Medulloblastoma, Supratentorial Primitive Neuroectodermal Tumor, or Atypical Teratoid Rhabdoid Tumor | Central Nervous System Neoplasm | Vincristine (NPC260909) | |
| NCT00002565 | Combination Chemotherapy in Treating Patients With Intermediate-Grade or Immunoblastic Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT03586999 | Nivolumab With Standard of Care Chemotherapy for Peripheral T Cell Lymphomas | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT00187161 | Treatment of Burkitt Lymphoma/Leukemia and B Large Cell NHL | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00001563 | EPOCH Chemotherapy +/- IL-12 for Previously Untreated and EPOCH Plus Rituximab for Previously Treated Patients With AIDS-Associated Lymphoma | Lymphoma, AIDS-Related | Vincristine (NPC260909) | |
| NCT04517435 | ME-401 and R-CHOP in Newly Diagnosed Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT05453500 | Chemotherapy (DA-EPOCH+/-R) and Targeted Therapy (Tafasitamab) for the Treatment of Newly-Diagnosed Philadelphia Chromosome Negative B Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT03201471 | Chidamide With R-CHOP Regimen for DLBCL Patients | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01645709 | Safety, Tolerability, and Efficacy of IA Verapamil in the Treatment of Joint Pain in Subjects With Osteoarthritis of the Knee | osteoarthritis, knee | Verapamil (NPC42793) | |
| NCT02518750 | Re-Induction Therapy for Relapsed Pediatric T-Cell Acute Lymphoblastic Leukemia or Lymphoma | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02449278 | The Palliative Benefit of Involved-site Radiotherapy for Patients With Advanced-stage Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01516567 | Intergroup Trial for Children or Adolescents With Primary Mediastinal Large B-Cell Lymphoma: DA-EPOCH-Rituximab Evaluation | lymphoma | Vincristine (NPC260909) | |
| NCT02212574 | Study Assessing the Feasibility of a Surgery and Chemotherapy-Only in Children With Wnt Positive Medulloblastoma | medulloblastoma | Vincristine (NPC260909) | |
| NCT04067037 | Camrelizumab Combined With AVD in the First-line Treatment for Patients With Advanced Classical Hodgkin's Lymphoma | classic Hodgkin lymphoma | Vincristine (NPC260909) | |
| NCT00211185 | A Study of ONTAK and CHOP in Newly Diagnosed, Peripheral T-Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00503594 | Methotrexate and Temozolomide Versus Methotrexate, Procarbazine, Vincristine and Cytarabine | lymphoma | Vincristine (NPC260909) | |
| NCT01483690 | A Pilot Study of Decitabine and Vorinostat With Chemotherapy for Relapsed ALL | precursor T-cell lymphoblastic leukemia-lymphoma | Vincristine (NPC260909) | |
| NCT01478542 | OPTIMAL>60 / DR. CHOP, Improvement of Therapy of Elderly Patients With CD20+ DLBCL Using Rituximab Optimized and Liposomal Vincristine | lymphoma | Vincristine (NPC260909) | |
| NCT04548700 | Liposomal Mitoxantrone Hydrochloride Injection,Cyclophosphamide, Vincristine and Prednisone in the Treatment of PTCL | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT04214626 | R-CHOP Combined With Lenalidomide in the First-line Treatment for Patients With Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00265031 | HD12 for Advanced Stages | lymphoma | Vincristine (NPC260909) | |
| NCT00193479 | Combination Chemotherapy and Rituximab With Pegfilgrastim Followed by Rituximab, in Large B-Cell Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT02116959 | Alternating Systemic Chemotherapy and Intra-Arterial Melphalan (IAM) Chemotherapy in Children With Intra-Ocular Retinoblastoma | retinoblastoma | Vincristine (NPC260909) | |
| NCT00877006 | Study of Bendamustine Hydrochloride and Rituximab (BR) Compared With R-CVP or R-CHOP in the First-Line Treatment of Patients With Advanced Indolent Non-Hodgkin's Lymphoma (NHL) or Mantle Cell Lymphoma (MCL) - Referred to as the BRIGHT Study | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT01659099 | GA In NEwly Diagnosed Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01356680 | HD17 for Intermediate Stage Hodgkin Lymphoma | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00945724 | Safety and Feasibility Study of Combination of State of Art Chemoimmunotherapy, Intensive Central Nervous System Prophylaxis and Scrotal Irradiation to Treat Primary Diffuse Large B-cell Lymphoma of Testis | lymphoma | Vincristine (NPC260909) | |
| NCT00003784 | S9911, Combination Chemotherapy Plus Monoclonal Antibody Therapy in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00001689 | A Pharmacokinetic and Pharmacodynamic Study of Vincristine in Children With Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01622439 | Valproate as First Line Therapy in Combination With Rituximab and CHOP in Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00930605 | The Effectiveness of Alemtuzumab Given in Combination With CHOP and ESHAP in Patients Newly Diagnosed With Peripheral T-Cell Lymphoma (PTCL) | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT01920737 | A Novel "Pediatric-Inspired" Regimen With Reduced Myelosuppressive Drugs for Adults (Aged 18-60) With Newly Diagnosed Ph Negative Acute Lymphoblastic Leukemia | leukemia | Vincristine (NPC260909) | |
| NCT05046314 | A Clinical Study of TK216 in Patients With Relapsed or Refractory Ewing's Sarcoma | Ewing sarcoma | Vincristine (NPC260909) | |
| NCT00115700 | Radiotherapy Versus Radiotherapy Plus Chemotherapy in Early Stage Follicular Lymphoma | follicular lymphoma | Vincristine (NPC260909) | |
| NCT01148446 | R-CHOP Versus R-mini-CEOP in Elderly Patients(>65)With DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00136084 | Treatment of Patients With Newly Diagnosed Acute Myeloid Leukemia or Myelodysplasia | acute myeloid leukemia | Vincristine (NPC260909) | |
| NCT01852435 | R-CEOP-90/R-CEOP-70 Versus R-CHOP-50 in the Treatment of Diffuse Large B-cell Lymphoma and Follicular Lymphoma Grade 3B | diffuse large B-cell lymphoma;follicular lymphoma | Vincristine (NPC260909) | |
| NCT05425654 | RL-MPV Followed by BBC HCT Using Autologous Stem Cells and Maintenance Therapy With Nivolumab for Newly Diagnosed PCNSL | lymphoma | Vincristine (NPC260909) | |
| NCT00026208 | Combination Chemotherapy Plus Low-Dose Radiation Therapy in Treating Patients With Stage I or Stage IIA Hodgkin's Lymphoma | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00136435 | A Study in Adults With Untreated Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02405676 | BNHL-2015 for Children or Adolescents in China | neoplasm of mature B-cells | Vincristine (NPC260909) | |
| NCT00337987 | A Pilot Study to Determine the Safety of the Combination of Ontak in Combination With CHOP in Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT02449252 | Efficacy of Consolidative Involved-site Radiotherapy for Patients With Limited-stage Follicular Lymphoma | follicular lymphoma | Vincristine (NPC260909) | |
| NCT01746173 | CHOEP + High Dose Therapy + Auto SCT for T-Cell Lymphoma | T-cell non-Hodgkin lymphoma | Vincristine (NPC260909) | |
| NCT00060346 | Rituximab and Combination Chemotherapy in Treating Patients With Newly Diagnosed Waldenstrom's Macroglobulinemia | lymphoma | Vincristine (NPC260909) | |
| NCT00004179 | Combination Chemotherapy With or Without Rituximab in Treating Patients With Relapsed Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT03782662 | Drug Interaction Study With RV521 in Healthy Volunteer Subjects | Respiratory Syncytial Virus Infection | Verapamil (NPC42793) | |
| NCT02163356 | Fenretinide Lym-X-Sorb + Ketoconazole + Vincristine for Recurrent or Resistant Neuroblastoma | neuroblastoma | Vincristine (NPC260909) | |
| NCT02596971 | A Study of Atezolizumab in Combination With Either Obinutuzumab Plus Bendamustine or Obinutuzumab Plus (+) Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) in Participants With Follicular Lymphoma (FL) or Rituximab + CHOP in Participants With Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00005867 | Combination Chemotherapy in Treating Patients With Aggressive Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT03003520 | A Study of Durvalumab in Combination With R-CHOP or Lenalidomide Plus R-CHOP in Previously Untreated High-Risk Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01724021 | A Study of Participant Preference With Subcutaneous Versus Intravenous MabThera/Rituxan in Participants With CD20+ Diffuse Large B-Cell Lymphoma or CD20+ Follicular Non-Hodgkin's Lymphoma Grades 1, 2 or 3a | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01735747 | Temozolomide, Nedaplatin, Vincristine, and Radiotherapy as First-line Treatment in Newly Diagnosed Primary CNS Lymphoma | Central Nervous System Neoplasm | Vincristine (NPC260909) | |
| NCT00002557 | Combination Chemotherapy in Patients With Advanced or Recurrent Mycosis Fungoides | lymphoma | Vincristine (NPC260909) | |
| NCT04978584 | Rituximab, Lenalidomide, Acalabrutinib, Tafasitamab Alone and With Combination Chemotherapy for the Treatment of Newly Diagnosed Non-germinal Center Diffuse Large B-Cell Lymphoma, Smart Stop Study | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00646854 | Alemtuzumab and CHOP in T-cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT03755804 | Pediatric Classical Hodgkin Lymphoma Consortium Study: cHOD17 | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT01974440 | A Study of PCI-32765 (Ibrutinib) in Combination With Either Bendamustine and Rituximab or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Participants With Previously Treated Indolent Non-Hodgkin Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00187005 | Total Therapy Study XIV for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00038610 | Study of Hyper-CVAD Plus Imatinib Mesylate for Philadelphia-Positive Acute Lymphocytic Leukemia | leukemia | Vincristine (NPC260909) | |
| NCT02012088 | Clinical Trial to Evaluate R-COMP Versus R-CHOP in Newly Diagnosed Patients With Non-localised Diffuse Large B-cell Lymphoma (DLBCL)/Follicular Lymphoma Grade IIIb | lymphoma | Vincristine (NPC260909) | |
| NCT02792491 | Phase II Prospective Trial of Addition of Rituximab to Reduced Dose CHOP Chemotherapy in DLBC L Patients Aged 65 Years and Over | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00556127 | Rituximab in Addition to Chemotherapy With Autologous Stem Cell Transplantation as Treatment Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00250718 | Study: Treatment of Relapsed Lymphoid Malignancies With an Anti-Angiogenic Approach | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00000556 | Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) | atrial fibrillation | Verapamil (NPC42793) | |
| NCT00476190 | ALL Adult Consortium Trial: Adult ALL Trial | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00970385 | Study About Treatment of Newly Diagnosed Non Cutaneous Peripheral T Cell Lymphoma | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT00450385 | Genes in Predicting Outcome of Patients With DLBCL Treated With Rituximab and Combination Chemotherapy (R-CHOP) | lymphoma | Vincristine (NPC260909) | |
| NCT03296800 | Study to Evaluate Effects of Probenecid, Rifampin and Verapamil on Bexagliflozin in Healthy Subjects | type 2 diabetes mellitus | Verapamil (NPC42793) | |
| NCT00083551 | UARK 98-026 TT II: Multiple Myeloma Evaluating Anti-Angiogenesis With Thalidomide and Post-Transplant Consolidation Chemotherapy | multiple myeloma | Vincristine (NPC260909) | |
| NCT00333008 | A Dose Study of Doxil in a Dose Dense, 14 Day CDOP/Rituximab Regimen for Patients With Diffuse Large B-Cell Non-Hodgkin Lymphoma (NHL)> 60 Years or With Compromised Cardiac Status. | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00003389 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00003150 | Combination Chemotherapy With or Without Monoclonal Antibody Therapy in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT02265770 | An International Clinical Program for the Diagnosis and Treatment of Children With Ependymoma | childhood ependymoma | Vincristine (NPC260909) | |
| NCT02855359 | Denintuzumab Mafodotin (SGN-CD19A) Combined With RCHOP or RCHP Versus RCHOP Alone in Diffuse Large B-Cell Lymphoma or Follicular Lymphoma | diffuse large B-cell lymphoma;follicular lymphoma | Vincristine (NPC260909) | |
| NCT00433537 | Combination Chemotherapy and Rituximab in Treating Patients With Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT02639650 | Study of Paclitaxel Plus Cisplatin as the First-line Chemotherapy in High Risk Gestational Trophoblastic Tumor | gestational trophoblastic neoplasm | Vincristine (NPC260909) | |
| NCT03860844 | Isatuximab in Combination With Chemotherapy in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia or Acute Myeloid Leukemia | acute lymphoblastic leukemia;acute myeloid leukemia | Vincristine (NPC260909) | |
| NCT02617485 | MabionCD20® Compared to MabThera® in Lymphoma Patients | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT05201248 | A Study to Evaluate Adverse Events and Change in Disease Activity of Subcutaneous (SC) Epcoritamab As Monotherapy or Combined With Standard of Care Therapies in Adult Participants in China With B-Cell Non-Hodgkin Lymphoma | neoplasm of mature B-cells | Vincristine (NPC260909) | |
| NCT00518947 | Pharmacotherapy of Treatment-Resistant Mania | bipolar disorder | Verapamil (NPC42793) | |
| NCT03571321 | Ruxolitinib and Chemotherapy in Adolescents and Young Adults With Ph-like Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00268853 | A Trial in Patients With Diffuse Large-B-cell Lymphoma Comparing Pixantrone Against Doxorubicin | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00003215 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Newly Diagnosed Aggressive Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00313053 | Study of mAb 216 With Chemotherapy for Treatment of Pediatric Relapsed or Refractory B-progenitor Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02753647 | Chidamide Plus R-CHOP in Elderly DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT04545151 | Verapamil SR in Adults With Type 1 Diabetes | type 1 diabetes mellitus | Verapamil (NPC42793) | |
| NCT01887587 | Vincristine, Doxorubicin, And Dexamethasone + Ixazomib in Acute Lymphoblastic Leukemia (ALL), Lymphoblastic Lymphoma Or Mixed Phenotype Acute Leukemia | acute lymphoblastic leukemia;lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT00809341 | R-ICE and High-Dose Cyclophosphamide With PET/CT for Diffuse Large B-Cell Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT03962465 | Phase I Study of Inotuzumab With Augmented BFM Re-Induction for Patients With Relapsed/Refractory B-cell ALL | B-cell acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02209155 | R-Verapamil for the Prophylaxis of Episodic Cluster Headache | pain | Verapamil (NPC42793) | |
| NCT01889069 | A Study to Evaluate Safety, Efficacy and Pharmacokinetics of Rituximab (MabThera/Rituxan) in Participants With Diffuse Large B Cell Lymphoma (DLBCL) or Follicular Lymphoma (FL) | lymphoma | Vincristine (NPC260909) | |
| NCT00613457 | Combination Chemotherapy Based on Risk of Relapse in Treating Young Patients With Acute Lymphoblastic Leukemia | leukemia | Vincristine (NPC260909) | |
| NCT00195871 | Safety and Efficacy of an Adult Acute Lymphoblastic Leukemia Chemotherapy for Adult Lymphoblastic Lymphoma | lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT03188198 | Risk Adapted Therapy in Diffuse Large B Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01925612 | Study of Brentuximab Vedotin Combined With RCHOP or RCHP in Front-line Treatment of Patients With Diffuse Large B-cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT02404220 | Safety and Efficacy of Entospletinib With Vincristine and Dexamethasone in Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00801281 | First-line R-CVP vs R-CHOP Induction Immunochemotherapy for Indolent Lymphoma and R Maintenance. | chronic lymphocytic leukemia;follicular lymphoma;lymphoplasmacytic lymphoma | Vincristine (NPC260909) | |
| NCT05008224 | Study of Safety and Efficacy of Pembrolizumab and Chemotherapy in Participants With Newly Diagnosed Classical Hodgkin Lymphoma (cHL) (MK-3475-C11/KEYNOTE-C11) | classic Hodgkin lymphoma | Vincristine (NPC260909) | |
| NCT03808610 | Low-Intensity Chemotherapy and Venetoclax in Treating Patients With Relapsed or Refractory B- or T-Cell Acute Lymphoblastic Leukemia | T-cell acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01871766 | Risk-Adapted Focal Proton Beam Radiation and/or Surgery in Patients With Low, Intermediate and High Risk Rhabdomyosarcoma Receiving Standard or Intensified Chemotherapy | rhabdomyosarcoma | Vincristine (NPC260909) | |
| NCT02912663 | Magnesium And Verapamil After Recanalization in Ischemia of the Cerebrum: a Clinical and Translational Study. | Ischemic stroke | Verapamil (NPC42793) | |
| NCT02568683 | Safety and Efficacy of Entospletinib (ENTO [GS-9973]) Combined With Vincristine (VCR) in Adult Participants With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma (NHL) | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00064116 | Combination Chemotherapy With or Without Rituximab in Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01516580 | Intergroup Randomized Trial for Children or Adolescents With B-Cell Non Hodgkin Lymphoma or B-Acute Leukemia: Rituximab Evaluation in High Risk Patients | leukemia;non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00494780 | Ofatumumab (Humax-CD20) With CHOP (Cyclophosphamide,Doxorubicin, Vincristine, Predisolone) in Follicular Lymphoma (FL) Patients | follicular lymphoma | Vincristine (NPC260909) | |
| NCT01397825 | MLN8237 in Patients With Relapsed or Refractory Aggressive B-Cell Lymphoma Treated With Rituximab +/- Vincristine | Burkitts lymphoma;diffuse large B-cell lymphoma;follicular lymphoma;Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT00101101 | Universal Granulocyte Macrophage-colony Stimulating Factor (GM-CSF)-Producing and GM.CD40L for Autologous Tumor Vaccine in Mantle Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01040871 | Study of the Combination of VELCADE, Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Non-Germinal Center B-Cell Subtype of Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00324467 | Tailoring Treatment for B Cell Non-hodgkin's Lymphoma Based on PET Scan Results Mid Treatment | neoplasm of mature B-cells;non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00131053 | Applying Pediatric Regimens to Younger Adult Patients With Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02035137 | 131I-MIBG Alone VS. 131I-MIBG With Vincristine and Irinotecan VS131I-MIBG With Vorinostat | neuroblastoma | Vincristine (NPC260909) | |
| NCT05458180 | CMOEP in the Treatment of Untreated Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT00313157 | RATe Control in Atrial Fibrillation | atrial fibrillation | Verapamil (NPC42793) | |
| NCT01468740 | Prospective Study on HIV-related Hodgkin Lymphoma | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00907348 | Prospective Multicenter Dose Finding Phase II Pilot Trial to Evaluate Efficacy and Safety of LR-CHOP21 for Elderly Patients With Untreated Diffuse Large B Cell Lymphoma | follicular lymphoma | Vincristine (NPC260909) | |
| NCT04835870 | Zanubrutinib Plus R-CHOP for Patients With Newly Diagnosed Untreated Non-GCB DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01427114 | R-CVP for the Treatment of Non-conjunctival Ocular Adnexal MALT Lymphoma (OAML) | lymphoma | Vincristine (NPC260909) | |
| NCT00722137 | Study of the Combination of Rituximab, Cyclophosphamide, Doxorubicin, VELCADE, and Prednisone or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Mantle Cell Lymphoma | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT03020030 | Treatment of Newly Diagnosed Acute Lymphoblastic Leukemia in Children and Adolescents | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02911142 | Lenalidomide Combined With Modified DA-EPOCH and Rituximab (EPOCH-R2) in Primary Effusion Lymphoma or KSHV-associated Large Cell Lymphoma | B-cell neoplasm | Vincristine (NPC260909) | |
| NCT03013933 | Brentuximab Vedotin, Cyclosporine, and Verapamil Hydrochloride in Treating Patients With Relapsed or Refractory Hodgkin Lymphoma | Hodgkins lymphoma | Verapamil (NPC42793) | |
| NCT02356159 | Study of Palifermin (Kepivance) in Persons Undergoing Unrelated Donor Allogeneic Hematopoietic Cell Transplantation | myelodysplastic syndrome;Hodgkins lymphoma;leukemia;multiple myeloma;non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00186875 | Therapy for Pediatric Relapsed or Refractory Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00993044 | A Study of Vincristine, Escalating Doses of Irinotecan, Temozolomide and Bevacizumab (Vit-b) in Pediatric and Adolescent Patients With Recurrent or Refractory Solid Tumors of Non-hematopoietic Origin | neoplasm | Vincristine (NPC260909) | |
| NCT04773366 | A Prospective Study for the Treatment of Children With Newly Diagnosed LCH Using a Cytarabine Contained Protocol | Langerhans Cell Histiocytosis | Vincristine (NPC260909) | |
| NCT02406092 | Safety Study of Rituximab (SC) Administered in Participants With CD20+ DLBCL or CD20+ Follicular NHL Grade 1 to 3A | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT01415765 | MLN4924 Compared With MLN4924 Plus Chemotherapy for Large B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00290433 | Efficacy of the HCVIDDOXIL Regimen in Patients With Newly Diagnosed Peripheral T-Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT02303821 | Study of Carfilzomib in Combination With Induction Chemotherapy in Children With Relapsed or Refractory Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00568464 | Study on VCD/IE in the Patients With Ewing's Sarcoma Family of Tumors (ESFT) | Ewing sarcoma | Vincristine (NPC260909) | |
| NCT00022945 | Safety and Efficacy Study of Iodine-131 Anti-B1 Antibody Plus CHOP For Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT00634179 | A Phase I/II Trial of VR-CHOP in Lymphoma Patients | follicular lymphoma | Vincristine (NPC260909) | |
| NCT00060385 | Combination Chemotherapy With or Without Etoposide in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00973752 | Treatment of Older Adults With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02566993 | Clinical Trial of Lurbinectedin (PM01183)/Doxorubicin Versus CAV or Topotecan as Treatment in Patients With Small-Cell Lung Cancer | small cell lung carcinoma | Vincristine (NPC260909) | |
| NCT02143414 | Blinatumomab and Combination Chemotherapy or Dasatinib, Prednisone, and Blinatumomab in Treating Older Patients With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT03952572 | Efficacy and Safety of CDOP vs CHOP for Newly Diagnosed Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT02626455 | Study of Copanlisib in Combination With Standard Immunochemotherapy in Relapsed Indolent Non-Hodgkin's Lymphoma (iNHL) | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT01287741 | A Study of Obinutuzumab in Combination With CHOP Chemotherapy Versus Rituximab With CHOP in Participants With CD20-Positive Diffuse Large B-Cell Lymphoma (GOYA) | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01321008 | Stage I/II Nasal NK Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT02228512 | Study of Pomalidomide Combined With Modified DA-EPOCH and Rituximab in KSHV-Associated Lymphomas | Castleman disease;diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT02684708 | Second International Inter-Group Study for Classical Hodgkin Lymphoma in Children and Adolescents | classic Hodgkin lymphoma | Vincristine (NPC260909) | |
| NCT03553537 | Efficacy and Safety of Decitabine Plus CHOP vs CHOP in Patients With Untreated Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT00284271 | Feasibility and Efficacy of BACOPP-21 for Patients > 60 Years With Intermediate or Advanced Hodgkins Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00054665 | PS-341 Alone and PS-341 Plus EPOCH Chemotherapy to Treat Non-Hodgkin's Lymphoma | neoplasm of mature B-cells | Vincristine (NPC260909) | |
| NCT03564704 | Precision Diagnosis Directing HDACi Chidamide Target Therapy for Adult T-LBL/ALL | lymphoblastic lymphoma | Vincristine (NPC260909) | |
| NCT01831505 | Feasibility of Assessing Lymphoma Response to Precise Local Injection of Candidate Chemotherapy Agents | lymphoma | Vincristine (NPC260909) | |
| NCT05200312 | A Phase II Study of Zanubrutinib, Lenalidomide Plus R-CHOP as the First-line Treatment for Diffused Large B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01788137 | A Study of Improving the Efficacy of Treatment in High Risk T Cell Lymphoma Patients | T-cell acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00441168 | Velcade (Bortezomib), Adriamycin Dexamethasone (PAD) or Vincristine Adriamycin Dexamethasone in Second Line Treatment of Multiple Myeloma | multiple myeloma | Vincristine (NPC260909) | |
| NCT04796012 | VITAS: Atezolizumab in Combination With Chemotherapy for Pediatric Relapsed/Refractory Solid Tumors | neoplasm | Vincristine (NPC260909) | |
| NCT01527422 | Cyclophosphamide, Doxorubicin, Vincristine, Prednisone, Rituximab Pateinets With Aggresive NHL | lymphoma | Vincristine (NPC260909) | |
| NCT05457829 | Doxorubicin Hydrochloride Liposome Combined With Irinotecan Versus VIT Regimen in the Treatment of Pediatric Rhabdomyosarcoma | rhabdomyosarcoma | Vincristine (NPC260909) | |
| NCT02428751 | R-CHOP Versus R-CDOP as First-line Treatment for Elderly Patients With Diffuse Large-B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT05428670 | The Efficacy and Safety of ZR2 Versus R-CHOP-like Regimen for Elderly Patients With Newly Diagnosed Diffuse Large B Cell Lymphoma. | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01878617 | A Clinical and Molecular Risk-Directed Therapy for Newly Diagnosed Medulloblastoma | medulloblastoma | Vincristine (NPC260909) | |
| NCT01719835 | CHOP vs GEM-P in 1st Line Treatment of T-cell Lymphoma, Multicentre Phase II Study | anaplastic large cell lymphoma;angioimmunoblastic T-cell lymphoma;unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT03853044 | Study Evaluating the Safety and Efficacy of C-CHOP in Untreated Subjects With Angioimmunoblastic T Cell Lymphoma | angioimmunoblastic T-cell lymphoma | Vincristine (NPC260909) | |
| NCT00379574 | Bortezomib Plus CHOP Every 2 Weeks for Advanced Stage DLBCL | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00201695 | Liposomal Doxorubicin, Vincristine, & Dexamethasone Plus Arsenic Trioxide in Untreated Symptomatic Multiple Myeloma | multiple myeloma | Vincristine (NPC260909) | |
| NCT00041132 | S0213 Chemotherapy Plus Rituximab in Treating Patients With Mantle Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01523977 | Everolimus With Multiagent Re-Induction Chemotherapy in Pediatric Patients With ALL | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00088530 | BBR 2778 for Relapsed, Aggressive Non-Hodgkin's Lymphoma (NHL) | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT02703272 | A Safety and Efficacy Study of Ibrutinib in Pediatric and Young Adult Participants With Relapsed or Refractory Mature B-cell Non-Hodgkin Lymphoma | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00671658 | Modified Hyper-CVAD (Cyclophosphamide, Vincristine, Adriamycin, and Dexamethasone) Program for Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00165178 | Treatment of Acute Lymphoblastic Leukemia in Children | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT04221035 | High-Risk Neuroblastoma Study 2 of SIOP-Europa-Neuroblastoma (SIOPEN) | neuroblastoma | Vincristine (NPC260909) | |
| NCT02595242 | Safety and Efficacy Study of CNOP Chemotherapy in Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT03617432 | Chidamide Combined With CHOPE Regimen for Peripheral T-cell Lymphoma Patients | neoplasm | Vincristine (NPC260909) | |
| NCT04922567 | Efficacy and Safety of Lenalidomide Plus CHOP vs CHOP in Patients With Untreated Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Vincristine (NPC260909) | |
| NCT01424982 | Combination Chemotherapy and Ponatinib Hydrochloride in Treating Patients With Acute Lymphoblastic Leukemia | Blast Phase Chronic Myelogenous Leukemia, BCR-ABL1 Positive | Vincristine (NPC260909) | |
| NCT02772354 | Radiofrequency Ablation of Symptomatic Frequent Ventricular Premature Complexes in Pediatric Population | Ventricular arrhythmia | Verapamil (NPC42793) | |
| NCT01435018 | Three Chemo Regimens as an Adjunct to ART for Treatment of Advanced AIDS-KS | HIV-1 infection | Vincristine (NPC260909) | |
| NCT00877110 | Anti-GD2 3F8 Antibody and Allogeneic Natural Killer Cells for High-Risk Neuroblastoma | neuroblastoma | Vincristine (NPC260909) | |
| NCT00549848 | Total Therapy Study XVI for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT03643276 | Treatment Protocol for Children and Adolescents With Acute Lymphoblastic Leukemia - AIEOP-BFM ALL 2017 | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT02398240 | Brentuximab for Newly Diagnosed Hodgkin Disease | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT04824092 | Tafasitamab + Lenalidomide + R-CHOP Versus R-CHOP in Newly Diagnosed High-intermediate and High Risk DLBCL Patients | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00555464 | Clinical Trial of Vincristine vs. Prednisolone for Treatment of Complicated Hemangiomas | hemangioma | Vincristine (NPC260909) | |
| NCT00184002 | Doxorubicin (Doxil) Combined With Rituxan, Cyclophosphamide, Vincristine and Prednisone in Newly Diagnosed Aggressive Non-Hodgkin's Lymphomas | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT05389423 | Pomalidomide and Dose-Adjusted EPOCH +/- Rituximab for HIV-Associated Lymphomas | Burkitts lymphoma;diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT03407144 | Safety and Efficacy of Pembrolizumab (MK-3475) in Children and Young Adults With Classical Hodgkin Lymphoma (MK-3475-667/KEYNOTE-667) | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00878254 | Rituximab and Combination Chemotherapy in Treating Patients With Previously Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT02285062 | Efficacy and Safety Study of Lenalidomide Plus R-CHOP Chemotherapy Versus Placebo Plus R-CHOP Chemotherapy in Untreated ABC Type Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT05382338 | A Study of Treatment for Medulloblastoma Using Sodium Thiosulfate to Reduce Hearing Loss | medulloblastoma | Vincristine (NPC260909) | |
| NCT00581776 | Phase II Study of VcR-CVAD With Rituximab Consolidation and Maintenance for Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT00299182 | Study of AMG 531 to Evaluate the Safety & Efficacy in Patients With Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00004112 | Combination Chemotherapy With or Without Rituximab in Treating Patients With Newly Diagnosed Non-Hodgkin's Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT01414855 | A Study of Obinutuzumab [RO5072759 (GA101)] in Combination With CHOP Chemotherapy in Patients With Previously Untreated Advanced Diffuse Large B-Cell Lymphoma (GATHER) | lymphoma | Vincristine (NPC260909) | |
| NCT01679119 | Treatment of Patients With Diffuse Large B Cell Lymphoma Who Are Not Suitable for Anthracycline Containing Chemotherapy | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01046825 | Mature B-Cell Lymphoma And Leukemia Study III | lymphoma | Vincristine (NPC260909) | |
| NCT00137111 | Therapy for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01855750 | A Study of the Bruton's Tyrosine Kinase Inhibitor, PCI-32765 (Ibrutinib), in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Non-Germinal Center B-Cell Subtype of Diffuse Large B-Cell Lymphoma | lymphoma | Vincristine (NPC260909) | |
| NCT00215943 | Phase III Randomized Trial of Thalidomide/Dexamethasone Versus Vincristine+Adriamycin+Dexamethasone (VAD) | multiple myeloma | Vincristine (NPC260909) | |
| NCT02449265 | Efficacy of Consolidative Involved-site Radiotherapy for Patients With Limited-stage Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00184041 | Intensified Post Remission Therapy Containing PEG-Asparaginase | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT04638790 | First Line Chemotherapy for Classical Hodgkin Lymphoma in Russia (HL-Russia-1) | Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT05422066 | Selinexor Plus R-CHOP in High-risk GCB-subtype Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00461747 | GEM05 for Patients With Multiple Myeloma Under 65 Years | multiple myeloma | Vincristine (NPC260909) | |
| NCT00209209 | Induction Chemotherapy (R-CHOP Vs. R-FC) Followed by Interferon Maintenance Versus Rituximab Maintenance in MCL | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT02951728 | Decitabine Plus R-CHOP in Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT01324180 | Vincristine, Dexamethasone, Doxorubicin, and PEG-asparaginase (VPLD) and Metformin for Relapsed Childhood Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT01200758 | A Study of Rituximab (MabThera) Subcutaneous (SC) Versus Rituximab (MabThera) Intravenous in Participannts With Follicular Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Vincristine (NPC260909) | |
| NCT00384111 | Phase 3 Study of Zevalin Following R-CVP in Previously Untreated Patients With Follicular Non Hodgkin's Lymphoma (NHL) | follicular lymphoma | Vincristine (NPC260909) | |
| NCT02343536 | A Phase 1, Open-label Trial of Oral Azacitidine (CC-486) Plus RCHOP in Subjects With Large B-Cell Lymphoma or Follicular Lymphoma or Transformed Lymphoma | diffuse large B-cell lymphoma;follicular lymphoma | Vincristine (NPC260909) | |
| NCT03274492 | A Study Comparing the Efficacy and Safety of Polatuzumab Vedotin With Rituximab-Cyclophosphamide, Doxorubicin, and Prednisone (R-CHP) Versus Rituximab-Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Participants With Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00209222 | Efficacy of R-CHOP vs R-CHOP/R-DHAP in Untreated MCL | Mantle cell lymphoma | Vincristine (NPC260909) | |
| NCT03384654 | A Study to Evaluate the Efficacy and Safety of Daratumumab in Pediatric and Young Adult Participants Greater Than or Equal to (>=)1 and Less Than or Equal to (<=) 30 Years of Age With Relapsed/Refractory Precursor B-cell or T-cell Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma | acute lymphoblastic leukemia | Vincristine (NPC260909) | |
| NCT00931918 | Study to Assess the Effectiveness of RCHOP With or Without VELCADE in Previously Untreated Non-Germinal Center B-Cell-like Diffuse Large B-Cell Lymphoma Patients | diffuse large B-cell lymphoma | Vincristine (NPC260909) | |
| NCT00133991 | Combination Chemotherapy and Rituximab in Treating Patients With Newly Diagnosed Burkitt's Lymphoma or Leukemia | leukemia;lymphoma | Vincristine (NPC260909) | |
| NCT01303887 | A Trial Looking at Rituximab and Chemotherapy as a Treatment for Follicular Lymphoma in Elderly Patients | follicular lymphoma | Vincristine (NPC260909) | |
| NCT00786669 | A Pilot Study of the Addition of Bevacizumab to VOIT Regimen for Relapsed/Refractory Pediatric Solid Tumors | neoplasm | Vincristine (NPC260909) |
❱❱❱ Associated Human Diseases and Detailed Association Evidence
How do we define the Plant-Targeted Human Disease Association?
Associated human diseases of an individual plant are summurized based on FOUR types of association evidence, these include:
❶ Association by Therapeutic Target: Bioactive protein targets of the plant were defined in "Molecular Targets" section, target-disease associations collected from TTD database were subsequently used to build the associations between the plant and its targeted human diseases.
❷ Association by Disease Gene Reversion: Plant and a specific disease will be associated when >= 1 plant target gene overlaped with disease's DEGs.
❸ Association by Clinical Trials of Plant: Plant and a specific disease will be associated when >= 1 clinical trial (the plant is the intervetion) can be matched in ClinicalTrials.gov database.
❹ Association by Clinical Trials of Plant Ingredients: Plant and a specific disease will be associated when >= 1 clinical trial (the plant ingredient is the intervetion) can be matched in ClinicalTrials.gov database.
Associated Disease of the Plant |
Association Type & Detailed Evidence |
|---|---|
LeukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60-2B33 |
NCT02660762,NCT01920737,NCT00133991,NCT02356159,NCT00038610,NCT00613457,NCT00072007,NCT01516580,NCT00109837
|
Multiple myelomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A83 |
DRD3
NCT02356159,NCT00461747,NCT00201695,NCT00083551,NCT00344422,NCT00215943,NCT00441168 |
RetinoblastomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D02.2 |
NCT02116959,NCT01899066,NCT01783535,NCT01906814,NCT04681417,NCT02137928,NCT02870907,NCT00186888
|
Mature B-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85 |
NCT03467373,NCT03677141,NCT03283202,NCT05201248,NCT03206671,NCT02405676,NCT00054665,NCT00324467
|
Adenocarcinoma of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.0 |
HTR2B,ABCB1,CACNA1C,ABCG2,LMNA,TUBB6,HTR2A,KCNH2
|
Mature T-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90 |
DRD3
NCT00006721,NCT01889069,NCT00841945,NCT00041132,NCT01974440,NCT00005867,NCT01055496,NCT01831505,NCT01046825,NCT00004112,NCT01855750,NCT00004179,NCT05425654,NCT00060346,NCT00290498,NCT02012088,NCT00284271,NCT00450385,NCT01427114,NCT00945724,NCT00299182,NCT00052936,NCT00069238,NCT00002557,NCT00002565,NCT00101101,NCT00787527,NCT00335140,NCT00854568,NCT03188198,NCT01390584,NCT00118209,NCT00211185,NCT00003215,NCT00265031,NCT00217425,NCT00133991,NCT00290433,NCT01399372,NCT04884035,NCT00003595,NCT00003389,NCT01414855,NCT00064116,NCT01321008,NCT00264953,NCT01478542,NCT00274924,NCT00324831,NCT00003541,NCT04980222,NCT02529852,NCT01430013,NCT00770224,NCT00004031,NCT01527422,NCT01516567,NCT00003150,NCT00003784,NCT00060385,NCT02918747,NCT00003578,NCT00450801,NCT00503594,NCT00577993,NCT00554164,NCT02359162,NCT00451178,NCT00646854 |
Ewing sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B52 |
NCT03359005,NCT01864109,NCT00001209,NCT02063022,NCT00038142,NCT05046314,NCT00568464
|
Neuroblastoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH85Z0 |
NCT01857934,NCT02035137,NCT03786783,NCT00877110,NCT02163356,NCT01704716,NCT04221035
|
Malignant neoplasms of biliary tract, distal bile ductDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C15 |
SLC6A4,CACNA1C,LMNA,TUBB6,CACNA1F,KCNH2,ADRA2A
|
Diffuse large B-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A81 |
NCT03201471,NCT01030900,NCT03003520,NCT00850512,NCT00931918,NCT02951728,NCT04517435,NCT02889523,NCT00135499,NCT04668365,NCT01009970,NCT02855359,NCT01004991,NCT04974996,NCT02449265,NCT02285062,NCT04824092,NCT03536039,NCT04529772,NCT05389423,NCT02449278,NCT03018626,NCT00268853,NCT01622439,NCT01659099,NCT03943901,NCT04835870,NCT05290090,NCT03274492,NCT01724021,NCT05422066,NCT01848132,NCT01925612,NCT01287741,NCT05428670,NCT01679119,NCT02343536,NCT01852435,NCT04231448,NCT03225924,NCT01040871,NCT00333008,NCT01415765,NCT05200312,NCT04660799,NCT02228512,NCT04139304,NCT02428751,NCT04978584,NCT03758989,NCT01397825,NCT01148446,NCT04025593,NCT04214626,NCT00556127,NCT02595242,NCT01285765,NCT02753647,NCT05351346,NCT00575406,NCT02617485,NCT02792491,NCT00379574,NCT02596971,NCT02531308
|
Attention deficit hyperactivity disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A05 |
SLC6A4,HTR2B,ADRA2A,DRD3,HTR2A,HTR2C
|
DepressionDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A70-6A7Z |
SLC6A4,HTR2C,HTR2A,DRD3,HTR2B,ADRA2A
|
Pain, unspecifiedDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG3Z |
HTR2C,KCNH2,ADRA2A,SLC6A4
NCT02209155,NCT00804895 |
Medulloblastoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH8P29 |
NCT05382338,NCT02212574,NCT00031590,NCT04402073,NCT01878617,NCT02724579
|
Burkitt lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH4KA9 |
NCT01397825,NCT00669877,NCT05270057,NCT00126191,NCT05389423,NCT01371630
|
Diffuse large B-cell lymphomasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A81 |
HTR2B,CACNA1C,LMNA,TUBB6,HTT,ADRA2A
|
Precursor cell lymphoblastic leukaemia, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5J37 |
NCT02776605,NCT02553460,NCT02535806,NCT02981628,NCT00905034,NCT02518750,NCT01324180,NCT02828358,NCT01371630,NCT00440726,NCT00973752,NCT02881086,NCT05192889,NCT05303792,NCT00176462,NCT00968253,NCT05453500,NCT03589326,NCT03817320,NCT03571321,NCT01186328,NCT00131027,NCT00187083,NCT00131053,NCT03504644,NCT03384654,NCT00184041,NCT01004497,NCT00313079,NCT04307576,NCT03553238,NCT03643276,NCT02003222,NCT00130195,NCT03117751,NCT03991884,NCT00187005,NCT00137111,NCT00187161,NCT02420717,NCT00186875,NCT02404220,NCT00476190,NCT01523977,NCT00136435,NCT04747912,NCT01887587,NCT03349281,NCT00001689,NCT00313053,NCT00671658,NCT00165178,NCT03860844,NCT03020030,NCT02303821,NCT00549848,NCT02143414,NCT02101853,NCT00890656
|
Alzheimer diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A20 |
HTR2A,CACNA1C,SLC6A4,HTR2C,DRD3
|
NeoplasmsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 02 |
NCT03617432,NCT04796012,NCT00186979,NCT00786669,NCT00993044
|
Adult T-cell lymphoma or leukaemia, human T-cell lymphotropic virus type 1-associatedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90.5 |
NCT02228772,NCT01000285,NCT03808610,NCT01788137,NCT03504644
|
HepatoblastomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.01 |
SLC6A4,CACNA1C,KCNH2
NCT03017326,NCT04478292 |
Rhabdomyosarcoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0GA1 |
NCT04625907,NCT01871766,NCT05457829,NCT04388839,NCT01355445
|
Mesothelioma of pleuraDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C26.0 |
ABCB1,LMNA,HTR2A,CACNA1F,ADRA2A
|
SchizophreniaDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A20 |
SLC6A4,HTR2A,HTR2C,DRD3
|
ParkinsonismDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A00 |
HTR2C,SLC6A4,HTR2A,DRD3
|
Classical Hodgkin lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B30.1 |
NCT05008224,NCT02661503,NCT02684708,NCT04067037
|
Cerebral ischaemic strokeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B11 |
NCT03347786,NCT02823106,NCT02235558,NCT02912663
|
PheochromocytomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH3854 |
CACNA1C,HTR2A,CACNA1F,ADRA2A
|
Adenocarcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.0 |
HTR2B,HTR2C,KCNH2,ADRA2A
|
Psychotic disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A20-6A25 |
HTR2C,HTR2A,HTR2B
|
AnxietyDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MB24.3 |
HTR2A,HTR2C,SLC6A4
|
Substance abuseDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C40 |
ADRA2A,SLC6A4,HTR2C
|
Sleep-wake disorderDisease Category: 07.Sleep-wake disordersDisease ICD-11 Code: 7A00-7B2Z |
KCNH2,HTR2C,HTR2A
|
Bipolar disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A60 |
HTR2A,DRD3
NCT00518947 |
Pituitary gland disorderDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A60-5A61 |
HTR2A,HTR2C,HTR2B
|
Malignant haematopoietic neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B33 |
HTR2C
NCT05359237,NCT00051311 |
Central nervous system diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A04-8D87 |
NCT00085202,NCT01735747,NCT00887146
|
Atrial fibrillationDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BC81.3 |
NCT00000556,NCT00589303,NCT00313157
|
Other specified malignant neoplasms of kidney, except renal pelvisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90.Y |
KCNH2,CACNA1F
NCT04322318 |
Angioimmunoblastic T-cell lymphomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH1J86 |
NCT00169156,NCT03853044,NCT01719835
|
Type1diabetesmellitusDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A10 |
NCT04233034,NCT04545151,NCT02372253
|
Serous cystadenoma,borderline malignancy of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.4 |
HTR2C,CACNA1F,KCNH2
|
Melanoma of skinDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30 |
HTR2C,ABCG2,KCNH2
|
Hepatocellular carcinoma of liverDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.02 |
HTR2C,KCNH2,KCNH2
|
Superficial ovarian endometriosisDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA10.B4 |
LMNA,ADRA2A,TUBB6
|
Malignant neoplasms of thymusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C27 |
HTR2A,HTR2C,KCNH2
|
Invasive carcinoma of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C61 |
SLC6A4,HTR2C,KCNH2
|
Heart failure, unspecifiedDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD1Z |
HTR2A,SLC6A4,ABCG2
|
Carpal tunnel syndromeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C10.0 |
HTR2B,TUBB6,HTR2A
|
Malignant neoplasms of adrenal glandDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D11 |
ADRA2A,HTR2C,KCNH2
|
Malignant lymphoma, not elsewhere classifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B33.5 |
NCT01777152,NCT02055820,NCT02568683,NCT00250718,NCT00201318,NCT02626455,NCT02406092,NCT00901069,NCT01746173,NCT00184002,NCT02356159,NCT00193479,NCT02703272,NCT00088530,NCT00719472,NCT00361621,NCT00809341,NCT03467373,NCT00324467,NCT00825149,NCT01516580,NCT01332968,NCT01200758
|
MigraineDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A80 |
HTR2C,SLC6A4
|
Sexual dysfunctionDisease Category: 17.Conditions related to sexual healthDisease ICD-11 Code: HA00-HA01 |
DRD3,HTR2A
|
Acute diabete complicationDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A2Y |
HTR2A,HTR2C
|
ObesityDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5B80-5B81 |
HTR2C,SLC6A4
|
Chronic insomniaDisease Category: 07.Sleep-wake disordersDisease ICD-11 Code: 7A00 |
HTR2A,HTR2C
|
Chronic painDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG30 |
HTR2A,SLC6A4
|
Psychoactive substances use disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C4G |
DRD3,HTR2C
|
Type 2 diabetes mellitusDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A11 |
HTR2A
NCT03296800 |
Brain cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00 |
DRD3
NCT01014767 |
InsomniaDisease Category: 07.Sleep-wake disordersDisease ICD-11 Code: 7A00-7A0Z |
HTR2A,SLC6A4
|
Solid tumour/cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00-2F9Z |
ABCB1,DRD3
|
Mood disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A60-6E23 |
SLC6A4,HTR2A
|
Heart failureDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD10-BD1Z |
KCNH2
NCT00589303 |
Choroid plexus tumoursDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.22 |
NCT01014767,NCT00500890
|
Burkitt-like lymphoma with 11q aberrationDisease Category: X.Extension CodesDisease ICD-11 Code: XH8NN2 |
NCT03962465,NCT02228772
|
Acute myeloid leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60 |
NCT00136084,NCT03860844
|
Chronic lymphocytic leukaemia or small lymphocytic lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A82.0 |
NCT00801281,NCT01463852
|
Lymphoid leukaemia, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH7Q12 |
NCT00866749,NCT02523976
|
Anaplastic large cell lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH1LC0 |
NCT01719835,NCT01309789
|
Carcinosarcoma of uterusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.43 |
SLC6A4,HTR2C
|
Dengue fever, unspecifiedDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D2Z |
DRD3,ABCG2
|
Cytomegaloviral diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D82 |
ABCB1,KCNH2
|
Glioblastoma of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.00 |
LMNA,TUBB6
|
Renal cell carcinoma, chromophobe typeDisease Category: X.Extension CodesDisease ICD-11 Code: XH6153 |
SLC6A4,KCNH2
|
Germ cell tumour of testisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C80.2 |
HTR2C,ABCG2
|
Follicular lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A80 |
NCT01852435,NCT02162771,NCT00907348,NCT00801281,NCT00384111,NCT01303887,NCT00494780,NCT00562965,NCT01397825,NCT05371093,NCT00115700,NCT01370694,NCT00774826,NCT00634179,NCT02449252,NCT02343536,NCT02889523,NCT02855359,NCT05058404
|
Hodgkin lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B30 |
NCT00352027,NCT04638790,NCT00026208,NCT03755804,NCT03013933,NCT01030900,NCT00736320,NCT00225173,NCT03407144,NCT00901069,NCT01356680,NCT01468740,NCT02356159,NCT02398240,NCT01251107
|
Precursor B-lymphoblastic neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A70 |
NCT03817320,NCT02881086,NCT05303792,NCT02981628,NCT00195871,NCT03991884,NCT04043494,NCT03564704,NCT02228772,NCT02845882,NCT01887587,NCT01451515,NCT00866749,NCT02535806
|
Peripheral T-cell lymphoma, not otherwise specifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90.C |
NCT00337987,NCT02809573,NCT00632827,NCT01719835,NCT03952572,NCT00970385,NCT04922567,NCT03023358,NCT05458180,NCT00791947,NCT03586999,NCT03553537,NCT04548700,NCT00930605
|
Mantle cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85.5 |
NCT00878254,NCT01397825,NCT00877006,NCT00581776,NCT00722137,NCT00209209,NCT00022945,NCT00209222,NCT00114738,NCT02427620,NCT00285389,NCT00477412,NCT00433537
|
Rheumatoid arthritisDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FA20 |
ABCG2
|
Soft tissue disorderDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FB56 |
SLC6A4
|
Social anxiety disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6B04 |
HTR2A
|
Chronic kidney diseaseDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GB61 |
HTR2A
|
Corneal diseaseDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9A76-9A78 |
SLC6A4
|
MalariaDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1F40-1F45 |
KCNH2
|
Bacterial infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A00-1C4Z |
ABCB1
|
Cardiovascular diseaseDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA00-BE2Z |
HTR2A
|
Multiple sclerosisDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A40 |
KCNH2
|
Nausea/vomitingDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MD90 |
SLC6A4
|
DementiaDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6D80-6D8Z |
HTR2A
|
GlaucomaDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9C61 |
HTR2A
|
CoughDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MD12 |
SLC6A4
|
Acute upper respiratory infectionDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA07 |
DRD3
|
General pain disorderDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8E43 |
SLC6A4
|
Ejaculatory dysfunctionDisease Category: 17.Conditions related to sexual healthDisease ICD-11 Code: HA03 |
SLC6A4
|
Lung cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25 |
ABCB1
|
Gastroduodenal motor/secretory disorderDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DA41 |
DRD3
|
Binge eating disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6B82 |
SLC6A4
|
BCR-ABL1-negative chronic myeloid leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A41 |
DRD3
|
Endometrial cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76 |
DRD3
|
Idiopathic interstitial pneumonitisDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB03 |
HTR2A
|
Stomach cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72 |
DRD3
|
Schizoaffective disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A21 |
HTR2A
|
Pulmonary hypertensionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BB01 |
HTR2B
|
Menopausal disorderDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA30 |
SLC6A4
|
Cocaine use disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C45 |
SLC6A4
|
Nasopharyngeal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6B |
DRD3
|
Pancreatic cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10 |
HTR2A
|
Irritable bowel syndromeDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DD91 |
ABCG2
|
Nicotine use disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C4A |
SLC6A4
|
Neurobehavioral disorderDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A00-8A0Z |
SLC6A4
|
concerning food/fluid intake symptomDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG43 |
SLC6A4
|
Tonus and reflex abnormalityDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MB47 |
HTR2C
|
Orthostatic hypotensionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA21 |
SLC6A4
|
Post-traumatic stress disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6B40 |
HTR2A
|
Cerebral ischaemiaDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B1Z |
HTR2A
|
Opioid use disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C43 |
ADRA2A
|
OsteosarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B51 |
NCT00001209
|
Optic nerve disorderDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9C40 |
NCT05278715
|
T lymphoblastic leukaemia/lymphomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH50W7 |
NCT01483690
|
Alveolar rhabdomyosarcomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH7099 |
NCT05304585
|
Lymphoplasmacytic lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85.4 |
NCT00801281
|
Castlemans diseaseDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4B2Y |
NCT02228512
|
Astrocytoma, anaplasticDisease Category: X.Extension CodesDisease ICD-11 Code: XH96C7 |
NCT00431561
|
Other specified gliomas of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.0Y |
NCT02265770
|
Malignant trophoblastic neoplasms of placentaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C75.0 |
NCT02639650
|
Nephroblastoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5QN3 |
NCT05384821
|
Malignant neoplasm metastasis, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E2Z |
NCT02677116
|
GanglioneuroblastomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH77W7 |
NCT03786783
|
Adult T-cell leukaemia/lymphoma (HTLV-1 positive)Disease Category: X.Extension CodesDisease ICD-11 Code: XH6TE2 |
NCT00145002
|
Immune thrombocytopenic purpuraDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3B64.10 |
NCT00774202
|
Myelodysplastic syndromeDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A37 |
NCT02356159
|
Ependymoma, anaplasticDisease Category: X.Extension CodesDisease ICD-11 Code: XH6922 |
NCT01096368
|
Osteoarthritis of kneeDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FA01 |
NCT01645709
|
Embryonal rhabdomyosarcoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH83G1 |
NCT05304585
|
Pneumonia due to Respiratory syncytial virusDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA40.11 |
NCT03782662
|
Primary neoplasms of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00 |
NCT01528046
|
Ventricular tachyarrhythmiaDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BC71 |
NCT02772354
|
COVID-19Disease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D6Y |
NCT04351763
|
Mature B-cell neoplasms, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A8Z |
NCT02911142
|
HemangiomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E81-2F2Y |
NCT00555464
|
Chronic myelogenous leukaemia, BCR-ABL1-positiveDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A20.0 |
NCT01424982
|
Small cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.1 |
NCT02566993
|
Crohn diseaseDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DD70 |
NCT01261286
|
Haemorrhage, not elsewhere classifiedDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG27 |
NCT04356196
|
Pleomorphic sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B54 |
NCT04199026
|
Human immunodeficiency virus type 1Disease Category: X.Extension CodesDisease ICD-11 Code: XN8LD |
NCT01435018
|
Malignant lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5FJ5 |
NCT00001563
|
Neuroendocrine carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C34 |
NCT00001339
|
Glioblastoma, primary, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0MB1 |
NCT00431561
|
Unknown or unspecified psychoactive substance dependence, substance and state of remission unspecifiedDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C4G.2Z |
NCT00593463
|
Langerhans cell histiocytosisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B31.2 |
NCT04773366
|
Dravet syndromeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A61.11 |
NCT01607073
|
Idiopathic pulmonary fibrosisDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB03.4 |
HTR2A
|
Adenocarcinoma of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.0 |
HTR2C
|
Malignant neoplasm metastasis in lymph nodes of head, face or neckDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D60.0 |
HTR2C
|
Myotonic dystrophyDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C71.0 |
ADRA2A
|
Squamous cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.2 |
HTR2C
|
Tetralogy of FallotDisease Category: 20.Developmental anomaliesDisease ICD-11 Code: LA88.2 |
ABCG2
|
Other specified malignant neoplasms ofcolonDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B90.Y |
HTR2C
|
Other specified malignant neoplasms of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.Y |
HTR2C
|
Urothelial carcinoma of bladderDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94.2 |
HTR2C
|
Malignant neoplasms of corpus uteri, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.Z |
HTR2C
|
Chikungunya virus diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D40 |
LMNA
|
Dilated cardiomyopathyDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BC43.0 |
ABCG2
|

