Collective Molecular Activities of the Plant: Symphytum Tuberosum

Plant ID: NPO9866
Plant Latin Name: Symphytum Tuberosum
Taxonomy Genus: Symphytum
Taxonomy Family: Boraginaceae
Plant External Links:
NCBI TaxonomyDB:
256504
Plant-of-the-World-Online:
n.a.
Country/Region:
China; SpainTraditional Medicine System:
TCMChina; Spain
Overview of Ingredients
47 All known Ingredients in Total
Unique ingredients have been isolated from this plant.Plant-Ingredients Associations were manually curated from publications or collected from other databases.
11 Ingredients with Acceptable Bioavailablity
Unique ingredients exhibit acceptable human oral bioavailablity, according to the criteria of SwissADME [PMID: 28256516] and HobPre [PMID: 34991690]. The criteria details:SwissADME: six descriptors are used by SwissADME to evaluate the oral bioavailability of a natural product:
☑ LIPO(Lipophility): -0.7 < XLOGP3 < +5.0
☑ SIZE: 150g/mol < MW < 500g/mol
☑ POLAR(Polarity): 20Ų < TPSA < 130Ų
☑ INSOLU(Insolubility): -6 < Log S (ESOL) < 0
☑ INSATU(Insaturation): 0.25 < Fraction Csp3 < 1
☑ FLEX(Flexibility): 0 < Num. rotatable bonds < 9
If 6 descriptors of a natural plant satisfy the above rules, it will be labeled high HOB.
HobPre: A natural plant ingredient with HobPre score >0.5 is labeled high human oral availability (HOB)
16 Ingredients with experimental-derived Activity
Unique ingredients have activity data available.Ingredient Structrual Cards

Ingredient ID: NPC92898
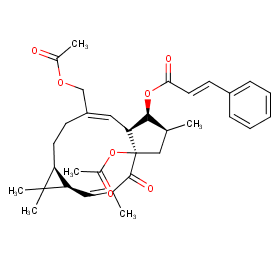
Ingredient ID: NPC8990
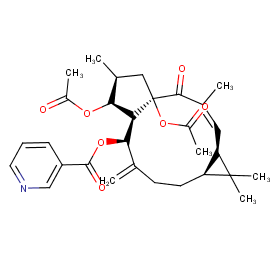
Ingredient ID: NPC7780
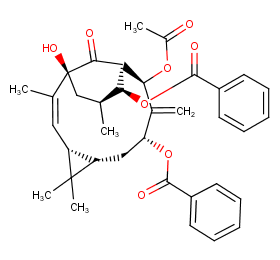
Ingredient ID: NPC76859
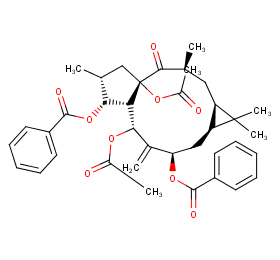
Ingredient ID: NPC74150
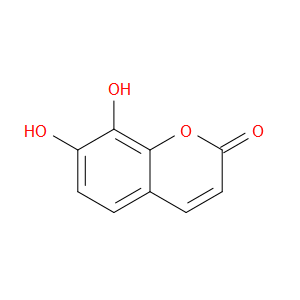
Ingredient ID: NPC73738
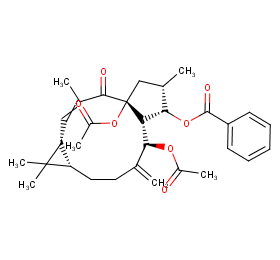
Ingredient ID: NPC70361
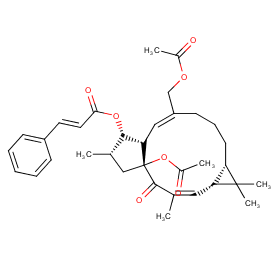
Ingredient ID: NPC59326
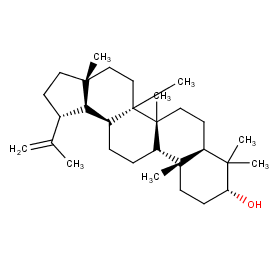
Ingredient ID: NPC53475
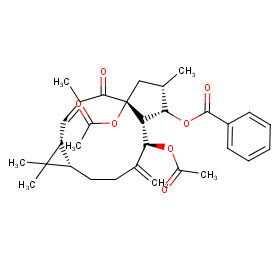
Ingredient ID: NPC42234
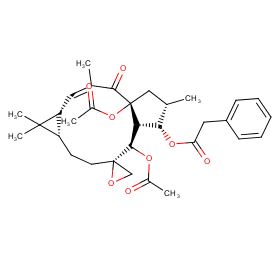
Ingredient ID: NPC3450
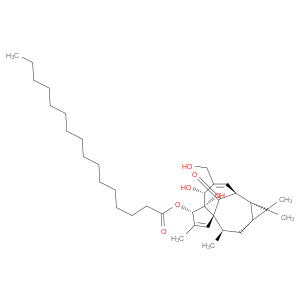
Ingredient ID: NPC308191
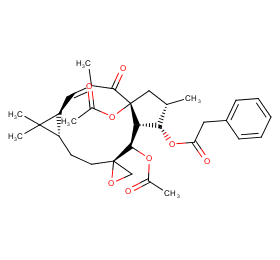
Ingredient ID: NPC282285
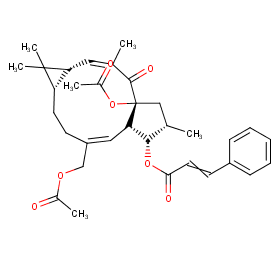
Ingredient ID: NPC281452
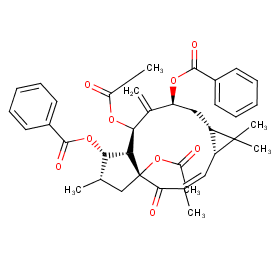
Ingredient ID: NPC276652
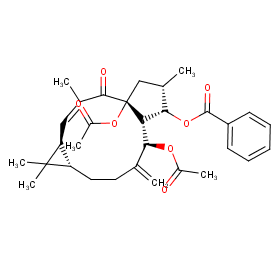
Ingredient ID: NPC27154
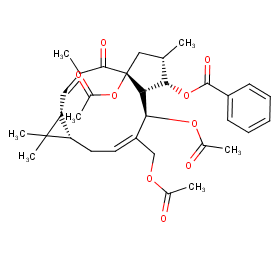
Ingredient ID: NPC270364
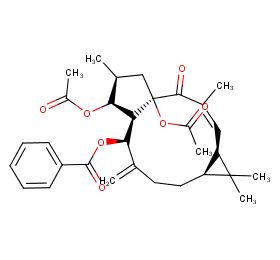
Ingredient ID: NPC264653
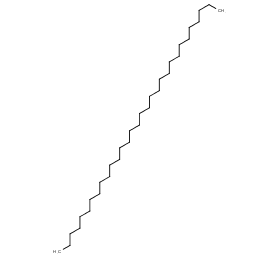
Ingredient ID: NPC26229
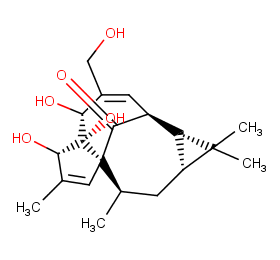
Ingredient ID: NPC261341
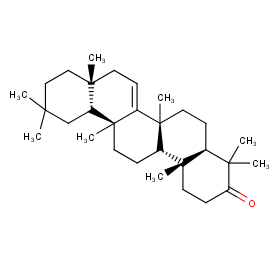
Ingredient ID: NPC259168
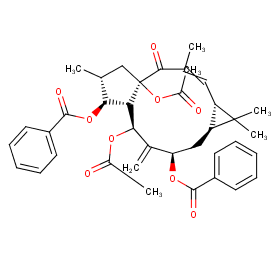
Ingredient ID: NPC252593
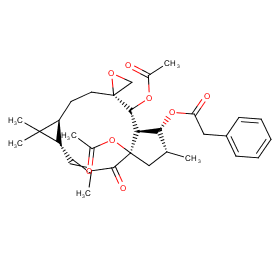
Ingredient ID: NPC24873
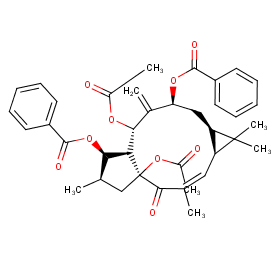
Ingredient ID: NPC24580
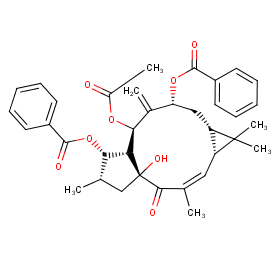
Ingredient ID: NPC243595
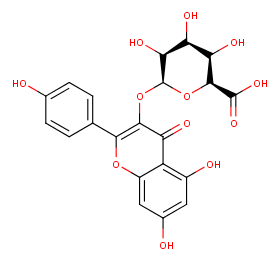
Ingredient ID: NPC242861
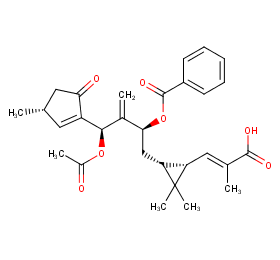
Ingredient ID: NPC23322
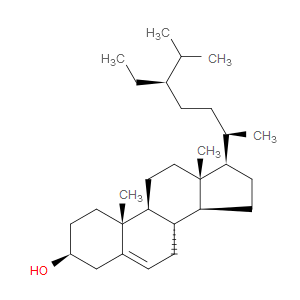
Ingredient ID: NPC230301
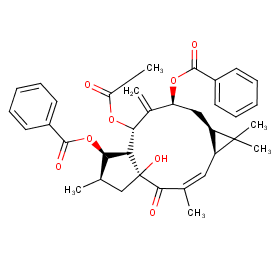
Ingredient ID: NPC215626
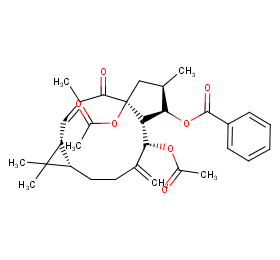
Ingredient ID: NPC212755
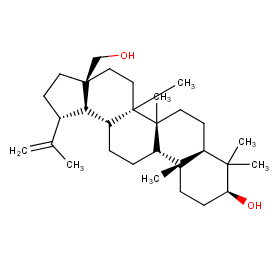
Ingredient ID: NPC206438
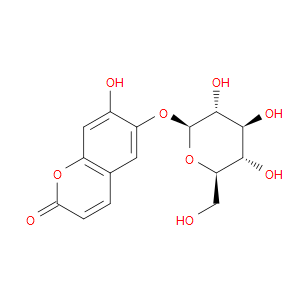
Ingredient ID: NPC206264
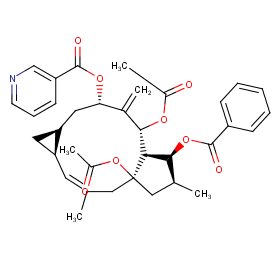
Ingredient ID: NPC202964
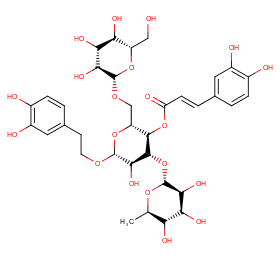
Ingredient ID: NPC175214
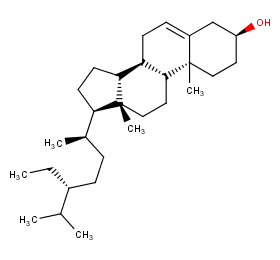
Ingredient ID: NPC159362
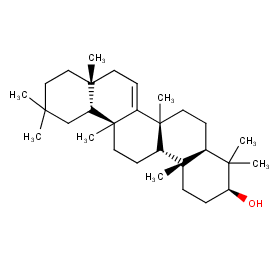
Ingredient ID: NPC156358
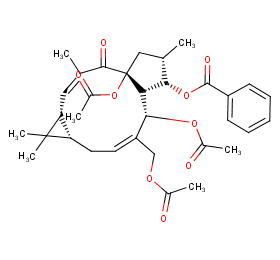
Ingredient ID: NPC151859
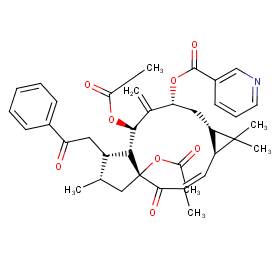
Ingredient ID: NPC145218
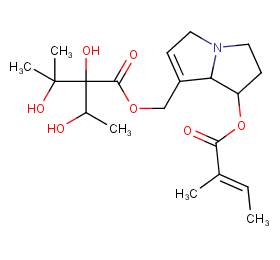
Ingredient ID: NPC144015
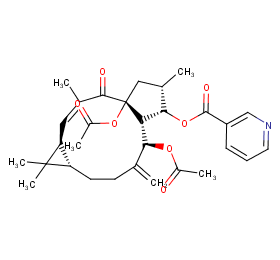
Ingredient ID: NPC143569
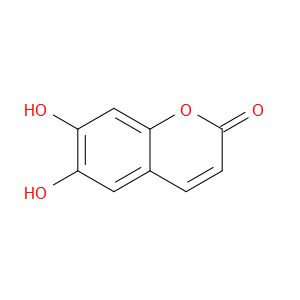
Ingredient ID: NPC137949
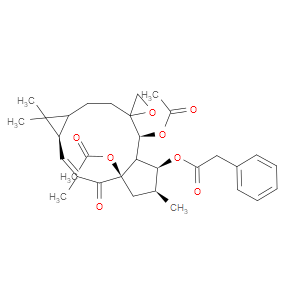
Ingredient ID: NPC122504
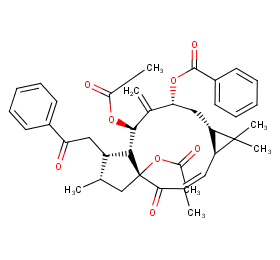
Ingredient ID: NPC11977
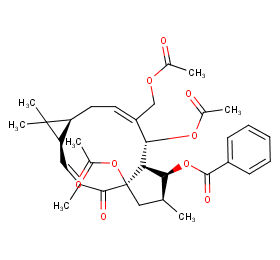
Ingredient ID: NPC118799
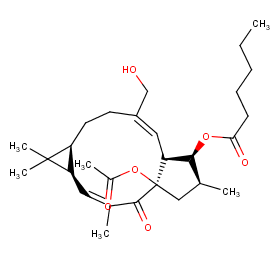
Ingredient ID: NPC118405
Ingredient ID: NPC11666
Ingredient ID: NPC105222
Classification of Human Proteins Collectively Targeted by the Plant
Detailed Information of Target Proteins
| Target Type | Protein Class | Gene ID | Protein Name | Uniprot ID | Target ChEMBL ID |
|---|---|---|---|---|---|
| Therapeutic Target | Lyase | CA9 | Carbonic anhydrase IX | Q16790 | CHEMBL3594 |
| Therapeutic Target | Lyase | CA12 | Carbonic anhydrase XII | O43570 | CHEMBL3242 |
| Therapeutic Target | Oxidoreductase | ALDH1A1 | Aldehyde dehydrogenase 1A1 | P00352 | CHEMBL3577 |
| Therapeutic Target | Phosphodiesterase | TDP1 | Tyrosyl-DNA phosphodiesterase 1 | Q9NUW8 | CHEMBL1075138 |
| Therapeutic Target | Protein Kinase | PRKCD | Protein kinase C delta | Q05655 | CHEMBL2996 |
Clinical trials associated with plant from natural product (NP) & plant level:
| Clinical trials type | Number of clinical trials | |
|---|---|---|
| 148 | ||
| NCT ID | Title | Condition | Form in clinical use | Associated by plant or compound |
|---|---|---|---|---|
| NCT05087914 | Novel Non-opioid Post-surgical Pain Treatment in Females | pain | Levodopa (NPC161593) | |
| NCT04513340 | WD-1603 PK Study Under Fasting and Fed Conditions in Healthy Subjects | Parkinson disease | Levodopa (NPC161593) | |
| NCT04082715 | Transition From Acute to Chronic Back Pain : Effect of L-dopa,Gender,and Associated Brain Plasticity | Back pain | Levodopa (NPC161593) | |
| NCT00335153 | Levodopa-Carbidopa Intestinal Gel Open-Label Study in Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01883505 | A Phase 2a Study Followed to Evaluate the Safety, Tolerability and Levodopa Pharmacokinetics in Levodopa-treated Parkinson's Disease Patients Receiving ND0612 | Parkinson disease | Levodopa (NPC161593) | |
| NCT00485069 | REQUIP (Ropinirole Hydrochloride) IR Long-Term Phase 4 Study | Parkinson disease | Levodopa (NPC161593) | |
| NCT03243552 | Proof of Mechanism Study for the Treatment of Social Anhedonia in ASD | autism spectrum disorder | Levodopa (NPC161593) | |
| NCT02169414 | Effect of Three Multiple-dose Regimens of BIA 9 1067 at Steady-state on the Levodopa Pharmacokinetics | Parkinson disease | Levodopa (NPC161593) | |
| NCT01519284 | Study of BIA 9-1067 to Investigate Its Effect on Levodopa Pharmacokinetic | Parkinson disease | Levodopa (NPC161593) | |
| NCT00660673 | Open Label Continuation Treatment Study With Levodopa-Carbidopa Intestinal Gel in Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00099268 | Efficacy and Safety of Carbidopa/Levodopa/Entacapone in Patients With Parkinson's Disease Requiring Initiation of Levodopa Therapy | Parkinson disease | Levodopa (NPC161593) | |
| NCT00634556 | Dopaminergic Effects on Cortical Function in Tourette's (Levodopa Protocol) | Tourette syndrome | Levodopa (NPC161593) | |
| NCT01479127 | Study of Safety, Tolerability, Pharmacokinetics, and Efficacy of ABT-SLV187 in Subjects With Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00829439 | Study on Tolerability of Levodopa/Carbidopa in Children With Angelman Syndrome | Angelman syndrome | Levodopa (NPC161593) | |
| NCT01725802 | A Phase I/IIa Study of Safety, Tolerability and Plasma Concentration of Subcutaneous Continuously-delivered Levodopa/Carbidopa Solution (ND0612) in PD Patients | Parkinson disease | Levodopa (NPC161593) | |
| NCT02170376 | The Effect of BIA 9-1067 at Steady-state on the Levodopa Pharmacokinetics | Parkinson disease | Levodopa (NPC161593) | |
| NCT00601978 | Carbidopa/Levodopa Versus Carbidopa/Levodopa/Entacapone on Markers of Event Related Potentials (ERPs) in Patients With Idiopathic Parkinson's Disease (PD) and End-of-dose Wearing Off | Parkinson disease | Levodopa (NPC161593) | |
| NCT04990284 | eArly levoDopa With Opicapone in Parkinson's paTients wIth motOr fluctuatioNs. | Parkinson disease | Levodopa (NPC161593) | |
| NCT00143026 | Study to Compare the Effect of Treatment With Carbidopa/Levodopa/Entacapone on the Quality of Life of Patients With Parkinson's Disease. This Study is Not Recruiting in the United States | Parkinson disease | Levodopa (NPC161593) | |
| NCT00279825 | Comparison of IPX054, IR Carbidopa-Levodopa, and CR Carbidopa-Levodopa in Subjects With Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01281475 | A Trial of Levodopa in Angelman Syndrome | Angelman syndrome | Levodopa (NPC161593) | |
| NCT03103399 | Efficacy and Tolerability of Nebicapone in Parkinson's Disease Patients With "Wearingoff" Phenomenon | Parkinson disease | Levodopa (NPC161593) | |
| NCT02312232 | Pharmacokinetic Study in Healthy Males | Parkinson disease | Levodopa (NPC161593) | |
| NCT00660387 | Study of Efficacy, Safety and Tolerability of Levodopa-Carbidopa Intestinal Gel in Levodopa-Responsive Parkinson's Subjects | Parkinson disease | Levodopa (NPC161593) | |
| NCT01568047 | Multicentre Study in Four Parallel Groups of Parkinson's Disease (PD) Patients | Parkinson disease | Levodopa (NPC161593) | |
| NCT01636037 | Antipsychotic Augmentation With L-Dopa | schizophrenia | Levodopa (NPC161593) | |
| NCT05471609 | Sustained Release Oral Formulation for Treatment of Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00914134 | Duodenal Levodopa Infusion, Quality of Life and Autonomic Nervous System in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01351168 | Use of Zolpidem in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT03000569 | A Study to Evaluate SAGE-217 in Participants With Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT04000919 | Effects of 5HTP and LDOPA on CNS Excitability After SCI | Spinal cord injury | Levodopa (NPC161593) | |
| NCT00625547 | A Study to Determine the Efficacy and Safety of Cabergoline for the Treatment of Patients With RLS | restless legs syndrome | Levodopa (NPC161593) | |
| NCT01484990 | A Pharmacokinetic Study of Levodopa and Carbidopa Intestinal Gel in Subjects With Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00004733 | Timing of Levodopa Treatment in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00253084 | Comparison of IPX054 and Immediate-Release Carbidopa-Levodopa in Patients With Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01770145 | Apokyn for Motor IMProvement of Morning AKinesia Trial (AM IMPAKT) | Parkinson disease | Levodopa (NPC161593) | |
| NCT01568034 | A Study to Investigate the Tolerability and Effect of Three Single-dose Regimens of BIA 9-1067 | Parkinson disease | Levodopa (NPC161593) | |
| NCT03022318 | Carbidopa-levodopa in Neovascular AMD | age-related macular degeneration | Levodopa (NPC161593) | |
| NCT02641054 | Efficacy Phase IIa Study of CVXL-0107 in Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT02837640 | Studying a Potential Protective Effect of L-Dopa on Retinitis Pigmentosa | retinitis pigmentosa | Levodopa (NPC161593) | |
| NCT00505843 | A Study of MK0657 in Parkinson's Disease Patients (0657-006) | Parkinson disease | Levodopa (NPC161593) | |
| NCT04952194 | Clinical Study of Stalevo in the Treatment of Early Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01387711 | PEP005 Gel - Biological Effects in Actinic Keratosis Assessed by Histology | actinic keratosis | Ingenol (NPC261341) | |
| NCT02080819 | Striatal Effective Connectivity to Predict Treatment Response in Cocaine Misuse | cocaine dependence | Levodopa (NPC161593) | |
| NCT02554734 | Pharmacokinetic Study in Healthy Volunteers | Parkinson disease | Levodopa (NPC161593) | |
| NCT02601586 | Effects of PR Oxycodone and of Levodopa, vs Placebo, on Central Neuropathic Pain in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT02633839 | A Study of the Safety and Levodopa Pharmacokinetics Following Single Dose Administration of CVT 301 (Levodopa Inhalation Powder) in Smoking and Non-Smoking Adults | nicotine dependence | Levodopa (NPC161593) | |
| NCT01484184 | Study to Assess Safety, Tolerability and MTD of a Central Pattern Generator-activating Tritherapy (SPINALON) in Patients With Chronic Spinal Cord Injury | Spinal cord injury | Levodopa (NPC161593) | |
| NCT00108667 | Talampanel to Treat Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT02812394 | A Study to Compare Two Dose Strengths of CVT-301 (Levodopa Inhalation Powder) With an Oral Dose of Sinemet® (Carbidopa-levodopa) Tablets | Parkinson disease | Levodopa (NPC161593) | |
| NCT00360568 | Safety/Efficacy Study of Levodopa-Carbidopa Intestinal Gel in Parkinson's Subjects | Parkinson disease | Levodopa (NPC161593) | |
| NCT01429077 | Augmenting Language Therapy for Aphasia: Levodopa | Aphasia;stroke | Levodopa (NPC161593) | |
| NCT00141518 | Long-term Study of Duodopa (Levodopa/Carbidopa) in Advanced Parkinson's: Health Outcomes & Net Economic Impact | Parkinson disease | Levodopa (NPC161593) | |
| NCT05128175 | Comparative Bioavailability Study of Carbidopa/Levodopa Extended-Release Tablets Under Fasting and Fed Conditions | Parkinson disease | Levodopa (NPC161593) | |
| NCT01171313 | A Efficacy, Safety and Pharmacokinetic Study of XP21279 and Sinemet® in Parkinson's Disease Subjects | Parkinson disease | Levodopa (NPC161593) | |
| NCT01515410 | Study in Advanced Parkinson's Disease Patients With Predictable Motor Fluctuations | Parkinson disease | Levodopa (NPC161593) | |
| NCT04558112 | Improving Therapeutic Learning for PTSD | post-traumatic stress disorder | Levodopa (NPC161593) | |
| NCT01736176 | A Study to Assess the Safety and Efficacy of Levodopa-carbidopa Intestinal Gel (LCIG) for the Treatment of Non-motor Symptoms in Patients With Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00144209 | Assess Efficacy and Safety of the Dopamine Agonist Pramipexole Versus Levodopa / Benserazide (Madopar® DR) in Patients With Restless Legs Syndrome | restless legs syndrome | Levodopa (NPC161593) | |
| NCT02242487 | Twelve Month Safety and Efficacy Study of CVT-301 In Parkinson's Disease Patients With OFF Episodes | Parkinson disease | Levodopa (NPC161593) | |
| NCT00845000 | Acute Effects of Preladenant (SCH 420814) on Dyskinesia and Parkinsonism in Levodopa Treated Participants (P05550) | Parkinson disease | Levodopa (NPC161593) | |
| NCT04006210 | Efficacy, Safety and Tolerability Study of ND0612 vs. Oral IR-LD/CD in Subjects With PD Experiencing Motor Fluctuations | Parkinson disease | Levodopa (NPC161593) | |
| NCT02769793 | Efficacy of Levodopa/Benserazide Dispersible Tablet on Response Fluctuations in PD Patients With Delayed ON | Parkinson disease | Levodopa (NPC161593) | |
| NCT03887884 | Pharmacokinetic Study of CVT-301 (Levodopa Inhalation Powder) | Parkinson disease | Levodopa (NPC161593) | |
| NCT04591535 | PK Study of WD-1603 in Healthy Subjects | Parkinson disease | Levodopa (NPC161593) | |
| NCT01960842 | A Study to Assess the Efficacy, Safety and Tolerability of ABT-SLV187 Monotherapy in Subjects With Advanced Parkinson's Disease (PD) and Persistent Motor Complications, Despite Optimized Treatment With Available Anti-Parkinsonian Medications | Parkinson disease | Levodopa (NPC161593) | |
| NCT03140956 | Pharmacokinetic of Levodopa Study in Healthy Males | Parkinson disease | Levodopa (NPC161593) | |
| NCT03496870 | A Study of the Pharmacokinetics, Pharmacodynamics, and Safety of Opicapone in Subjects With Parkinson's Disease Taking Levodopa. | Parkinson disease | Levodopa (NPC161593) | |
| NCT01227655 | Efficacy and Safety of BIA 9-1067 in Idiopathic Parkinson's Disease Patients. | Parkinson disease | Levodopa (NPC161593) | |
| NCT01663935 | Vision Response to Dopamine Replacement | oculocutaneous albinism | Levodopa (NPC161593) | |
| NCT00006077 | Effects of Monoamine Reuptake Inhibitor NS2330 in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00076674 | Levetiracetam Treatment of L-dopa Induced Dyskinesias | Parkinson disease | Levodopa (NPC161593) | |
| NCT03119636 | Safety and Efficacy Study of Human ESC-derived Neural Precursor Cells in the Treatment of Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT03576638 | Study to Assess Pharmacokinetics of Accordion Pill Carbidopa-Levodopa Compared to Immediate Release Carbidopa-Levodopa in Parkinson's Disease Patients | Parkinson disease | Levodopa (NPC161593) | |
| NCT01176435 | Trial of L-DOPA as a Treatment to Improve Vision in Albinism | albinism | Levodopa (NPC161593) | |
| NCT00558337 | Efficacy, Safety, and Pharmacokinetics/Pharmacodynamic Study of L-Dopa/Carbidopa To Treat Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00406588 | SLV308 for Treatment of Patients With Parkinson's Disease Experiencing Motor Fluctuations | Parkinson disease | Levodopa (NPC161593) | |
| NCT00642356 | Carbidopa/Levodopa/Entacapone Versus Immediate Release (IR) Carbidopa/Levodopa on Non-motor Symptoms in Patients With Idiopathic Parkinson's Disease and Demonstrating Non-motor Symptoms of Wearing Off | Parkinson disease | Levodopa (NPC161593) | |
| NCT00218075 | Behavioral Therapy Combined With Carbidopa/Levodopa for the Treatment of Cocaine Dependence | cocaine dependence | Levodopa (NPC161593) | |
| NCT02873351 | A Safety and Efficacy Study of Carbidopa-levodopa in Patients With Macular Degeneration | age-related macular degeneration | Levodopa (NPC161593) | |
| NCT02486432 | A Single Period Investigation to Assess the Tolerability of Healthy Subjects to Oral Sinemet® (Levodopa/Carbidopa) | Parkinson disease | Levodopa (NPC161593) | |
| NCT00590122 | Parcopa Versus Carbidopa-levodopa in a Single Dose Cross-over Comparison Study | Parkinson disease | Levodopa (NPC161593) | |
| NCT00040209 | JP-1730 to Treat Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT05132660 | Treating Early Stage Diabetic Retinopathy | diabetic retinopathy | Levodopa (NPC161593) | |
| NCT02352363 | Randomized Safety Study of CVT-301 Compared to an Observational Control Group | Parkinson disease | Levodopa (NPC161593) | |
| NCT00009048 | EMD 128130 for the Treatment of Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01070628 | Levodopa Concentration Profile With Stalevo 75/125 mg | Parkinson disease | Levodopa (NPC161593) | |
| NCT00918177 | An Evaluation of the Pharmacokinetics and Pharmacodynamics of AP09004 in Patients With Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01468012 | Imaging the Neurobiology of Behavioral and Medication Treatment for Cocaine Dependence | cocaine dependence | Levodopa (NPC161593) | |
| NCT01617135 | Safety, Pharmacokinetics and Efficacy Study of CVT-301 Inpatients With Parkinson's Disease and "Off" Episodes | Parkinson disease | Levodopa (NPC161593) | |
| NCT03929068 | Sinemet for Spasticity and Function in Amyotrophic Lateral Sclerosis and Primary Lateral Sclerosis | amyotrophic lateral sclerosis | Levodopa (NPC161593) | |
| NCT02847442 | Efficacy and Safety of Opicapone in Clinical Practice | Parkinson disease | Levodopa (NPC161593) | |
| NCT00745277 | High and Low Dose Carbidopa Treatment of Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT02807675 | A Study of the Safety and Tolerability of a Single Dose Administration of CVT-301 (Levodopa Inhalation Powder) | Parkinson disease | Levodopa (NPC161593) | |
| NCT00789672 | Pilot Study to Evaluate Levodopa as Treatment for Residual Amblyopia | amblyopia | Levodopa (NPC161593) | |
| NCT02073773 | Combinational Rehabilitative Therapy and Functional Brain Imaging for Patients Recovering From Motor Stroke | stroke | Levodopa (NPC161593) | |
| NCT03205956 | Measuring Parkinson's Disease Progression | Parkinson disease | Levodopa (NPC161593) | |
| NCT01777555 | Efficacy and Safety Study of Inhaled CVT 301 in Parkinson's Disease Patients for Treatment of OFF Episodes | Parkinson disease | Levodopa (NPC161593) | |
| NCT02096601 | A Pharmacokinetic Study of ND0612 Delivered as a Continuous Subcutaneous in Parkinson's Disease Patients | Parkinson disease | Levodopa (NPC161593) | |
| NCT00888186 | Different Dyskinesias in Parkinson's Disease and Their Relation to Levodopa Pharmacokinetics | Parkinson disease | Levodopa (NPC161593) | |
| NCT00357994 | Study of Efficacy, Safety and Tolerability of Levodopa-Carbidopa Intestinal Gel in Levodopa-Responsive Parkinson's Subjects | Parkinson disease | Levodopa (NPC161593) | |
| NCT02240030 | Efficacy and Safety Study of CVT-301 In Parkinson's Disease Patients With OFF Episodes | Parkinson disease | Levodopa (NPC161593) | |
| NCT00914602 | An Exploratory Study of XP21279 (With Lodosyn®) and Sinemet® in Parkinson's Disease Subjects | Parkinson disease | Levodopa (NPC161593) | |
| NCT01568073 | Efficacy and Safety of BIA 9-1067 in Idiopathic Parkinson's Disease Patients With "Wearing-off" Phenomenon | Parkinson disease | Levodopa (NPC161593) | |
| NCT02271503 | A Study to Assess the PK and Pharmacodynamics of IPX203 in Patients With Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01393457 | Cognitive Remediation for Cocaine Dependence | cocaine dependence | Levodopa (NPC161593) | |
| NCT00399477 | A Non-Blinded Study Demonstrating the Effectiveness and Safety of Azilect Alone or in Combination Therapy in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00391898 | Efficacy of Levodopa/Carbidopa/Entacapone vs Levodopa/Carbidopa in Parkinson's Disease Patients With Early Wearing-off | Parkinson disease | Levodopa (NPC161593) | |
| NCT02778594 | Pharmacokinetic-pharmacodynamic Interaction Between BIA 3-202 and Levodopa/Benserazide | Parkinson disease | Levodopa (NPC161593) | |
| NCT02347059 | L-dopa Versus Dopamine Agonists After Subthalamic Nucleus Deep Brain Stimulation in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT02549092 | A Study to Examine the Effect of Levodopa-Carbidopa Intestinal Gel (LCIG) Therapy Relative to That of Optimized Medical Treatment (OMT) on Non-motor Symptoms (NMS) Associated With Advanced Parkinson's Disease (PD) | Parkinson disease | Levodopa (NPC161593) | |
| NCT02116790 | Efficacy of Co-administration of an NSAID With a Dopamine Agonist In Healthy Subjects | pain | Levodopa (NPC161593) | |
| NCT02741947 | Levodopa Benserazide Generic Formulation Versus the Originator | Parkinson disease | Levodopa (NPC161593) | |
| NCT03007888 | A Study to Assess the PK and Pharmacodynamics of IPX203 in Subjects With Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT04380142 | Study Comparing Continuous Subcutaneous Infusion Of ABBV-951 With Oral Carbidopa/Levodopa Tablets For Treatment Of Motor Fluctuations In Adult Participants With Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT03023059 | Dose Ranging Study of Carbidopa-levodopa | age-related macular degeneration | Levodopa (NPC161593) | |
| NCT00174239 | Study Of Cabaser and Sinemet CR For The Treatment Of Nighttime Symptoms Associated With Parkinson's Disease. | Parkinson disease | Levodopa (NPC161593) | |
| NCT00272688 | Continuous Delivery of Levodopa in Patients With Advanced Idiopathic Parkinsons Disease - Cost-benefit | Parkinson disease | Levodopa (NPC161593) | |
| NCT00134966 | A Study to Evaluate Fixed Dose Carbidopa/Levodopa/Entacapone Versus Immediate Release Carbidopa/Levodopa | Parkinson disease | Levodopa (NPC161593) | |
| NCT00218023 | Medications for Stopping Cocaine Dependence and Preventing Relapse | cocaine dependence | Levodopa (NPC161593) | |
| NCT02633007 | A Study of the Safety and Pharmacokinetics of Levodopa Following Administration of CVT 301 (Levodopa Inhalation Powder) in Adults With Asthma | asthma | Levodopa (NPC161593) | |
| NCT02480803 | INfusion VErsus STimulation in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT02764125 | Efficacy and Safety Proof of Concept Study in Patients With Parkinson's Disease and End-of-dose Wearing-off (COMPOC) | Parkinson disease | Levodopa (NPC161593) | |
| NCT00006337 | KW-6002 to Treat Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00713583 | Carbidopa/Levodopa Combined With Behavioral Therapy for the Treatment of Cocaine Dependence | cocaine dependence | Levodopa (NPC161593) | |
| NCT01646255 | Rotigotine Versus Placebo, A Study To Evaluate The Efficacy In Advanced Stage Idiopathic Parkinson's Disease Patients | Parkinson disease | Levodopa (NPC161593) | |
| NCT00086294 | ACP-103 to Treat Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT05036473 | A Study of the Efficacy and Safety of Carbidopa-Levodopa Extended-Release Tablets in Patients With Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT01026428 | A Study to Assess the Effect of Safinamide on Levodopa Pharmacokinetics | Parkinson disease | Levodopa (NPC161593) | |
| NCT03451500 | Carbidopa-Levodopa in Dry AMD With Geographic Atrophy | age-related macular degeneration | Levodopa (NPC161593) | |
| NCT01190813 | Levodopa for the Treatment of Residual Amblyopia | amblyopia | Levodopa (NPC161593) | |
| NCT00089622 | Lisuride Patch to Treat Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT03735901 | Enhancement of Stroke Rehabilitation With Levodopa | stroke | Levodopa (NPC161593) | |
| NCT02577523 | A Clinical Study of Efficacy, Safety, Tolerability and PK of ND0612H in Subjects With Advanced Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT04052776 | Acute Effects of Pharmacological Neuromodulation on Leg Motor Activity in Patients With SCI Treated With EES | Spinal cord injury | Levodopa (NPC161593) | |
| NCT02799381 | A Study Comparing Efficacy of Levodopa-Carbidopa Intestinal Gel/Carbidopa-Levodopa Enteral Suspension and Optimized Medical Treatment on Dyskinesia in Subjects With Advanced Parkinson's Disease (DYSCOVER) | Parkinson disease | Levodopa (NPC161593) | |
| NCT00219284 | Effects of Carbidopa/Levodopa/Entacapone on Motor Function and Quality of Life in Patients With Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00125567 | Stalevo in Early Wearing-Off Patients | Parkinson disease | Levodopa (NPC161593) | |
| NCT05285683 | The Role of Brain Dopamine in Chronic Pain | Chronic pain | Levodopa (NPC161593) | |
| NCT05369533 | Dopaminergic Enhancement of Rehabilitation Therapy Early After Stroke | stroke | Levodopa (NPC161593) | |
| NCT01533116 | Effect of BIA 9-1067 at Steady-state on the Pharmacokinetics of Levodopa/Carbidopa and Levodopa/Benserazide | Parkinson disease | Levodopa (NPC161593) | |
| NCT00013624 | Riluzole to Treat Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00906828 | Pharmacokinetics of Levodopa/Carbidopa Infusion With and Without Oral Catechol-O-methyl Transferase (COMT) Inhibitors | Parkinson disease | Levodopa (NPC161593) | |
| NCT01504178 | Evaluation of the Role of the Noradrenergic System in Pain Perception in Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT04244058 | Changes in Glutamatergic Neurotransmission of Severe TBI Patients | brain injury | Levodopa (NPC161593) | |
| NCT00153972 | Dopamine Turnover Rate as Surrogate Parameter for Diagnosis of Early Parkinson's Disease | Parkinson disease | Levodopa (NPC161593) | |
| NCT00223691 | Treatment of Orthostatic Hypotension in Autonomic Failure | orthostatic hypotension | Levodopa (NPC161593) | |
| NCT01283594 | Safety and Efficacy Study of SYN115 in Parkinson's Patients Using Levodopa to Treat End of Dose Wearing Off | Parkinson disease | Levodopa (NPC161593) |
❱❱❱ Associated Human Diseases and Detailed Association Evidence
How do we define the Plant-Targeted Human Disease Association?
Associated human diseases of an individual plant are summurized based on FOUR types of association evidence, these include:
❶ Association by Therapeutic Target: Bioactive protein targets of the plant were defined in "Molecular Targets" section, target-disease associations collected from TTD database were subsequently used to build the associations between the plant and its targeted human diseases.
❷ Association by Disease Gene Reversion: Plant and a specific disease will be associated when >= 1 plant target gene overlaped with disease's DEGs.
❸ Association by Clinical Trials of Plant: Plant and a specific disease will be associated when >= 1 clinical trial (the plant is the intervetion) can be matched in ClinicalTrials.gov database.
❹ Association by Clinical Trials of Plant Ingredients: Plant and a specific disease will be associated when >= 1 clinical trial (the plant ingredient is the intervetion) can be matched in ClinicalTrials.gov database.
Associated Disease of the Plant |
Association Type & Detailed Evidence |
|---|---|
Cocaine use disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C45 |
NCT00218023,NCT01393457,NCT00218075,NCT01468012,NCT00713583,NCT02080819
|
Ischaemic/haemorrhagic strokeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B20 |
F2
NCT01429077,NCT03735901,NCT02073773,NCT05369533 |
Diffuse large B-cell lymphomasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A81 |
HSD17B10,ALDH1A1,CA9,CA12,TDP1
|
Macular degenerationDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9B75 |
NCT03022318,NCT02873351,NCT03023059,NCT03451500
|
Serous cystadenoma,borderline malignancy of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.4 |
PRKCD,CA9,CA12,TDP1
|
Hepatocellular carcinoma of liverDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.02 |
CA9,CA12,CA9,CA12
|
Injury of spinal cord, level unspecifiedDisease Category: 22.Injury, poisoning or certain other consequences of external causesDisease ICD-11 Code: ND51.2 |
NCT01484184,NCT04052776,NCT04000919
|
Carcinosarcoma of uterusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.43 |
CA9,TDP1,HSD17B10
|
Other specified malignant neoplasms of kidney, except renal pelvisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90.Y |
CA9,CA9,F2
|
Squamous cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.2 |
CA9,CA12,F2
|
Adenocarcinoma of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.0 |
CA9,F2,PRKCD
|
Mesothelioma of pleuraDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C26.0 |
CA9,CA12,TDP1
|
Adenocarcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.0 |
CA9,CA12,F2
|
Solid tumour/cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00-2F9Z |
CA9,CA12
|
Myocardial infarctionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA41-BA43 |
PRKCD,F2
|
Breast cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C60-2C6Y |
CA9,CA12
|
AsthmaDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA23 |
F2
NCT02633007 |
Pain, unspecifiedDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG3Z |
NCT02116790,NCT05087914
|
Impairment of binocular functionsDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9D46 |
NCT01190813,NCT00789672
|
Angelman syndromeDisease Category: 20.Developmental anomaliesDisease ICD-11 Code: LD90.0 |
NCT00829439,NCT01281475
|
Restless legs syndromeDisease Category: 07.Sleep-wake disordersDisease ICD-11 Code: 7A80 |
NCT00144209,NCT00625547
|
Malignant neoplasms of biliary tract, distal bile ductDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C15 |
CA9,PRKCD
|
Malignant neoplasm metastasis in lymph nodes of head, face or neckDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D60.0 |
CA9,F2
|
Other specified malignant neoplasms ofcolonDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B90.Y |
CA9,F2
|
Other specified malignant neoplasms of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.Y |
CA9,CA12
|
Malignant neoplasms of corpus uteri, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.Z |
CA9,F2
|
Malignant neoplasms of thymusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C27 |
CA9,HSD17B10
|
Invasive carcinoma of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C61 |
CA9,CA12
|
Glioblastoma of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.00 |
CA9,CA12
|
Renal cell carcinoma, chromophobe typeDisease Category: X.Extension CodesDisease ICD-11 Code: XH6153 |
CA12,PRKCD
|
Germ cell tumour of testisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C80.2 |
CA12,F2
|
Malignant neoplasms of adrenal glandDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D11 |
CA9,CA12
|
ParkinsonismDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A00 |
NCT00918177,NCT00099268,NCT02312232,NCT01568047,NCT01568073,NCT03007888,NCT01519284,NCT00253084,NCT00406588,NCT01883505,NCT02601586,NCT01484990,NCT02480803,NCT00660387,NCT00914134,NCT02271503,NCT01171313,NCT00006077,NCT00279825,NCT03103399,NCT00601978,NCT00399477,NCT03496870,NCT00086294,NCT02096601,NCT00642356,NCT00888186,NCT00219284,NCT00360568,NCT02847442,NCT04952194,NCT02242487,NCT00013624,NCT03000569,NCT01515410,NCT02486432,NCT01725802,NCT00153972,NCT02799381,NCT00906828,NCT02554734,NCT02352363,NCT00391898,NCT00845000,NCT01777555,NCT00089622,NCT01568034,NCT00272688,NCT02641054,NCT03140956,NCT01646255,NCT01504178,NCT03205956,NCT05128175,NCT01026428,NCT02769793,NCT00590122,NCT00141518,NCT00485069,NCT04513340,NCT01070628,NCT01736176,NCT00134966,NCT00335153,NCT01770145,NCT04591535,NCT04990284,NCT00009048,NCT01533116,NCT01617135,NCT02764125,NCT02549092,NCT05471609,NCT01960842,NCT00040209,NCT04006210,NCT00125567,NCT01283594,NCT04380142,NCT05036473,NCT02778594,NCT00076674,NCT02812394,NCT00174239,NCT02741947,NCT01227655,NCT00108667,NCT02240030,NCT02347059,NCT03119636,NCT00006337,NCT00004733,NCT00660673,NCT00914602,NCT02170376,NCT02577523,NCT01351168,NCT00558337,NCT01479127,NCT02169414,NCT03576638,NCT02807675,NCT00357994,NCT00745277,NCT00505843,NCT03887884,NCT00143026
|
Cardiovascular diseaseDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA00-BE2Z |
F2
|
Coronary thrombosisDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA43 |
F2
|
Seborrhoeic dermatitisDisease Category: 14.Diseases of the skinDisease ICD-11 Code: EA81 |
CA12
|
Bleeding disorderDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA20-GA21 |
F2
|
ThrombocytopeniaDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3B64 |
F2
|
Bacterial infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A00-1C4Z |
CA12
|
Cerebral ischaemic strokeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B11 |
F2
|
Coagulation defectDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3B10 |
F2
|
Mature T-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90 |
CA9
|
Nutritional deficiencyDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5B50-5B71 |
F2
|
Renal cell carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90 |
CA9
|
Angina pectorisDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA40 |
F2
|
Type 2 diabetes mellitusDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A11 |
F2
|
Substance abuseDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C40 |
ALDH1A1
|
Multiple sclerosisDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A40 |
F2
|
ThrombosisDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DB61-GB90 |
F2
|
AphasiaDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MA80.0 |
NCT01429077
|
Neuropathy, ataxia, and retinitis pigmentosaDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C73.1 |
NCT02837640
|
Actinic keratosisDisease Category: X.Extension CodesDisease ICD-11 Code: XH36H6 |
NCT01387711
|
Chronic painDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG30 |
NCT05285683
|
Orthostatic hypotensionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA21 |
NCT00223691
|
Amyotrophic lateral sclerosisDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B60.0 |
NCT03929068
|
Tourette syndromeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A05.00 |
NCT00634556
|
Intracranial injury, unspecifiedDisease Category: 22.Injury, poisoning or certain other consequences of external causesDisease ICD-11 Code: NA07.Z |
NCT04244058
|
OculocutaneousalbinismDisease Category: 14.Diseases of the skinDisease ICD-11 Code: EC23.20 |
NCT01663935
|
Post-traumatic stress disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6B40 |
NCT04558112
|
SchizophreniaDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A20 |
NCT01636037
|
Albinism or other specified genetically-determined hypomelanotic disordersDisease Category: 14.Diseases of the skinDisease ICD-11 Code: EC23.2 |
NCT01176435
|
Nicotine use disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C4A |
NCT02633839
|
Spinal painDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: ME84 |
NCT04082715
|
RetinopathyDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9B71 |
NCT05132660
|
Autism spectrum disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6A02 |
NCT03243552
|
Osteoarthritis, unspecifiedDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FA0Z |
CA12
|
HepatoblastomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.01 |
CA12
|
Adenocarcinoma of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.0 |
F2
|
Tetralogy of FallotDisease Category: 20.Developmental anomaliesDisease ICD-11 Code: LA88.2 |
F2
|
Melanoma of skinDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30 |
ALDH1A1
|
Urothelial carcinoma of bladderDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94.2 |
CA9
|
Chronic rhinosinusitisDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA0A |
CA9
|
Malignant neoplasms of thyroid gland, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D10.Z |
CA12
|
Parkinson diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A00.0 |
ALDH1A1
|

