Collective Molecular Activities of the Plant: Rubus Occidentalis

Country/Region:
United StatesTraditional Medicine System:
Medicinal Functions:
Astringent; Cathartic; Ophthalmic; Pectoral; Salve; TB; VD
Canada; United States; Georgia
Overview of Ingredients
34 All known Ingredients in Total
Unique ingredients have been isolated from this plant.Plant-Ingredients Associations were manually curated from publications or collected from other databases.
29 Ingredients with Acceptable Bioavailablity
Unique ingredients exhibit acceptable human oral bioavailablity, according to the criteria of SwissADME [PMID: 28256516] and HobPre [PMID: 34991690]. The criteria details:SwissADME: six descriptors are used by SwissADME to evaluate the oral bioavailability of a natural product:
☑ LIPO(Lipophility): -0.7 < XLOGP3 < +5.0
☑ SIZE: 150g/mol < MW < 500g/mol
☑ POLAR(Polarity): 20Ų < TPSA < 130Ų
☑ INSOLU(Insolubility): -6 < Log S (ESOL) < 0
☑ INSATU(Insaturation): 0.25 < Fraction Csp3 < 1
☑ FLEX(Flexibility): 0 < Num. rotatable bonds < 9
If 6 descriptors of a natural plant satisfy the above rules, it will be labeled high HOB.
HobPre: A natural plant ingredient with HobPre score >0.5 is labeled high human oral availability (HOB)
10 Ingredients with experimental-derived Activity
Unique ingredients have activity data available.Ingredient Structrual Cards
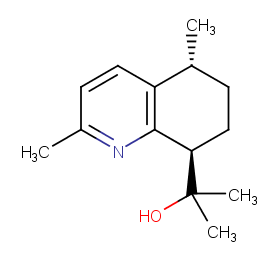
Ingredient ID: NPC97727
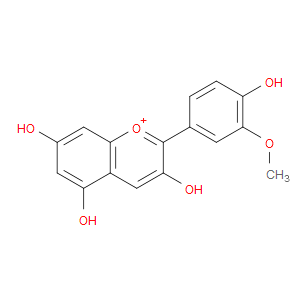
Ingredient ID: NPC86655
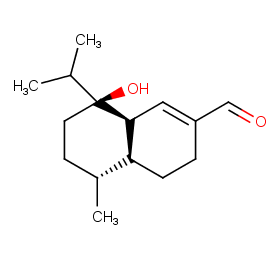
Ingredient ID: NPC84454
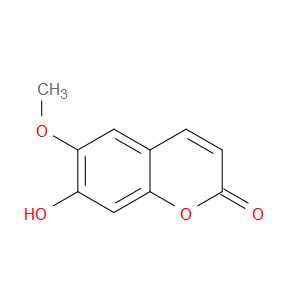
Ingredient ID: NPC76336
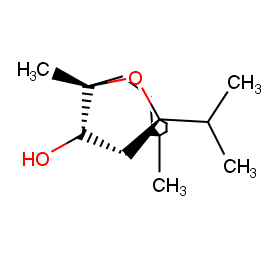
Ingredient ID: NPC71004
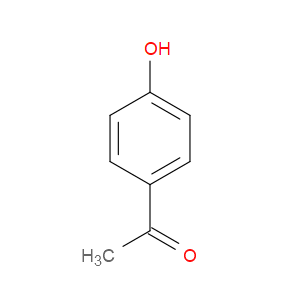
Ingredient ID: NPC6984
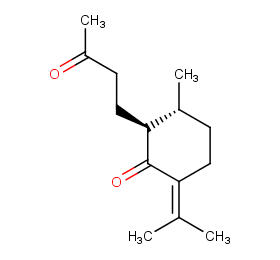
Ingredient ID: NPC67599
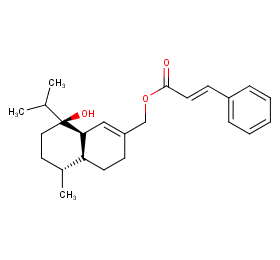
Ingredient ID: NPC45483
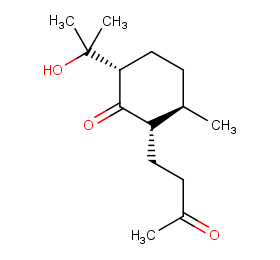
Ingredient ID: NPC41500
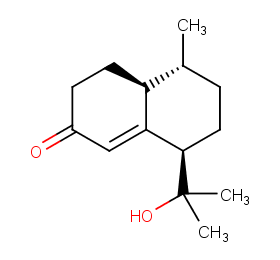
Ingredient ID: NPC32421
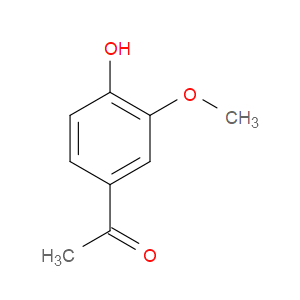
Ingredient ID: NPC32163
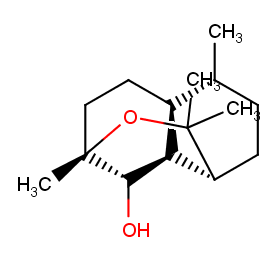
Ingredient ID: NPC31584
Ingredient ID: NPC27526
Ingredient ID: NPC262047
Ingredient ID: NPC252655
Ingredient ID: NPC235224
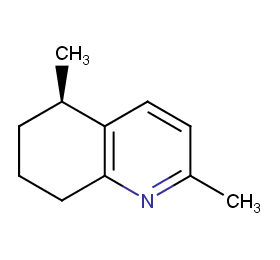
Ingredient ID: NPC230007
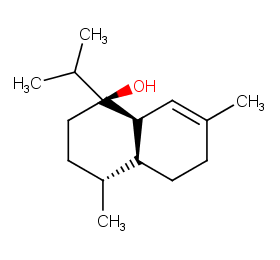
Ingredient ID: NPC228069
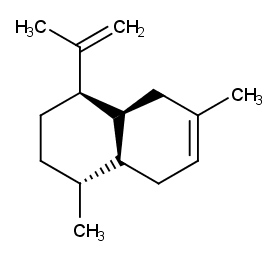
Ingredient ID: NPC213312
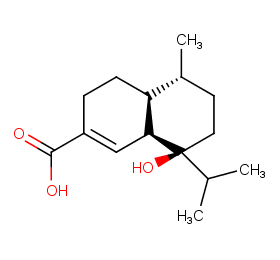
Ingredient ID: NPC195172
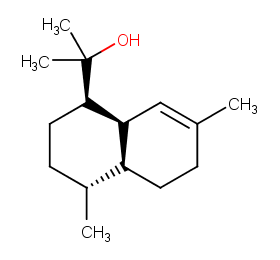
Ingredient ID: NPC195170
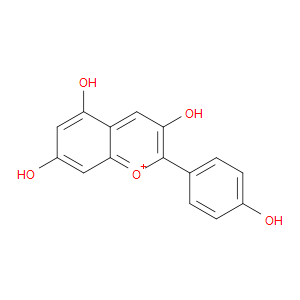
Ingredient ID: NPC190454
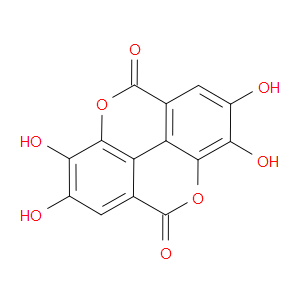
Ingredient ID: NPC178134
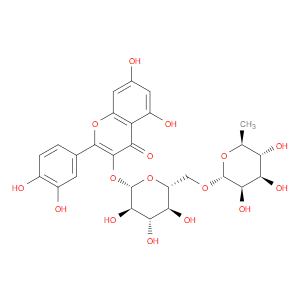
Ingredient ID: NPC176740
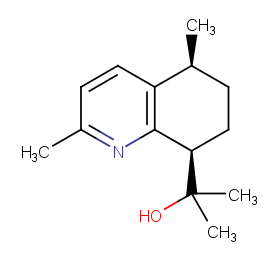
Ingredient ID: NPC175492
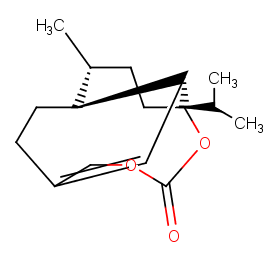
Ingredient ID: NPC159115
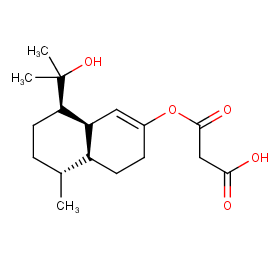
Ingredient ID: NPC15692
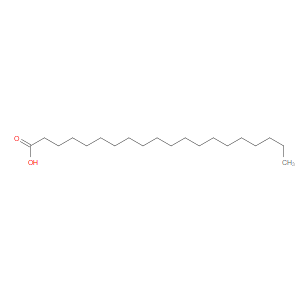
Ingredient ID: NPC149184
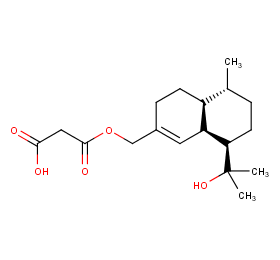
Ingredient ID: NPC145538
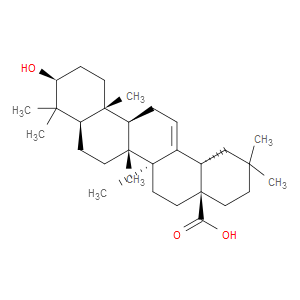
Ingredient ID: NPC142415
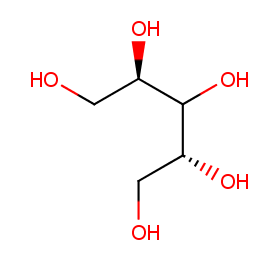
Ingredient ID: NPC127074
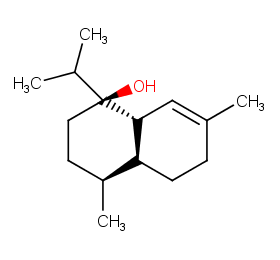
Ingredient ID: NPC118843
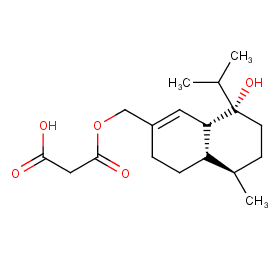
Ingredient ID: NPC117727
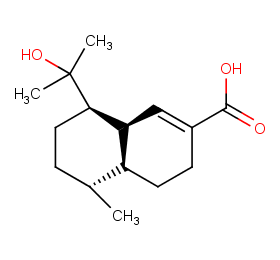
Ingredient ID: NPC104354
Classification of Human Proteins Collectively Targeted by the Plant
Detailed Information of Target Proteins
| Target Type | Protein Class | Gene ID | Protein Name | Uniprot ID | Target ChEMBL ID |
|---|---|---|---|---|---|
| Drug Transporter | SLC superfamily of solute carriers | SLC22A6 | Solute carrier family 22 member 6 | Q4U2R8 | CHEMBL1641347 |
| Therapeutic Target | Enzyme | HPGD | 15-hydroxyprostaglandin dehydrogenase [NAD+] | P15428 | CHEMBL1293255 |
| Therapeutic Target | Enzyme | CES1 | Acyl coenzyme A:cholesterol acyltransferase | P23141 | CHEMBL2265 |
| Therapeutic Target | Enzyme | APEX1 | DNA-(apurinic or apyrimidinic site) lyase | P27695 | CHEMBL5619 |
| Therapeutic Target | Enzyme | RECQL | ATP-dependent DNA helicase Q1 | P46063 | CHEMBL1293236 |
| Therapeutic Target | Enzyme | POLB | DNA polymerase beta | P06746 | CHEMBL2392 |
| Therapeutic Target | Hydrolase | ACHE | Acetylcholinesterase | P22303 | CHEMBL220 |
| Therapeutic Target | Lyase | CA9 | Carbonic anhydrase IX | Q16790 | CHEMBL3594 |
| Therapeutic Target | Nuclear hormone receptor subfamily 1 | NR1H4 | Bile acid receptor FXR | Q96RI1 | CHEMBL2047 |
| Therapeutic Target | Other cytosolic protein | HSPA1A | Heat shock 70 kDa protein 1 | P0DMV8 | CHEMBL5460 |
Clinical trials associated with plant from natural product (NP) & plant level:
| Clinical trials type | Number of clinical trials | |
|---|---|---|
| 7 | ||
| 4 | ||
| NCT ID | Title | Condition | Form in clinical use | Associated by plant or compound |
|---|---|---|---|---|
| NCT00455416 | Dietary Intervention in Follicular Lymphoma | follicular lymphoma | Ellagic Acid (NPC178134) | |
| NCT00992667 | Inhaled Apocynin Decreases Reactive Oxygen Species Concentrations in Exhaled Breath Condensate in Mild Asthmatics | asthma | Apocynin (NPC32163) | |
| NCT01402297 | Hydrogen Peroxide and Nitrite Reduction in Exhaled Breath Condensate of COPD Patients | chronic obstructive pulmonary disease | Apocynin (NPC32163) | |
| NCT01465776 | Freeze-Dried Black Raspberries in Treating Patients With Oral Squamous Cell Cancer Undergoing Surgery | Squamous Cell Carcinoma of Mouth | Rubus Occidentalis | As single plant |
| NCT01504932 | Freeze-Dried Black Raspberries in Preventing Oral Cancer Recurrence in High At-Risk Appalachian Patients Oral Cancer Survivors | Stage I Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage I Squamous Cell Carcinoma of the Oropharynx|Stage I Verrucous Carcinoma of the Oral Cavity|Stage II Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage II Squamous Cell Carcinoma of the Oropharynx|Stage II Verrucous Carcinoma of the Oral Cavity|Stage III Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage III Squamous Cell Carcinoma of the Oropharynx|Stage III Verrucous Carcinoma of the Oral Cavity|Stage IVA Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage IVA Squamous Cell Carcinoma of the Oropharynx|Stage IVA Verrucous Carcinoma of the Oral Cavity|Stage IVB Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage IVB Squamous Cell Carcinoma of the Oropharynx|Stage IVB Verrucous Carcinoma of the Oral Cavity|Stage IVC Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage IVC Squamous Cell Carcinoma of the Oropharynx|Stage IVC Verrucous Carcinoma of the Oral Cavity|Tongue Cancer | Rubus Occidentalis | As single plant |
| NCT01705652 | Study to Assess the Activity of Nexrutine® in Prostate Cancer Patients | prostate cancer | Rutin (NPC176740) | |
| NCT03140280 | Hypomethylating Properties of Freeze-dried Black Raspberries (BRB) in Patients With Myelodysplastic Syndrome or Myelodysplastic Syndrome/Myeloproliferative Neoplasm (MDS/MPN) | Myelodysplastic Syndromes | Rubus Occidentalis | As single plant |
| NCT03680573 | The Effect of Antioxidants on Skin Blood Flow-BH4 | cardiovascular disease | Apocynin (NPC32163) | |
| NCT03680638 | The Effect of Antioxidants on Skin Blood Flow During Local Heating | cardiovascular disease | Apocynin (NPC32163) | |
| NCT04011618 | Effect of Ellagic Ácid on the Components of Metabolic Syndrome, Insulin Sensitivity and Insulin Secretion | metabolic syndrome | Ellagic Acid (NPC178134) |
❱❱❱ Associated Human Diseases and Detailed Association Evidence
How do we define the Plant-Targeted Human Disease Association?
Associated human diseases of an individual plant are summurized based on FOUR types of association evidence, these include:
❶ Association by Therapeutic Target: Bioactive protein targets of the plant were defined in "Molecular Targets" section, target-disease associations collected from TTD database were subsequently used to build the associations between the plant and its targeted human diseases.
❷ Association by Disease Gene Reversion: Plant and a specific disease will be associated when >= 1 plant target gene overlaped with disease's DEGs.
❸ Association by Clinical Trials of Plant: Plant and a specific disease will be associated when >= 1 clinical trial (the plant is the intervetion) can be matched in ClinicalTrials.gov database.
❹ Association by Clinical Trials of Plant Ingredients: Plant and a specific disease will be associated when >= 1 clinical trial (the plant ingredient is the intervetion) can be matched in ClinicalTrials.gov database.
Associated Disease of the Plant | Association Type & Detailed Evidence |
|---|---|
Acute diabete complicationDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A2Y |
INSR,PTPN1,KDR,AKR1B1
|
Acute myeloid leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60 |
SRC,PTPRC
|
Acute respiratory distress syndromeDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB00 |
F3
|
Adenocarcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.0 |
ACHE,GPR35,AKR1B10,CA9
|
Adenocarcinoma of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.0 |
NUAK1,TEK,ACHE,AKR1B10,CA9,MET,PTPRC,PTPN1,POLB,CDC25B,RECQL,CES1
|
Adenocarcinoma of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.0 |
MET,CDC25B
|
Alzheimer diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A20 |
KDR,MET,ACHE,INSR
NCT05680532 |
Angina pectorisDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA40 |
EGFR
|
AsthmaDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA23 |
NCT00992667
|
Bacterial infection of unspecified siteDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1C41 |
NCT05680532
|

