Collective Molecular Activities of the Plant: Amentotaxus Argotaenia
Unknown.
Vietnam; China; Cambodia; Laos
Overview of Ingredients
43 All known Ingredients in Total
Unique ingredients have been isolated from this plant.Plant-Ingredients Associations were manually curated from publications or collected from other databases.
30 Ingredients with Acceptable Bioavailablity
Unique ingredients exhibit acceptable human oral bioavailablity, according to the criteria of SwissADME [PMID: 28256516] and HobPre [PMID: 34991690]. The criteria details:SwissADME: six descriptors are used by SwissADME to evaluate the oral bioavailability of a natural product:
☑ LIPO(Lipophility): -0.7 < XLOGP3 < +5.0
☑ SIZE: 150g/mol < MW < 500g/mol
☑ POLAR(Polarity): 20Ų < TPSA < 130Ų
☑ INSOLU(Insolubility): -6 < Log S (ESOL) < 0
☑ INSATU(Insaturation): 0.25 < Fraction Csp3 < 1
☑ FLEX(Flexibility): 0 < Num. rotatable bonds < 9
If 6 descriptors of a natural plant satisfy the above rules, it will be labeled high HOB.
HobPre: A natural plant ingredient with HobPre score >0.5 is labeled high human oral availability (HOB)
25 Ingredients with experimental-derived Activity
Unique ingredients have activity data available.Ingredient Structrual Cards
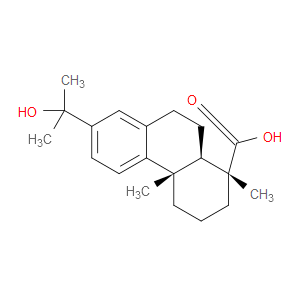
Ingredient ID: NPC95126
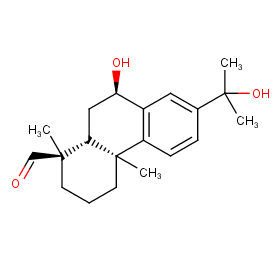
Ingredient ID: NPC94939
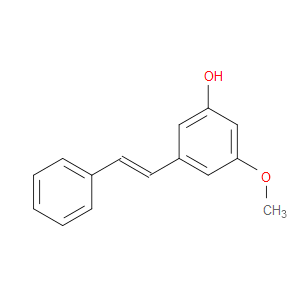
Ingredient ID: NPC94045
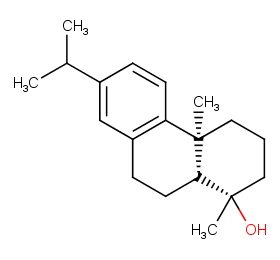
Ingredient ID: NPC93829

Ingredient ID: NPC9129
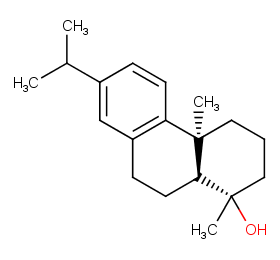
Ingredient ID: NPC9007
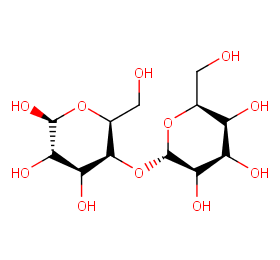
Ingredient ID: NPC50855
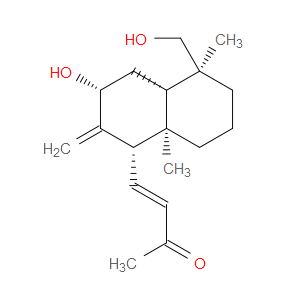
Ingredient ID: NPC488961
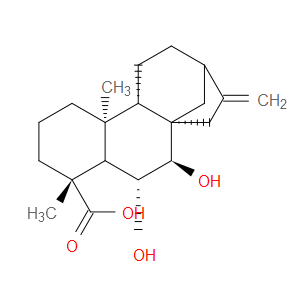
Ingredient ID: NPC488960
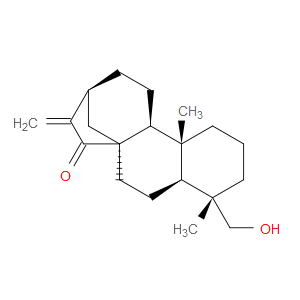
Ingredient ID: NPC488959
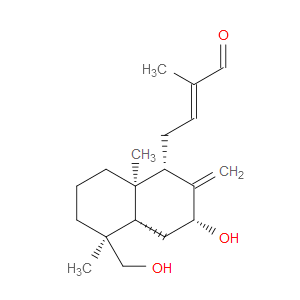
Ingredient ID: NPC488958
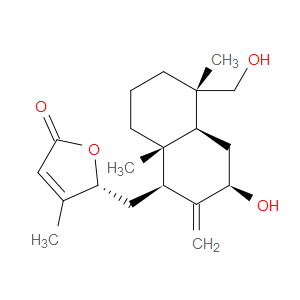
Ingredient ID: NPC488957
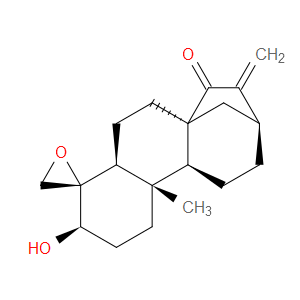
Ingredient ID: NPC488956
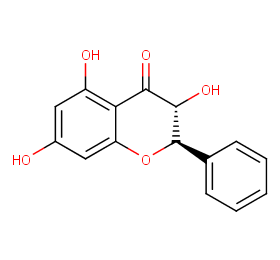
Ingredient ID: NPC4152
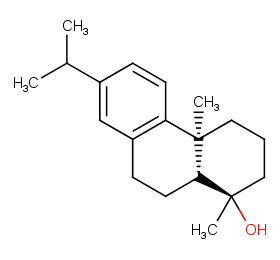
Ingredient ID: NPC38807
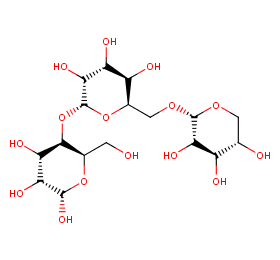
Ingredient ID: NPC37592
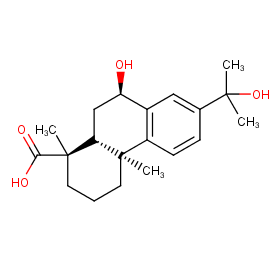
Ingredient ID: NPC369
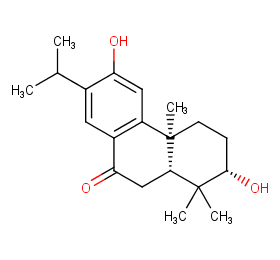
Ingredient ID: NPC36765
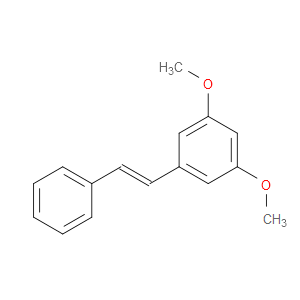
Ingredient ID: NPC35543
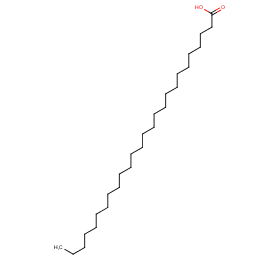
Ingredient ID: NPC32021
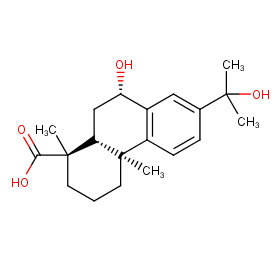
Ingredient ID: NPC293831
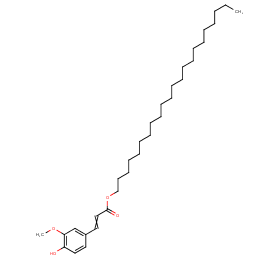
Ingredient ID: NPC283700
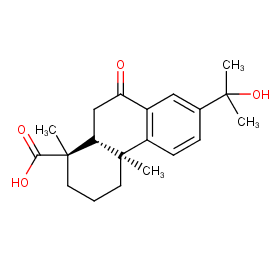
Ingredient ID: NPC269923
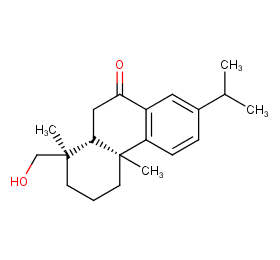
Ingredient ID: NPC265513
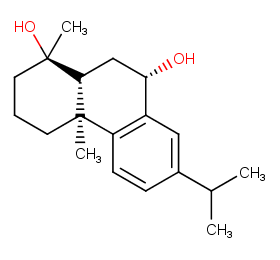
Ingredient ID: NPC24446
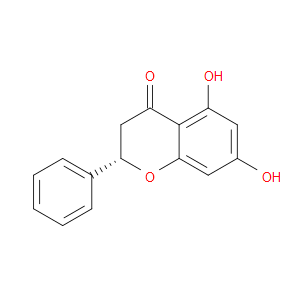
Ingredient ID: NPC243083
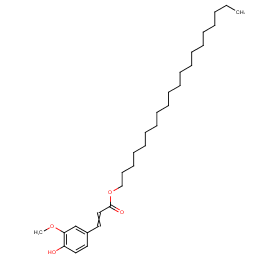
Ingredient ID: NPC236777
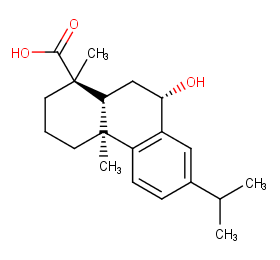
Ingredient ID: NPC234337
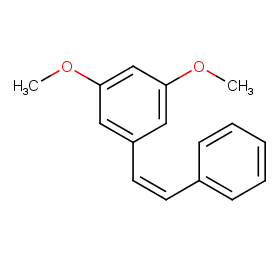
Ingredient ID: NPC2290
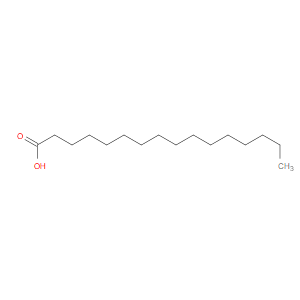
Ingredient ID: NPC216630
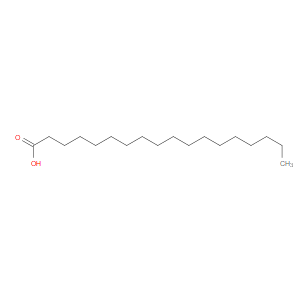
Ingredient ID: NPC209970
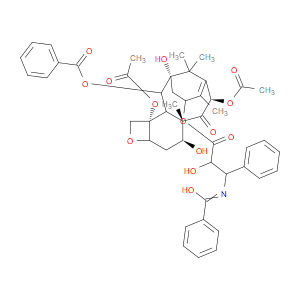
Ingredient ID: NPC208553
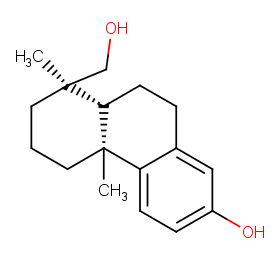
Ingredient ID: NPC189468
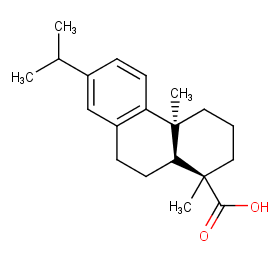
Ingredient ID: NPC189078
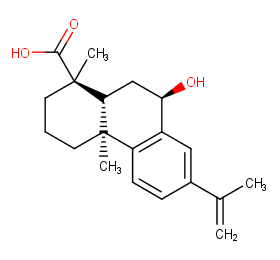
Ingredient ID: NPC183339
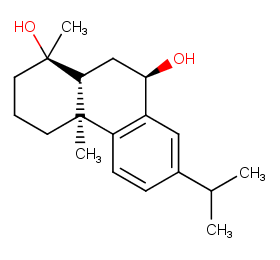
Ingredient ID: NPC181782

Ingredient ID: NPC176308
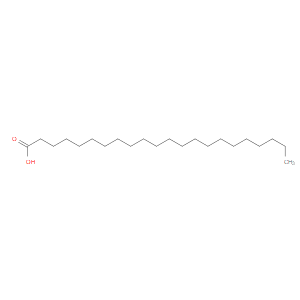
Ingredient ID: NPC171736

Ingredient ID: NPC15999
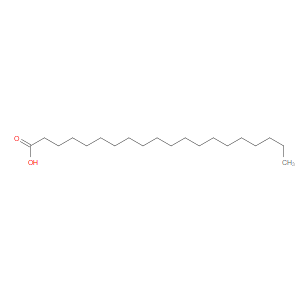
Ingredient ID: NPC149184
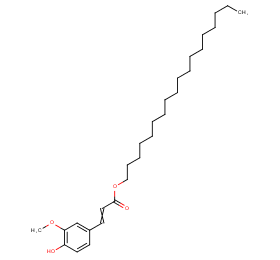
Ingredient ID: NPC141836
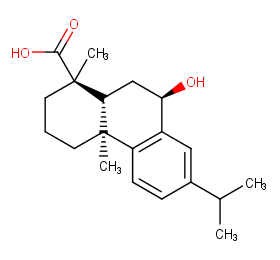
Ingredient ID: NPC133389
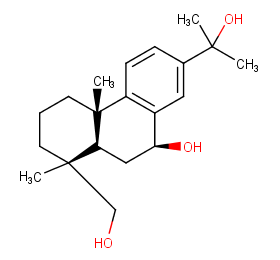
Ingredient ID: NPC104551
Classification of Human Proteins Collectively Targeted by the Plant
Detailed Information of Target Proteins
| Target Type | Protein Class | Gene ID | Protein Name | Uniprot ID | Target ChEMBL ID |
|---|---|---|---|---|---|
| Cytochrome P450 | Cytochrome P450 family 19 | CYP19A1 | Cytochrome P450 19A1 | P11511 | CHEMBL1978 |
| Drug Transporter | SLC superfamily of solute carriers | SLCO1B1 | Solute carrier organic anion transporter family member 1B1 | Q9Y6L6 | CHEMBL1697668 |
| Drug Transporter | SLC superfamily of solute carriers | SLCO1B3 | Solute carrier organic anion transporter family member 1B3 | Q9NPD5 | CHEMBL1743121 |
| Therapeutic Target | Enzyme | KARS1 | Lysyl-tRNA synthetase | Q15046 | CHEMBL5575 |
| Therapeutic Target | Fatty acid binding protein family | FABP4 | Fatty acid binding protein adipocyte | P15090 | CHEMBL2083 |
| Therapeutic Target | Hydrolase | RAB9A | Ras-related protein Rab-9A | P51151 | CHEMBL1293294 |
| Therapeutic Target | Nuclear hormone receptor subfamily 1 | THRB | Thyroid hormone receptor beta-1 | P10828 | CHEMBL1947 |
| Therapeutic Target | Protein Kinase | MTOR | Serine/threonine-protein kinase mTOR | P42345 | CHEMBL2842 |
| Therapeutic Target | Structural protein | LMNA | Prelamin-A/C | P02545 | CHEMBL1293235 |
| Therapeutic Target | Structural protein | TUBB3 | Tubulin beta-3 chain | Q13509 | CHEMBL2597 |
Clinical trials associated with plant from natural product (NP) & plant level:
| Clinical trials type | Number of clinical trials | |
|---|---|---|
| 2101 | ||
| NCT ID | Title | Condition | Form in clinical use | Associated by plant or compound |
|---|---|---|---|---|
| NCT00001272 | A Phase I Study of Taxol, Cisplatin, Cyclophosphamide and Granulocyte Colony-Stimulating Factor (G-CSF) in Previously Nontreated Ovarian Cancer Patients | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00001383 | A Phase I Study of Infusional Paclitaxel With the P-Glycoprotein Antagonist PSC 833 | renal cell carcinoma;lymphoma;ovarian cancer;breast cancer | Paclitaxel (NPC208553) | |
| NCT00001384 | A Pilot Trial of AC (Adriamycin, Cyclophosphamide) Chemotherapy With G-CSF (Granulocyte Colony-Stimulating Factor) Followed by Infusional Taxol (Paclitaxel) as Adjuvant Treatment for High Risk Stage II and Stage III Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT00001387 | Phase I and Pharmacokinetic Trial of Paclitaxel (Taxol) Given as a 3-Hour Infusion in Pediatric Patients With Refractory Malignancy | neoplasm | Paclitaxel (NPC208553) | |
| NCT00001426 | A Multi-Institutional Phase II Study of Cyclophosphamide, Paclitaxel, Cisplatin With G-CSF for Patients With Newly Diagnosed Advanced Stage Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00001442 | A Pilot Study of Paclitaxel With Radiation Therapy for Locally Advanced Head and Neck Cancer | squamous cell carcinoma;upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT00001450 | Phase II Trial of a 96-Hour Continuous Infusion of Paclitaxel Followed by Cisplatin for Patients With Stage III/IV and Relapsed NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00001498 | A Pilot Trial of Sequential Chemotherapy With Antimetabolite Induction, High-Dose Alkylating Agent Consolidation With Peripheral Blood Progenitor Cell Support, and Intensification With Paclitaxel and Doxorubicin for Patients With High-Risk Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00001499 | Phase II Neoadjuvant Trial of a Continuous Infusion of Paclitaxel Plus Cisplatin Followed by Chest Radiotherapy for Patients With Stage III Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00001569 | Phase I Trial of Continuous Hyperthermic Peritoneal Perfusion (CHPP) With Cisplatin Plus Early Postoperative Intraperitoneal Paclitaxel and 5-FU for Peritoneal Carcinomatosis | peritoneal neoplasm;carcinoma | Paclitaxel (NPC208553) |
❱❱❱ Associated Human Diseases and Detailed Association Evidence
How do we define the Plant-Targeted Human Disease Association?
Associated human diseases of an individual plant are summurized based on FOUR types of association evidence, these include:
❶ Association by Therapeutic Target: Bioactive protein targets of the plant were defined in "Molecular Targets" section, target-disease associations collected from TTD database were subsequently used to build the associations between the plant and its targeted human diseases.
❷ Association by Disease Gene Reversion: Plant and a specific disease will be associated when >= 1 plant target gene overlaped with disease's DEGs.
❸ Association by Clinical Trials of Plant: Plant and a specific disease will be associated when >= 1 clinical trial (the plant is the intervetion) can be matched in ClinicalTrials.gov database.
❹ Association by Clinical Trials of Plant Ingredients: Plant and a specific disease will be associated when >= 1 clinical trial (the plant ingredient is the intervetion) can be matched in ClinicalTrials.gov database.
Associated Disease of the Plant | Association Type & Detailed Evidence |
|---|---|
Actinic keratosisDisease Category: X.Extension CodesDisease ICD-11 Code: XH36H6 |
NCT03083470
|
Adenocarcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.0 |
SLCO1B1,SLCO1B3,GMNN
|
Adenocarcinoma of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.0 |
NPC1,OR51E2,SLCO1B3,TUBB6,PMP22,LMNA,ITGAV
|
Adenocarcinoma of prostateDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C82.0 |
OR51E2,SLCO1B1,FABP5,SLCO1B3
NCT04221828,NCT00521781 |
Adenocarcinoma of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.0 |
SLCO1B1,CYP19A1,SLCO1B3
NCT03281369,NCT02615730,NCT02625623,NCT02448329,NCT01336062,NCT04077255,NCT04209686,NCT04604132,NCT01641783,NCT04190745,NCT03990103,NCT02449655,NCT04572542,NCT03193918,NCT04510064 |
Adenocarcinoma, endocervical typeDisease Category: X.Extension CodesDisease ICD-11 Code: XH0GS9 |
NCT00295789,NCT02020707,NCT04723875,NCT04516616,NCT01755897,NCT00064077
|
AdenocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D40 |
NCT05042128,NCT02732938,NCT04821284,NCT04827953,NCT00384826,NCT03915444,NCT04134468,NCT02186847,NCT03517176,NCT04634539,NCT04935359,NCT05257993,NCT01506973,NCT00005838,NCT04929041,NCT02487277,NCT02303977,NCT03345810,NCT00955305,NCT00042835,NCT02993731,NCT01253525,NCT00040794,NCT01676259,NCT01578551,NCT02715804,NCT03496662,NCT02047513,NCT04390763,NCT02583477,NCT00368992,NCT00226746,NCT02926183,NCT00201734,NCT00021060,NCT02243007,NCT05254171,NCT02501902,NCT02754726,NCT04329949,NCT02101021,NCT04581343,NCT00002852,NCT02048943,NCT02468557,NCT01431794,NCT04469556,NCT04481204,NCT04046887,NCT02426281,NCT03908333,NCT03361319,NCT02723331,NCT00369551,NCT01218516,NCT04257448,NCT03697239,NCT02059967,NCT03410030
|
Adrenal cortical carcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH4KH2 |
NCT00786110
|
Allergic/hypersensitivity disorderDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4A80-4A8Z |
NCT00277043
|
AnemiaDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3A00-3A9Z |
NCT00189371
|

