Collective Molecular Activities of the Plant: Selaginella Moellendorffii
Overview of Ingredients
20 All known Ingredients in Total
Unique ingredients have been isolated from this plant.Plant-Ingredients Associations were manually curated from publications or collected from other databases.
7 Ingredients with Acceptable Bioavailablity
Unique ingredients exhibit acceptable human oral bioavailablity, according to the criteria of SwissADME [PMID: 28256516] and HobPre [PMID: 34991690]. The criteria details:SwissADME: six descriptors are used by SwissADME to evaluate the oral bioavailability of a natural product:
☑ LIPO(Lipophility): -0.7 < XLOGP3 < +5.0
☑ SIZE: 150g/mol < MW < 500g/mol
☑ POLAR(Polarity): 20Ų < TPSA < 130Ų
☑ INSOLU(Insolubility): -6 < Log S (ESOL) < 0
☑ INSATU(Insaturation): 0.25 < Fraction Csp3 < 1
☑ FLEX(Flexibility): 0 < Num. rotatable bonds < 9
If 6 descriptors of a natural plant satisfy the above rules, it will be labeled high HOB.
HobPre: A natural plant ingredient with HobPre score >0.5 is labeled high human oral availability (HOB)
12 Ingredients with experimental-derived Activity
Unique ingredients have activity data available.Ingredient Structrual Cards
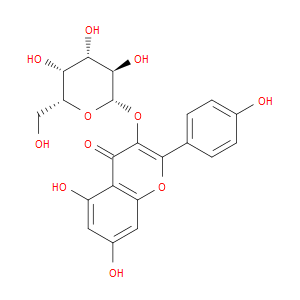
Ingredient ID: NPC78263

Ingredient ID: NPC75844
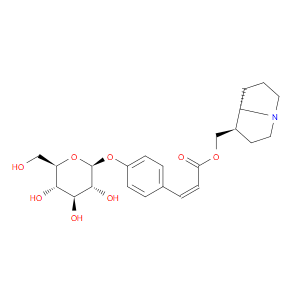
Ingredient ID: NPC50539
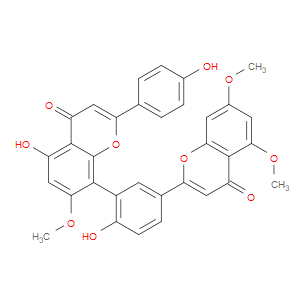
Ingredient ID: NPC485525
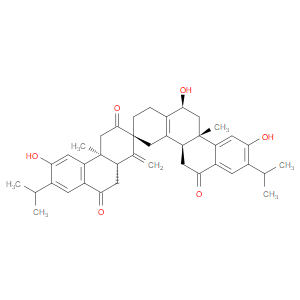
Ingredient ID: NPC479611

Ingredient ID: NPC479610
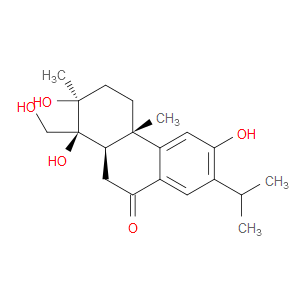
Ingredient ID: NPC479609
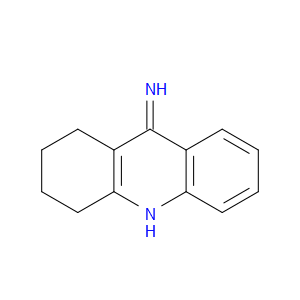
Ingredient ID: NPC479379
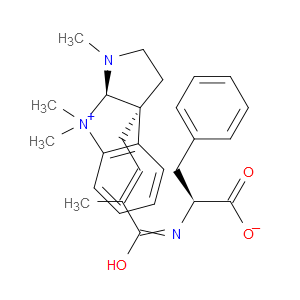
Ingredient ID: NPC476172
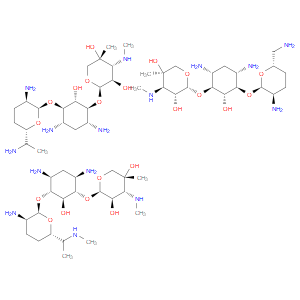
Ingredient ID: NPC471628
Ingredient ID: NPC303485
Ingredient ID: NPC265624
Ingredient ID: NPC261012

Ingredient ID: NPC240272
Ingredient ID: NPC208553
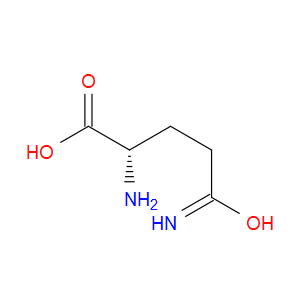
Ingredient ID: NPC197087
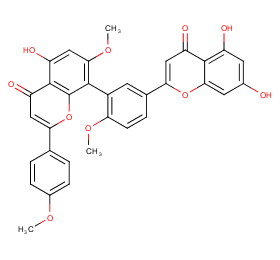
Ingredient ID: NPC186227
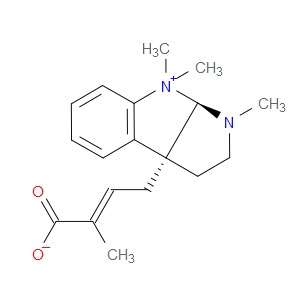
Ingredient ID: NPC14903
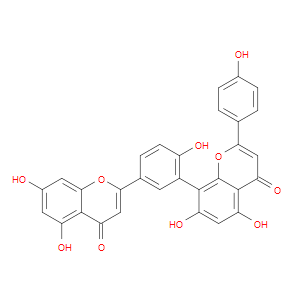
Ingredient ID: NPC138299
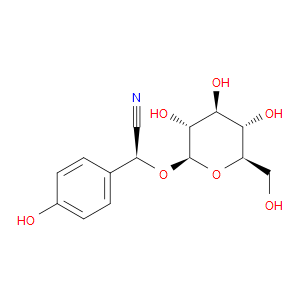
Ingredient ID: NPC109759
Classification of Human Proteins Collectively Targeted by the Plant
Detailed Information of Target Proteins
| Target Type | Protein Class | Gene ID | Protein Name | Uniprot ID | Target ChEMBL ID |
|---|---|---|---|---|---|
| Therapeutic Target | Aspartic protease | BACE1 | Beta-secretase 1 | P56817 | CHEMBL4822 |
| Therapeutic Target | Enzyme | BLM | Bloom syndrome protein | P54132 | CHEMBL1293237 |
| Therapeutic Target | Hydrolase | RAB9A | Ras-related protein Rab-9A | P51151 | CHEMBL1293294 |
| Therapeutic Target | Hydrolase | ACHE | Acetylcholinesterase | P22303 | CHEMBL220 |
| Therapeutic Target | Hydrolase | BCHE | Butyrylcholinesterase | P06276 | CHEMBL1914 |
| Therapeutic Target | Isomerase | TOP2A | DNA topoisomerase II alpha | P11388 | CHEMBL1806 |
| Therapeutic Target | Membrane receptor | APP | Beta amyloid A4 protein | P05067 | CHEMBL2487 |
| Therapeutic Target | Nicotinic acetylcholine receptor | CHRNE | Acetylcholine receptor protein epsilon chain | Q04844 | CHEMBL2484 |
| Therapeutic Target | Nuclear hormone receptor subfamily 1 | THRB | Thyroid hormone receptor beta-1 | P10828 | CHEMBL1947 |
| Therapeutic Target | Peptide receptor (family A GPCR) | TSHR | Thyroid stimulating hormone receptor | P16473 | CHEMBL1963 |
| Therapeutic Target | Peptide receptor (family A GPCR) | OPRD1 | Delta opioid receptor | P41143 | CHEMBL236 |
| Therapeutic Target | Protein Kinase | AURKA | Serine/threonine-protein kinase Aurora-A | O14965 | CHEMBL4722 |
| Therapeutic Target | Protein Kinase | MTOR | Serine/threonine-protein kinase mTOR | P42345 | CHEMBL2842 |
| Therapeutic Target | Secreted protein | THPO | Thrombopoietin | P40225 | CHEMBL1293256 |
| Drug Transporter | SLC superfamily of solute carriers | SLC22A2 | Solute carrier family 22 member 2 | O15244 | CHEMBL1743122 |
| Drug Transporter | SLC superfamily of solute carriers | SLCO1B1 | Solute carrier organic anion transporter family member 1B1 | Q9Y6L6 | CHEMBL1697668 |
| Drug Transporter | SLC superfamily of solute carriers | SLCO1B3 | Solute carrier organic anion transporter family member 1B3 | Q9NPD5 | CHEMBL1743121 |
| Therapeutic Target | Structural protein | TUBB3 | Tubulin beta-3 chain | Q13509 | CHEMBL2597 |
| Therapeutic Target | Structural protein | LMNA | Prelamin-A/C | P02545 | CHEMBL1293235 |
| Therapeutic Target | Unclassified protein | GMNN | Geminin | O75496 | CHEMBL1293278 |
| Therapeutic Target | Unclassified protein | PMP22 | Peripheral myelin protein 22 | Q01453 | CHEMBL1293298 |
Clinical trials associated with plant from natural product (NP) & plant level:
| Clinical trials type | Number of clinical trials | |
|---|---|---|
| 4138 | ||
| NCT ID | Title | Condition | Form in clinical use | Associated by plant or compound |
|---|---|---|---|---|
| NCT03083470 | Study of SOR007 Ointment for Actinic Keratosis | actinic keratosis | Paclitaxel (NPC208553) | |
| NCT00111904 | Paclitaxel in Treating Patients With Unresectable Locally Advanced or Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00687817 | Study of Bavituximab Plus Paclitaxel and Carboplatin in Patients With Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02845908 | POF Versus FOLFOX Versus FOLFOX Plus ip Paclitaxel in AGC | gastric cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01258634 | A Study of Pre-Operative Treatment of Newly-Diagnosed, Surgically-Resectable Osteosarcoma With Doxorubicin, Ifosfamide, Etoposide, and Cisplatin With Early Metabolic Assessment of Response | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT02279134 | Phase II/III Study Compare Adjuvant Chemoradiotherapy, Radiotherapy and Surgery Alone for Esophageal Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT02172976 | Randomized Multicenter Phase II/III Study With Adjuvant Gemcitabine Versus Neoadjuvant / Adjuvant FOLFIRINOX for Resectable Pancreas Carcinoma | carcinoma | Fluorouracil (NPC75844) | |
| NCT00003907 | Chemoembolization in Treating Patients With Primary Liver Cancer or Metastases to the Liver | liver cancer | Doxorubicin (NPC261012) | |
| NCT00581776 | Phase II Study of VcR-CVAD With Rituximab Consolidation and Maintenance for Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02741856 | Study of Chemoradiotherapy in Oesophageal Cancer Including PET Response and Dose Escalation | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04544943 | Safety, Tolerability, Pharmacokinetics and Efficacy of Twice Daily Application of Topical BioLexa in Adult Healthy Subjects and Patients With Mild to Moderate Atopic Dermatitis | atopic eczema | Gentamicin (NPC471628) | |
| NCT00580333 | Preoperative Cisplatin and Bevacizumab in ER-, PR-, HER2 Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02192021 | Micro Needle Array-Doxorubicin (MNA-D) in Patients With Cutaneous T-cell Lymphoma (CTCL) | Cutaneous T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00006721 | S0016 Combination Chemotherapy With Monoclonal Antibody Therapy in Newly Diagnosed Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00278122 | Paclitaxel and GM-CSF in Treating Patients With Stage III or Stage IV Melanoma That Cannot Be Removed By Surgery | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT00031954 | Combination Chemotherapy in Treating Patients With Ovarian Epithelial, Fallopian Tube, or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00456846 | First Line Therapy for Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003209 | Radiation Therapy With or Without Chemotherapy After Surgery in Treating Patients With Stage IB or Stage IIA Cervical Cancer | cervical cancer | Fluorouracil (NPC75844) | |
| NCT00879190 | Ampicillin / Sulbactam vs. Ampicillin / Gentamicin for Treatment of Chorioamnionitis | chorioamnionitis | Gentamicin (NPC471628) | |
| NCT01444547 | A Study on Predictive Value of ERCC1 in Esophageal Cancer Patients Treated With Paclitaxel and Cisplatin | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04974944 | First-line Treatment With Camrelizumab + Apatinib Versus Chemotherapy + Bevacizumab in Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02423226 | Computed Tomography-guided Brachytherapy Plus Chemotherapy for Locally Recurrent Rectum Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00309478 | Randomized Study Comparing CMF and Goserelin + Tamoxifen in Premenopausal Receptor-positive Patients | breast cancer | Fluorouracil (NPC75844) | |
| NCT02566993 | Clinical Trial of Lurbinectedin (PM01183)/Doxorubicin Versus CAV or Topotecan as Treatment in Patients With Small-Cell Lung Cancer | small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT00417976 | Gemcitabine, Infusional 5 Fluorouracil and Bevacizumab in Patients With Advanced Pancreas Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT04027764 | Toripalimab Combined With S1 and Albumin Paclitaxel in Patients With Advanced Biliary Tract Cancer | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT00816816 | Phase Ⅱ Study of Neoadjuvant Chemotherapy Followed by Concurrent Chemoradiation for Stage ⅣAB Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00006115 | Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00112489 | Paclitaxel and Carboplatin in Treating Patients With Persistent or Recurrent Stage III or Stage IV Uterine Cancer | sarcoma | Paclitaxel (NPC208553) | |
| NCT04982237 | A Study of AK104 Plus Platinum-containing Chemotherapy±Bevacizumab as First-line Treatment for Persistent, Recurrent, or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00520000 | Carboplatin and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01754623 | GTX-RT in Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00755261 | Phase II Study of Doxorubicin and Avastin® in Sarcoma. | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02676349 | Neoadjuvant mFolfirinox With or Without Preoperative Concomitant Chemoradiotherapy in Patients With Borderline Resectable Pancreatic Carcinoma (PANDAS-PRODIGE 44) | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT04006041 | Combination of Toripalimab and Neoadjuvant Chemoradiotherapy in Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00976768 | Biweekly FOLFIRI in Advanced Gastric Cancer (AGC) With Failure of Prior Taxane, Fluoropyrimidine, and Cisplatin | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02723955 | Dose Escalation and Expansion Study of GSK3359609 in Participants With Selected Advanced Solid Tumors (INDUCE-1) | neoplasm | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00370552 | A Trial of 2 Schedules of Ixabepilone Plus Bevacizumab and Paclitaxel Plus Bevacizumab for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00112697 | Radiation Therapy and Docetaxel With Either Fluorouracil or Cisplatin as First-Line Therapy in Treating Patients With Metastatic Pancreatic Cancer That Cannot Be Removed By Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT05446467 | Pembrolizumab in Combination With Low-dose PFas Neoadjuvant Treatment for Locally Advanced HNSCC | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT03800693 | 2 Versus 6 Hour Oxaliplatin Infusions in Patients With Gastrointestinal Cancers | digestive system cancer | Fluorouracil (NPC75844) | |
| NCT01200342 | Genasense, Carboplatin, Paclitaxel (GCP) Combination in Uveal Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT03520790 | Paricalcitol Plus Gemcitabine and Nab-paclitaxel in Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05358704 | Preoperative ChemoRadiation And FOLFOXIRI for Rectal Cancer (CRAFTER) for Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00331630 | Abraxane and Lapatinib in Treating Patients With Stage I, Stage II, or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03197935 | A Study to Investigate Atezolizumab and Chemotherapy Compared With Placebo and Chemotherapy in the Neoadjuvant Setting in Participants With Early Stage Triple Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT00003896 | S9912 Combination Chemo in Stage III Ovarian Cancer, | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT02383966 | Phase III Trial to Assess Efficacy and Safety of Cetuximab for the Treatment of Chinese Participants With Head and Neck Cancer | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT04420884 | A Study of TAK-676 as Single Agent and TAK-676 in Combination With Pembrolizumab in Adults With Advanced or Metastatic Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT00108745 | Paclitaxel, Polyglutamate Paclitaxel, or Observation in Treating Patients With Stage III or Stage IV Ovarian Epithelial, Peritoneal Cancer, or Fallopian Tube Cancer | endometrioid carcinoma;undifferentiated carcinoma | Paclitaxel (NPC208553) | |
| NCT04745949 | PACIFIC: Primary Mediastinal Large B-cell Lymphoma Treated With Antibody Therapy, Checkpoint Inhibitor in Frontline With ImmunoChemotherapy | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT00004003 | Combination Chemotherapy in Treating Patients With Stage III or Stage IV Pancreatic Cancer That Cannot Be Removed by Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02734771 | A Study of Brentuximab Vedotin, Rituximab, and Dose Attenuated CHP in Elderly Patients With Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02124148 | A Study of Prexasertib (LY2606368) With Chemotherapy or Targeted Agents in Participants With Advanced Cancer | colorectal neoplasm;metastasis | Fluorouracil (NPC75844) | |
| NCT03544567 | A Study of Oraxol in Subjects With Cutaneous Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT02125344 | A Phase III Trial Comparing Two Dose-dense, Dose-intensified Approaches (ETC and PM(Cb)) for Neoadjuvant Treatment of Patients With High-risk Early Breast Cancer (GeparOcto) | inflammatory breast carcinoma | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT03605706 | A Trial of SHR-1210 (an Anti-PD-1 Inhibitor) in Combination With FOLFOX4 in Subjects With Advanced HCC Who Have Never Received Prior Systemic Treatment. | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00237900 | Gefitinib in Combination With Chemoradiation in Resectable Gastric Cancer | gastric carcinoma | Fluorouracil (NPC75844) | |
| NCT02467907 | Safety and Efficacy of Bevacizumab in Combination With Carboplatin and Paclitaxel for Metastatic, Recurrent or Persistent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01684878 | ICON8: Weekly Chemotherapy in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00610714 | AZD0530 Phase II Study in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01325441 | A Study of BBI608 Administered With Paclitaxel in Adult Patients With Advanced Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00002784 | High-Dose Combination Chemotherapy Plus Peripheral Stem Cell Transplantation Compared With Standard Combination Chemotherapy in Treating Women With High-Risk Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02383251 | Study of Metformin With Carboplatin/Paclitaxel Chemotherapy in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00023751 | Surgery With or Without Chemotherapy and Radiation Therapy in TreatingPatients With Stage I Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01246193 | CKD-828(80/2.5mg) Pharmacokinetic Study | hypertension | Paclitaxel (NPC208553) | |
| NCT00203372 | Neoadjuvant TAC Plus or Minus Bevacizumab(AVF3299) | breast cancer | Doxorubicin (NPC261012) | |
| NCT04077255 | EGFR-targeted Therapy for Gastric Cancer | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02141295 | A Study Comparing the Efficacy and Safety of Vanucizumab and FOLFOX With Bevacizumab and FOLFOX in Participants With Untreated Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01650701 | A Phase 3 Open Label Randomized Study to Compare the Efficacy and Safety of Rituximab Plus Lenalidomide (CC-5013) Versus Rituximab Plus Chemotherapy Followed by Rituximab in Subjects With Previously Untreated Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02193633 | Phase I Dose Escalation of Oral BAY1161909 in Combination With Intravenous Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT04129996 | A Trial of Camrelizumab in Combination With Nab-paclitaxel and Famitinib as a First Line Treatment in Patients With Unresectable Locally Advanced or Metastatic Immunomodulatory Triple Negative Breast Cancer(FUTURE-C-PLUS) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT04731038 | Combination Therapy for First Line Treatment of Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00832819 | E7080 in Combination With Carboplatin and Paclitaxel in Patients With Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02590367 | Estimate the Efficacy of HD6610 Granule for Oxaliplatin-induced Peripheral Neuropathy | peripheral neuropathy | Fluorouracil (NPC75844) | |
| NCT01934634 | Phase I Trial of LCL161 and Gemcitabine Plus Nab-Paclitaxel in Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01083537 | Study to Compare the Efficacy and Safety of Olaparib When Given in Combination With Carboplatin and Paclitaxel, Compared With Carboplatin and Paclitaxel in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00471484 | Combination Chemotherapy and Interferon Alfa-2b in Treating Patients With Nonmetastatic Liver Cancer That Cannot Be Removed by Surgery | liver cancer | Fluorouracil (NPC75844) | |
| NCT04826679 | Neoadjuvant Camrelizumab in Combination With Cisplatin and Nab-paclitaxel in Resectable HNSCC | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01216644 | 5-FU, Leucovorin, Oxaliplatin and Docetaxel (FLOT) Versus Epirubicin, Cisplatin and 5-FU (ECF) in Patients With Locally Advanced, Resectable Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00003784 | S9911, Combination Chemotherapy Plus Monoclonal Antibody Therapy in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01376310 | GSK1120212 Rollover Study | cancer | Paclitaxel (NPC208553) | |
| NCT01775501 | Sorafenib + mFOLFOX for Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT04804696 | Toripalimab With Paclitaxel and Cisplatin as Neoadjuvant Treatment for Esophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05389423 | Pomalidomide and Dose-Adjusted EPOCH +/- Rituximab for HIV-Associated Lymphomas | Burkitts lymphoma;diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01992341 | AMG 386 Drug-Drug Interaction Study With Paclitaxel | neoplasm | Paclitaxel (NPC208553) | |
| NCT01465451 | Intra-operative Chemotherapy With 5-FU for Colorectal Cancer Patients Receiving Curative Resection: Efficacy and Safety | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02488564 | A Study of Liposomal Doxorubicin + Docetaxel + Trastuzumab + Metformin in Operable and Locally Advanced HER2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00479674 | Phase II Study With Abraxane, Bevacizumab and Carboplatin in Triple Negative Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01056679 | Adriamycin, Vinblastine, DTIC and Revlimid in Elderly Hodgkin Lymphoma Patients | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03468231 | HAIC of FOLFOX vs. HAIC of OXA for Advanced HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT01391572 | A Trial Estimating The Optimal Radiation Volume Of Postsurgical Radiation For Patients With Esophageal Cancer | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00276796 | Paclitaxel, Topotecan, and Cisplatin in Treating Patients With Advanced, Persistent, or Recurrent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01100359 | Liposome-Encapsulated Doxorubicin Citrate and Carboplatin in Treating Patients With Advanced or Metastatic Recurrent Endometrial Cancer | endometrial cancer | Doxorubicin (NPC261012) | |
| NCT00448591 | A Study of Avastin (Bevacizumab) Plus Taxane-Based Therapy in Patients With Locally Recurrent or Metastatic Breast Cancer. | breast cancer | Paclitaxel (NPC208553) | |
| NCT00600483 | Safety and Efficacy of an Antibiotic Implant in Cardiac Surgical Subjects at Higher Risk for Sternal Wound Infection | infection | Gentamicin (NPC471628) | |
| NCT00265811 | Combination Chemotherapy With or Without Cetuximab in Treating Patients With Stage III Colon Cancer That Was Completely Removed By Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00343083 | Evaluation of Cetuximab (ERBITUX) and Concurrent Carboplatin, Paclitaxel & Radiotherapy in the Management of Patients With Advanced Locoregional Squamous Cell Carcinomas of the Head and Neck (GCC 0442) | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00055887 | Chemotherapy and Radiation Therapy With or Without Efaproxiral in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03201861 | Addition of Cisplatin to Adjuvant Chemotherapy for Early Stage Breast Cancer in High-Risk Women | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03564704 | Precision Diagnosis Directing HDACi Chidamide Target Therapy for Adult T-LBL/ALL | lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00003165 | Doxorubicin in Treating Women With Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00508625 | A Study of AMG 951 [rhApo2L/TRAIL] in Subjects With Previously Untreated Non-Small Cell Lung Cancer (NSCLC) Treated With Chemotherapy +/- Bevacizumab | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01821859 | Bevacizumab Beyond Progression in Platinum Sensitive Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00004979 | Topotecan and Paclitaxel in Treating Patients With Advanced Non-small Cell Lung Cancer or Other Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00556413 | Cetuximab and Combination Chemotherapy as First-Line Therapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03990103 | S1+ Paclitaxel (IV&IP) + Bevacizumab (IP) Versus S1+Oxaliplatin as First-line Treatment in Gastric Cancer With Malignant Ascites | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00215514 | Adjuvant Chemoradiation Therapy for Gastric or Gastroesophageal Junction Adenocarcinoma | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00004859 | Carboplatin, Paclitaxel, and Radiation Therapy With or Without Thalidomide in Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01381211 | Transarterial RAdioembolization Versus ChemoEmbolization for the Treatment of Hepatocellular Carcinoma (HCC) | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT03604965 | GP Induction Chemotherapy us TPF Adjuvant Chemotherapy Combined With DDP Concurrent Chemoradiotherapy in the Treatment of Locally Advanced NPC | nasopharyngeal neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00301028 | Cetuximab, Carboplatin, and Paclitaxel Followed by Radiation Therapy, With or Without Cisplatin, in Treating Patients With Metastatic Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00024427 | Triacetyluridine and Fluorouracil Compared With Gemcitabine in Treating Patients With Unresectable Locally Advanced, or Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00384176 | First Line Metastatic Colorectal Cancer Therapy in Combination With FOLFOX | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00087802 | Gemcitabine/Oxaliplatin (GEMOX) vs Carboplatin/Paclitaxel (CP) in Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01969578 | Androgen Deprivation Therapy in Advanced Salivary Gland Cancer | salivary gland cancer | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT03888677 | Dose-adjusted Adjuvant FEC Compared to Standard FEC for Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00016198 | Fluorouracil and Leucovorin With or Without Oxaliplatin in Treating Patients With Recurrent Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01402271 | A Study of MK-1775 in Combination With Paclitaxel and Carboplatin Versus Paclitaxel and Carboplatin Alone for Participants With Platinum-Sensitive Ovarian Tumors With the P53 Gene Mutation (MK-1775-004) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00536393 | Treatment of Disseminated High Grade Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003853 | 4'-Iodo-4'-Deoxydoxorubicin in Treating Patients With Primary Systemic Amyloidosis | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00005647 | SU5416 and Paclitaxel in Treating Patients With Recurrent, Locally Advanced or Metastatic Cancer of the Head and Neck | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00849472 | A Study to Evaluate Safety and Efficacy of Caelyx in Combination With Cyclophosphamide in the Treatment of Metastatic Breast Cancer (P02948) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT04211012 | A Study on Toripalimab Plus Nab-Paclitaxel With or Without Cisplatin as First-line Treatment of Urothelial Carcinoma | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT04203641 | L-DOS47 Plus Doxorubicin in Advanced Pancreatic Cancer | pancreatic carcinoma | Doxorubicin (NPC261012) | |
| NCT01612351 | Multimodality Risk Adapted Tx Including Induction Chemo for SCCHN Amenable to Transoral Surgery | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02477826 | An Investigational Immuno-therapy Trial of Nivolumab, or Nivolumab Plus Ipilimumab, or Nivolumab Plus Platinum-doublet Chemotherapy, Compared to Platinum Doublet Chemotherapy in Patients With Stage IV Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02654119 | Cyclophosphamide, Paclitaxel, and Trastuzumab in Treating Patients With Stage I-II HER2/Neu Positive Breast Cancer After Surgery | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03553238 | Precision Diagnosis Directing HDACi Chidamide Target Therapy for Adult ETP-ALL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00003313 | Amifostine in Treating Patients With Stage II or Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02725268 | A Study of Sapanisertib, Combination of Sapanisertib With MLN1117, Paclitaxel and Combination of Sapanisertib With Paclitaxel in Women With Endometrial Cancer | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT00949325 | Safety and Efficacy Study of Torisel and Liposomal Doxorubicin for Patients With Recurrent Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT03636308 | Nab-paclitaxel Plus S-1(AS) Versus Nab-paclitaxel Plus Gemcitabine(AG) in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00508664 | Docetaxel, Cisplatin (TP) + Radiation +/- Cetuximab in Larynx Carcinoma (CA) | hypopharyngeal carcinoma;laryngeal carcinoma | Fluorouracil (NPC75844) | |
| NCT05024773 | Study of ONCOFID-P-B (PACLITAXEL-HYALURONIC ACID) | urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT02942563 | Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT02597036 | A Study of LY3127804 With Ramucirumab in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01494363 | Phase II Study of FOLFOXIRI in Patients With Locally Advanced or Metastatic Biliary Tract Cancer | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT00123929 | Genetic Expression and Prediction of Response to Neoadjuvant Docetaxel or Doxorubicin in Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00776724 | Tailored Neoadjuvant Chemotherapy for Stage II/III Breast Cancer With Tumor Size More Than 2 cm | breast cancer | Fluorouracil (NPC75844) | |
| NCT00002893 | Palliative Chemotherapy in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02605915 | Safety and Pharmacokinetics of Atezolizumab Combination Treatments in Participants With HER2-Positive and HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01654146 | Dose-finding Study in Platinum-Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00467142 | Bevacizumab and Combination Chemotherapy as First-Line Therapy in Treating Patients With Metastatic Colorectal Cancer That Cannot Be Removed by Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00003160 | Weekly Infusions of Paclitaxel in Treating Women With Stage III or Stage IV Ovarian Cancer Refractory to Paclitaxel and Platinum | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04757363 | A Study of Nivolumab Combined With FOLFOX and Regorafenib in People Who Have HER2-Negative Esophagogastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00958737 | Combination Chemotherapy for 3 Months or 6 Months in Treating Patients With Stage III Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01980472 | Chemotherapy Plus Bevacizumab in Elderly Non-small Cell Lung Cancer Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01383343 | Sorafenib Tosylate, Bevacizumab, Irinotecan Hydrochloride, Leucovorin Calcium, and Fluorouracil in Treating Patients With Metastatic Colorectal Cancer | colorectal adenocarcinoma;malignant colon neoplasm;rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04850235 | Nab-paclitaxel Based TPX Neoadjuvant Chemotherapy for NPC Patients: a Dose-escalation Study | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00336960 | Celecoxib, Fluorouracil, and Radiation Therapy in Treating Patients With Stage II or Stage III Rectal Cancer That Can Be Removed By Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00886028 | Palliative Treatment With Liposomal Doxorubicin Plus Cisplatin for Patients With Malignant Pleural Mesothelioma | malignant pleural mesothelioma | Doxorubicin (NPC261012) | |
| NCT01649856 | A Study of Subcutaneous Versus Intravenous MabThera/Rituxan (Rituximab) in Combination With CHOP Chemotherapy in Patients With Previously Untreated CD20-Positive Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02723994 | A Phase 2 Study of Ruxolitinib With Chemotherapy in Children With Acute Lymphoblastic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT00002717 | Paclitaxel and Cisplatin in Treating Patients With Stage III or Stage IV Ovarian Cancer or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00005838 | Combination Chemotherapy Plus Radiation Therapy With or Without AE-941 in Treating Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Removed By Surgery | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05383196 | Onvansertib + Paclitaxel In TNBC | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00003881 | Trastuzumab Plus Chemotherapy in Treating Patients With Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04428333 | Study of GSK3359609 With Pembrolizumab and 5-fluorouracil (5-FU)-Platinum Chemotherapy in Participants With Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma | head and neck neoplasia | Fluorouracil (NPC75844) | |
| NCT04428151 | Lenvatinib (E7080/MK-7902) in Combination With Pembrolizumab (MK-3475) vs. Standard Chemotherapy and Lenvatinib Monotherapy in Participants With Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma That Progressed After Platinum Therapy and Immunotherapy (MK-7902-009/E7080-G000-228/LEAP-009) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04165772 | Study of Induction PD-1 Blockade in Subjects With Locally Advanced Mismatch Repair Deficient Solid Tumors | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01765582 | Sequential and Concurrent FOLFOXIRI/Bevacizumab Regimens Versus FOLFOX/Bevacizumab in First-Line Metastatic Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00095914 | Paclitaxel and ABI-007 in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00864253 | A Trial of ABI-007 Versus Dacarbazine in Previously Untreated Patients With Metastatic Malignant Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT04337879 | A Study of AL2846 Capsule Combined With Standard Chemotherapy Regimen in Subjects With Advanced Colorectal Cancer | colorectal cancer | Fluorouracil (NPC75844) | |
| NCT00110084 | ABI-007 (Nab-Paclitaxel) and Gemcitabine in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00016653 | Creatine and Glutamine in Steroid-Naive Duchenne Muscular Dystrophy | Duchenne muscular dystrophy | Glutamine (NPC197087) | |
| NCT00312208 | Docetaxel in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00446030 | Neoadjuvant Study With Chemotherapy, Lapatinib And Trastuzumab In Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00527735 | Phase II Study for Previously Untreated Subjects With Non Small Cell Lung Cancer (NSCLC) or Small Cell Lung Cancer (SCLC) | small cell lung carcinoma;non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00097227 | Trial of Carboplatin/Paclitaxel/Cetuximab in Stage IIIB/IV Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00483782 | Carboplatin and Paclitaxel With or Without Bevacizumab in Treating Patients With Newly Diagnosed Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cavity Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00725231 | Immunotherapy in Peripheral T Cell Lymphoma - the Role of Alemtuzumab in Addition to Dose Dense CHOP | angioimmunoblastic T-cell lymphoma;extranodal nasal NK/T cell lymphoma;unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00401635 | END-1: First Line Chemotherapy for Advanced or Recurrent Endometrial Carcinoma With Carboplatin and Liposomal Doxorubicin | endometrial cancer | Doxorubicin (NPC261012) | |
| NCT02735239 | Study of Anti-PD-L1 in Combination With Chemo(Radio)Therapy for Oesophageal Cancer | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00084214 | STA-4783/Paclitaxel or Paclitaxel Alone in Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00022191 | Cisplatin Plus Gemcitabine With or Without Paclitaxel in Treating Patients With Stage IV Urinary Tract Cancer | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01249443 | Paclitaxel and Carboplatin in Treating Patients With Metastatic or Recurrent Solid Tumors and HIV Infection | anus cancer;hypopharyngeal carcinoma;verrucous carcinoma;laryngeal squamous cell carcinoma;malignant epithelial tumor of ovary;oropharyngeal carcinoma;esophageal cancer;gastric cancer;HIV infection;non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00591851 | Phase II Study of Dose-Dense Doxurubicin and Cyclophosphamide (AC) Followed By Paclitaxel With Trastuzumab in HER2/ NEU-Amplified Breast Cancer: Feasibility | breast cancer | Paclitaxel (NPC208553) | |
| NCT00756470 | Phase II Neoadjuvant in Inflammatory Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03423979 | Optilume™ BPH Prostatic Drug Coated Balloon Dilation Catheter | benign prostatic hyperplasia | Paclitaxel (NPC208553) | |
| NCT01990352 | Correlate BRCA1 Protein Expression With Response to DNA Damaging Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT00613080 | Combination Chemotherapy and Intensity-Modulated Radiation Therapy in Treating Patients Undergoing Surgery for Locally Advanced Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04063683 | Paclitaxel and DDP Combined With Anlotinib in the First-line Treatment for Patients With Advanced Esophageal Squamous Cell Carcinoma(ESCC). | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01364012 | A Study of Bevacizumab Versus Placebo in Combination With Carboplatin/Paclitaxel in Participants With Advanced or Recurrent Non-Squamous Non-Small Cell Lung Cancer Who Have Not Received Previous Chemotherapy | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01205022 | Radiolabeled Monoclonal Antibody Therapy, Combination Chemotherapy, and Bevacizumab in Treating Patients With Metastatic Colorectal Cancer | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT00201708 | Dose-Dense Docetaxel Before or After Doxorubicin/Cyclophosphamide in Axillary Node-Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03742102 | 9-ING-41 in Patients With Advanced Cancers | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00980460 | Risk-Based Therapy in Treating Younger Patients With Newly Diagnosed Liver Cancer | Hepatoblastoma | Fluorouracil (NPC75844) | |
| NCT01354717 | Bioequivalence Study of Generic Fluorouracil 0.5% Cream and 0.5% Carac® and Placebo | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT00559845 | A Study of Avastin (Bevacizumab) in Patients With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00532857 | Phase II Study of Primary Chemotherapy With Paclitaxel, Gemcitabine, and Trastuzumab | breast cancer | Paclitaxel (NPC208553) | |
| NCT01402271 | Study of AMG 386 in Combination With Paclitaxel and Carboplatin in Subjects With Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00696488 | Measuring Adherence To Topical 5-Fluorouracil in a Clinic Population | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT00581971 | Radiosensitization With Celecoxib and Chemoradiation for Head and Neck Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT01225523 | Perioperative Vs. Preoperative Chemotherapy With Surgery in the Squamous Carcinoma of Esophagus | esophageal squamous cell carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03322566 | A Study of Pembrolizumab Plus Epacadostat With Platinum-based Chemotherapy Versus Pembrolizumab Plus Platinum-based Chemotherapy Plus Placebo in Metastatic Non-Small Cell Lung Cancer (KEYNOTE-715-06/ECHO-306-06) | lung cancer | Paclitaxel (NPC208553) | |
| NCT02327169 | A Study of RO6927005 Either As Monotherapy (Part A) or in Combination With Gemcitabine and Nab-Paclitaxel (Part B) to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Clinical Activity in Patients With Mesothelin-positive Metastatic and/or Locally Advanced Malignant Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT02827201 | FIrst Line Treatment of Metastatic Pancreatic Cancer: Sequential Nab-paclitaxel + Gemcitabine/FOLFIRI.3 VS Nab-paclitaxel + Gemcitabine | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT01183663 | Lenalidomide in Combination With Bevacizumab, Sorafenib, Temsirolimus, or 5-Fluorouracil, Leucovorin, Oxaliplatin (FOLFOX) | cancer | Fluorouracil (NPC75844) | |
| NCT00065117 | Safety, Tolerability and Efficacy of ZD6126 in Combination With Oxaliplatin, 5-Fluorouracil and Leucovorin in Subjects With Metastatic Colorectal Cancer. | colorectal neoplasm;metastasis | Fluorouracil (NPC75844) | |
| NCT00549848 | Total Therapy Study XVI for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01440088 | A Trial of TH-302 in Combination With Doxorubicin Versus Doxorubicin Alone to Treat Patients With Locally Advanced Unresectable or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01146795 | Trial of Best Supportive Care and Either Cisplatin or Paclitaxel to Treat Patients With Primary Ovarian Cancer, Primary Peritoneal Cancer or Fallopian Tube Cancer and Inoperable Malignant Bowel Obstruction | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02317419 | Nab-Paclitaxel as Salvage Treatment in Locally Advanced or Metastatic Gastric Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT00003394 | Combination Chemotherapy in Treating Patients With Advanced Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT01778803 | A Study of Bevacizumab (Avastin) in Neoadjuvant Therapy in Participants With International Federation of Gynecology and Obstetrics (FIGO) Stage IIIC/IV Ovarian, Tubal, or Peritoneal Cancer, Initially Unresectable | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00522834 | Elesclomol (STA-4783) With Paclitaxel Versus Paclitaxel Alone in Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00544232 | Preoperative Epirubicin Paclitaxel Aranesp Study (PREPARE) | breast cancer | Paclitaxel (NPC208553) | |
| NCT00052468 | Carboplatin/Paclitaxel +/-Gemcitabine in Treating Patients With Ovarian Epithelial or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05350943 | HAIC Combined With Toripalimab and Donafenib for Advanced BTC | biliary tract neoplasm | Fluorouracil (NPC75844) | |
| NCT02494583 | Study of Pembrolizumab (MK-3475) as First-Line Monotherapy and Combination Therapy for Treatment of Advanced Gastric or Gastroesophageal Junction Adenocarcinoma (MK-3475-062/KEYNOTE-062) | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03169790 | QUILT-3.052: NANT Non-Hodgkin Lymphoma (NHL) Vaccine: Combination Immunotherapy in Subjects With Relapsed CD20-positive NHL | non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT02092363 | GANNET53: Ganetespib in Metastatic, p53-mutant, Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00512980 | PVAG-14 Pilot for Intermediate Stages Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04143711 | Study of DF1001 in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01994031 | Dose Escalation and Pharmacokinetic Study of Paclitaxel Liposome Injection in Treating Patients With Advanced Solid Tumor After Failure From Conventional Treatments | neoplasm | Paclitaxel (NPC208553) | |
| NCT00343291 | A Study of Cetuximab and Bevacizumab in Combination With Paclitaxel and Carboplatin in Stage IIIb/IV NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01057342 | Paclitaxel, Carboplatin, and Dimethylxanthenone Acetic Acid in Treating Patients With Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00384878 | Study of Cetuximab Plus P-HDFL for the First-Line Treatment of Advanced Gastric Cancer | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT04213937 | Nab-paclitaxel Versus Topotecan As Second-Line Treatment for Patients With Extensive Stage Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05227664 | A Study of AK117/AK112 in Metastatic Triple-Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00002570 | Immediate Compared With Delayed Chemotherapy in Advanced Colorectal Cancer Without Signs or Symptoms of Disease | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00252798 | ZD1839 (Iressa™) and Concurrent Chemo-Radiation in Patients With Locally Advanced Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003898 | Glutamine in Treating Side Effects in Children Who Are Undergoing Stem Cell Transplantation | neoplasm | Glutamine (NPC197087) | |
| NCT04241185 | Efficacy and Safety of Pembrolizumab (MK-3475) in Combination With Chemoradiotherapy (CRT) Versus CRT Alone in Muscle-invasive Bladder Cancer (MIBC) (MK-3475-992/KEYNOTE-992) | bladder tumor | Fluorouracil (NPC75844) | |
| NCT02366949 | A Study MLN2480 in Combination With MLN0128 or Alisertib, or Paclitaxel, or Cetuximab, or Irinotecan in Adult Participants With Advanced Nonhematologic Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00006468 | Comparing Two Combination Chemotherapy Regimens in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00574080 | UARK 2006-15: A Study of Tandem Transplants With or Without Bortezomib and Thalidomide | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00112294 | Study of Taxane/Carboplatin +/- Cetuximab as First-Line Treatment for Patients With Advanced/Metastatic Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01489566 | Study of Tyroserleutide for Injection in Hepatocellular Carcinoma (HCC) Patients | carcinoma | Fluorouracil (NPC75844) | |
| NCT00533936 | Trastuzumab or Observation After Combination Chemotherapy and Trastuzumab in Treating Patients Undergoing Surgery for Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02479971 | Effect of Peritoneal Lavage With Clindamycin-gentamicin on Laparoscopic Sleeve Gastrectomy | pain | Gentamicin (NPC471628) | |
| NCT00176254 | Paclitaxel, Carboplatin and Radiotherapy as Induction Therapy in Locally Advanced Head and Neck Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02471820 | Lenalidomide & Adriamycin & Dexamethasone (RAD) in Newly Diagnosed, Multiple Myeloma Patients | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01451515 | NHL16: Study For Newly Diagnosed Patients With Acute Lymphoblastic Lymphoma | lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT04996160 | Palbociclib in Combination With Chemotherapy in Pediatric Patients With Relapsed or Refractory Acute Lymphoblastic Leukemia (RELPALL2) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT02115152 | Neoadjuvant Trial of Capecitabine for Axillary Lymph Node Positive Operable Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT03484299 | Chemotherapy and Irreversible Electroporation in the Treatment of Advanced Pancreatic Adenocarcinoma | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01804790 | Efficacy of Neoadjuvant Folfirinox Regimen in Patients With Resectable Locally Advanced Rectal Cancer | rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04213898 | SHR-1210 Combined With Albumin-bound Paclitaxel and Epirubicin Neoadjuvant for Triple Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00453167 | Weekly Paclitaxel Plus Gemcitabine as Second-line in Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02574663 | TGR-1202 Alone and in Combination With Either Nab-paclitaxel + Gemcitabine or With FOLFOX in Patients With Select Relapsed or Refractory Solid Tumors | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00005819 | Combination Chemotherapy in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03331640 | A Health Service Research Study to Investigate Survival of Metastatic Pancreatic Cancer Patients After Sequential Chemotherapy | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03785873 | Phase Ib/II Trial of Nal-Irinotecan and Nivolumab as Second-Line Treatment in Patients With Advanced Biliary Tract Cancer | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT00002783 | Combination Chemotherapy Before and After Surgery in Treating Patients With Stomach Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02622074 | Effect of Trastuzumab on Disease Free Survival in Early Stage HER2-Negative Breast Cancer Patients With ERBB2 Expressing Disseminated Tumor Cells | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00356681 | A Study of AMG 706 or Bevacizumab, in Combination With Paclitaxel Chemotherapy, as Treatment for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04949256 | Efficacy and Safety of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) Plus Chemotherapy in Participants With Metastatic Esophageal Carcinoma (MK-7902-014/E7080-G000-320/LEAP-014) | esophageal squamous cell carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03201471 | Chidamide With R-CHOP Regimen for DLBCL Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00006245 | Flavopiridol and Paclitaxel in Treating Patients With Locally Advanced or Metastatic Esophageal Cancer That Has Not Responded to Previous Paclitaxel | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02677597 | Cisplatin Combined With S-1 or Paclitaxel as First-line Treatment for Metastatic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00138151 | Isotretinoin, Interferon Alpha-2b, and Paclitaxel in Stage IV, Recurrent, or Persistent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01193452 | S-1/Leucovorin (SL) Versus sLV5FU2 as the First-line Treatment for Elderly Patients With Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00024310 | Paclitaxel, Folic Acid, and Lometrexol in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00953394 | 5FU and Octreotide Long-acting Release (LAR) for Neuroendocrine Tumors | neuroendocrine neoplasm | Fluorouracil (NPC75844) | |
| NCT00976677 | Carboplatin, Paclitaxel, and Bevacizumab With or Without Erlotinib Hydrochloride in Treating Non-Smokers With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01196741 | Neoadjuvant Therapy for Ovarian Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00275119 | Gemcitabine and Oxaliplatin Followed By Radiation Therapy, Fluorouracil, and Oxaliplatin in Treating Patients With Locally Advanced Pancreatic Cancer That Cannot Be Removed By Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03830606 | The Efficacy and Safety of Nab-paclitaxel Plus S-1 in First-line Treatment of Advanced Biliary Tract Adenocarcinoma | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT02053597 | TRIal evalUating the Menstrual and Ovarian Function of Young Breast Cancer Patients Treated With a cycloPHosphamide-free Regimen | breast cancer | Doxorubicin (NPC261012) | |
| NCT00878800 | A Phase I/II Clinical Trial of PXD101 in Combination With Doxorubicin in Patients With Soft Tissue Sarcomas | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02938442 | Vaccination of Triple Negative Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT03443492 | SLOG vs mFOLFIRINOX as the First-line Treatment in Locally Advanced Uncresectable or Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00034541 | Study of Cetuximab in Combination With Carboplatin-Paclitaxel in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03169790 | QUILT-3.052: NANT Non-Hodgkin Lymphoma (NHL) Vaccine: Combination Immunotherapy in Subjects With Relapsed CD20-positive NHL | non-Hodgkins lymphoma | Fluorouracil (NPC75844) | |
| NCT04228601 | A Study of Fluzoparib in Combination With mFOLFIRINOX in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00460265 | Study of Panitumumab Efficacy in Patients With Recurrent and/or Metastatic Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT02442362 | TOF Versus SOX in Metastatic Gastric Cancer | gastric cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT01270373 | NeoSAMBA: Neoadjuvant: Does the Sequence of Anthracycline and Taxane Matters: Before or After? | breast cancer | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT01649336 | TRINOVA-3: A Study of AMG 386 or AMG 386 Placebo in Combination With Paclitaxel and Carboplatin to Treat Ovarian Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00003956 | Combination Chemotherapy in Treating Patients With Advanced Cancer | lymphoma | Fluorouracil (NPC75844) | |
| NCT02016417 | Induction Chemotherapy With GP Versus TPF in the Treatment of Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT01855828 | Phase 2 Trial of Pertuzumab and Trastuzumab With Weekly Paclitaxel and Chemotherapy for HER2 Positive Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT05427383 | KN026 in Combination With Chemotherapy in the Second Line Treatment of HER-2 Positive Advanced or Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00478946 | A Study of Picoplatin in Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01712919 | Chemoradiation Plus Weekly c225 Against Locoregionally Advanced Nasopharyngeal Carcinoma (NPC) | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT01167725 | Standard Therapy With or Without Surgery and Mitomycin C in Treating Patients With Advanced Limited Peritoneal Dissemination of Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01659099 | GA In NEwly Diagnosed Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05268510 | Chemotherapy and Pembrolizumab, Followed by Pembrolizumab and Olaparib as Firstline Therapy in Her-2 Negative Gastric/GEJ Adenocarcinoma | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00025142 | Gefitinib and Combination Chemotherapy in Treating Patients With Advanced Solid Tumors or Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03007147 | Imatinib Mesylate and Combination Chemotherapy in Treating Patients With Newly Diagnosed Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia | T-cell acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01718873 | Optimization of Bevacizumab Scheduling With Chemotherapy for Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00736944 | Trial of 2 Cycles of Induction Chemo With Abraxane, Cetuximab, Cisplatin, & 5-FU for Advanced Head and Neck Cancer | head and neck squamous cell carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT05406401 | A Study of Zilovertamab Vedotin (MK-2140) in Combination With Cyclophosphamide, Doxorubicin, and Prednisone Plus Rituximab or Rituximab Biosimilar (Truxima) (R-CHP) in Participants With Diffuse Large B-Cell Lymphoma (DLBCL) (MK-2140-007) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02059967 | Phase I IGART Study Using Active Breathing Control and Simultaneous Boost for Patients With NSCLC | lung adenocarcinoma;squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01938846 | BI 860585 Dose Escalation Single Agent and in Combination With Exemestane or With Paclitaxel in Patients With Various Advanced and/or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00797472 | Study Comparing R-mabHD and a Combination of ABVD in Hodgkin's Disease | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01446458 | Phase I Study of Stereotactic Body Radiation Therapy and FOLFIRINOX in the Neoadjuvant Therapy of Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01622439 | Valproate as First Line Therapy in Combination With Rituximab and CHOP in Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04835896 | Study of M7824 and Paclitaxel Combination as a Second-line Treatment in Patients With Recurrent/Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01434069 | Phase I Trial of Combination of FOLFIRI and SOM 230 | digestive system neoplasm | Fluorouracil (NPC75844) | |
| NCT04196283 | Nab-paclitaxel in Combination With Gemcitabine for Pediatric Relapsed and Refractory Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT03758989 | A Study of PET Adapted Therapy and Non-invasive Monitoring for Previously Untreated Limited Stage Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02919787 | Nordic Pancreatic Cancer Trial (NorPACT) - 1 | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03641183 | Folfirinox or Gemcitabine-Nab Paclitaxel Followed by Stereotactic Body Radiotherapy for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00002524 | Combination Chemotherapy in Treating Patients With AIDS-Related Lymphoma | Lymphoma, AIDS-Related | Fluorouracil (NPC75844) | |
| NCT02431559 | Phase 1/2 Study of Motolimod, Doxorubicin, and Durvalumab in Recurrent, Platinum-Resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT04282070 | SHR-1701 in Patients With Recurrent/Metastatic Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00030654 | Hormone Therapy Plus Chemotherapy in Treating Patients With Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT05449366 | Intraperitoneal Paclitaxel for Patients With Primary Malignant Peritoneal Mesothelioma | malignant peritoneal mesothelioma | Paclitaxel (NPC208553) | |
| NCT03475615 | A Study of Intraperitoneal Paclitaxel in Combination With SOX Compared With SOX Alone in Gastric Cancer With Malignant Ascites | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03693612 | GSK3359609 Plus Tremelimumab for the Treatment of Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03278626 | Immune Checkpoint Therapy With Nivolumab Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04825938 | Neoadjuvant Toripalimab in Combination With Carboplatin and Nab-paclitaxel in Untreated Salivary Gland Malignant Neoplasms | salivary gland neoplasm | Paclitaxel (NPC208553) | |
| NCT03563144 | QUILT-3.088: NANT Pancreatic Cancer Vaccine | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00002852 | Surgery With or Without Chemotherapy in Treating Patients With Stage I Non-small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00734877 | UARK 2013-13, Total Therapy 4B - Formerly 2008-01 - A Phase III Trial for Low Risk Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00262990 | Patupilone Versus Doxorubicin in Patients With Ovarian, Primary Fallopian, or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneal neoplasm | Doxorubicin (NPC261012) | |
| NCT01154920 | Paclitaxel, Carboplatin and Cetuximab (PCC) With Cetuximab, Docetaxel, Cisplatin and Fluorouracil (C-TPF) in Previously Untreated Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT03554044 | T-VEC With Chemotherapy or Endocrine Therapy in Treating Participants With HER2- Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00198354 | Stage I/II NSCLC Perioperative Chemotherapy | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00002551 | SWOG-9304 Chemotherapy Plus Radiation Therapy in Treating Patients With Rectal Cancer That Has Been Surgically Removed | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01964391 | A Study of Participant Satisfaction and Safety With Subcutaneously Administered Trastuzumab (Herceptin) in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01126216 | Reduced Radiotherapy With Pac/Cis vs Standard Radiotherapy With 5-FU/Cis in Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT02296671 | Personalized Therapy for Esophagogastric Cancer Using Thymidylate Synthase Genetic Markers | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00553462 | Carboplatin and Paclitaxel Albumin-Stabilized Nanoparticle Formulation Followed by Radiation Therapy and Erlotinib in Treating Patients With Stage III Non-Small Cell Lung Cancer That Cannot Be Removed By Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT02121990 | Metformin Hydrochloride, Carboplatin, and Paclitaxel in Treating Patients With Recurrent Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01166542 | Efficacy Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin in Platinum-Refractory Head and Neck Cancers | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02071069 | Efficacy and Tolerance of Maintenance Therapy in Patients With Incurable Advanced Colorectal Cancer | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00003118 | Surgery With or Without Chemotherapy and Radiation Therapy in Treating Patients With Cancer of the Esophagus | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT02051751 | A Study to Evaluate the Potential Benefit of the Addition of BYL719 to Paclitaxel in the Treatment of Breast Cancer and Head-and-neck Cancer | upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT00806286 | Study of Carboplatin/Paclitaxel With or Without Investigational Drug (CS-7017) in Subjects With Metastatic Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02406092 | Safety Study of Rituximab (SC) Administered in Participants With CD20+ DLBCL or CD20+ Follicular NHL Grade 1 to 3A | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00005051 | Combination Chemotherapy in Treating Patients With Advanced Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00865189 | A Study of Bevacizumab (Avastin) in Participants With Newly Diagnosed Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01214668 | Dose-Escalation Study of LY573636-sodium and Liposomal Doxorubicin in Patients With Advanced Solid Tumors | neoplasm | Doxorubicin (NPC261012) | |
| NCT04535713 | GALLANT: Metronomic Gemcitabine, Doxorubicin, Docetaxel and Nivolumab for Advanced Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT05018520 | The Safety and Effectiveness of 4R-CHOP+4R vs 6R-CHOP+2R in Newly Diagnosed Patients With DLBCL in Low Risk | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00964626 | Combination Chemotherapy With CS-1008 to Treat Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03641976 | A Study of Bevacizumab, Infusional Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan (A-FOLFOXIRI) Compared With Bevacizumab, Infusional Fluorouracil, Leucovorin, and Irinotecan/Oxaliplatin (A-FOLFIRI/FOLFOX) as First-line Treatment for Metastatic Right-sided Colon Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00003701 | Combination Chemotherapy in Treating Patients With Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT03469479 | Neoadjuvant HAIC for Resectable Hepatocellular Carcinoma Beyond Milan Criteria | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00983424 | Cyclosporine A in Combination With Nab-Paclitaxel in Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00025480 | Tipifarnib Plus Radiation Therapy After Combination Chemotherapy in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05253846 | Short-course Radiotherapy Followed by Consolidation Chemotherapy. 2021-001206-29 | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00006108 | Capecitabine, Paclitaxel, and Trastuzumab in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01911598 | A Study of MEHD7945A in Combination With Cisplatin and 5-Fluorouracil (5-FU) or Paclitaxel and Carboplatin in Participants With Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M SCCHN) | head and neck malignant neoplasia | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00611858 | Cetuximab, 5-FU and Radiation as Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00525759 | Investigating the Biological Effects of the Addition of Zoledronic Acid to Pre-operative Chemotherapy in Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01063192 | A Study of Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00004067 | Doxorubicin and Cyclophosphamide Plus Paclitaxel With or Without Trastuzumab in Treating Women With Node-Positive Breast Cancer That Overexpresses HER2 | breast cancer | Doxorubicin (NPC261012) | |
| NCT00735345 | Chemotherapy Induction and Chemoradiotherapy in Patients With Esophageal Carcinoma | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00003896 | S9912 Combination Chemo in Stage III Ovarian Cancer, | fallopian tube cancer | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT00612586 | Study of Enzastaurin With 5-Fluorouracil/Leucovorin (5-FU/LV) Plus Bevacizumab as Maintenance Regimen Following First Line Therapy for Metastatic Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03005015 | Lenvatinib in Second Line Endometrial Carcinoma | endometrial neoplasm | Doxorubicin (NPC261012) | |
| NCT02506803 | Phase I Study of Neoadjuvant Chemotherapy for Patients With Borderline Resectable Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00002581 | Tamoxifen With or Without Combination Chemotherapy in Treating Postmenopausal Women With Operable Invasive Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT05428670 | The Efficacy and Safety of ZR2 Versus R-CHOP-like Regimen for Elderly Patients With Newly Diagnosed Diffuse Large B Cell Lymphoma. | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03647072 | PPI Versus Histamine Antagnists as Adjuvant to Chemotherapy | lymphoma | Doxorubicin (NPC261012) | |
| NCT03712202 | Brentuximab Vedotin and Nivolumab in Treating Patients With Early Stage Classic Hodgkin Lymphoma | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00045630 | S0219, Combination Chemotherapy Followed By Observation or Surgery in Patients With Stage II or Stage III Cancer of the Urothelium | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00003577 | Combination Chemotherapy With or Without Epirubicin in Treating Women With Stage I or Stage II Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01836029 | Chemotherapy Plus Cetuximab in Combination With VTX-2337 in Patients With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT03718624 | A Study of Intraperitoneal and Intravenous Paclitaxel Plus Apatinib and S-1 Conversion Therapy for Gastric Cancer With Positive Exfoliative Cancer Cells | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03101748 | Neratinib and Paclitaxel With or Without Pertuzumab and Trastuzumab Before Combination Chemotherapy in Treating Patients With Metastatic or Locally Advanced Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00484341 | Phase II Study of NGR-hTNF in Combination With Doxorubicin in Patients Affected by Soft Tissue Sarcomas. | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01429701 | Effectiveness of Polymyxin B Sulphate + Prednisolone + Benzocaine + Clioquinol in Acute and Sub-acute Dermatitis Eczematous | Eczema | Gentamicin (NPC471628) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | renal cell carcinoma | Doxorubicin (NPC261012) | |
| NCT00024141 | Irinotecan Followed by Fluorouracil in Treating Patients With Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT00868491 | Induction Chemotherapy Followed by Chemoradiation With Cetuximab in Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT02099240 | Patients Response to Early Switch To Oral:Osteomyelitis Study | osteomyelitis | Gentamicin (NPC471628) | |
| NCT01534585 | Safety and Efficacy Study of Icotinib With Intensity-modulated Radiotherapy in Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT01394354 | Vorinostat in Combination With Bortezomib, Doxorubicin and Dexamethasone (VBDD) in Patients With Refractory or Relapsed Multiple Myeloma (MM) | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00022945 | Safety and Efficacy Study of Iodine-131 Anti-B1 Antibody Plus CHOP For Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00441168 | Velcade (Bortezomib), Adriamycin Dexamethasone (PAD) or Vincristine Adriamycin Dexamethasone in Second Line Treatment of Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01650428 | Bevacizumab And Combination Chemotherapy in Rectal Cancer Until Surgery | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00005065 | Chemotherapy, Radiation Therapy, and Surgery in Treating Patients With Stage IIIA Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00016315 | Gemcitabine, Carboplatin or Paclitaxel Plus Radiation Therapy in Treating Patients With Stage IIIA or IIIB Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03991884 | Inotuzumab Ozogamicin and Chemotherapy in Treating Patients With Recurrent or Refractory B-cell Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT01633970 | A Study of Atezolizumab Administered in Combination With Bevacizumab and/or With Chemotherapy in Participants With Locally Advanced or Metastatic Solid Tumors | cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00236899 | Phase III Study of Two Different Schedules (Weekly and Tri-weekly) of Combination of Gemcitabine and Two Taxanes in MBC | breast cancer | Paclitaxel (NPC208553) | |
| NCT03579784 | Biomarker-oriented Study of Durvalumab (MEDI4736) in Combination With Olaparib and Paclitaxel in Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02311907 | Glutathione in Preventing Peripheral Neuropathy Caused by Paclitaxel and Carboplatin in Patients With Ovarian Cancer, Fallopian Tube Cancer, and/or Primary Peritoneal Cancer | neuropathy;pain | Paclitaxel (NPC208553) | |
| NCT05029973 | HAIC Combined With Sintilimab and Bevacizumab Biosimilar for Unresectable HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02845882 | LBL-2016 for Children or Adolescents in China | lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00002498 | Combination Chemotherapy Compared With Mitoxantrone in Treating Older Patients With Advanced Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02325986 | Concurrent Radiotherapy and Weekly Chemotherapy of PF for Postoperative Locoregional Recurrence of Esophageal Cancer | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT02632071 | ACY-1215 + Nab-paclitaxel in Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03727061 | Porfimer Sodium Interstitial Photodynamic Therapy With or Without Standard of Care Chemotherapy in Treating Patients With Locally Advanced or Recurrent Head and Neck Cancer | head and neck carcinoma | Fluorouracil (NPC75844) | |
| NCT03778593 | FOLFIRINOX for 2nd-line Treatment of BTC | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT00607438 | A Phase II Study Of Abraxane and Nexavar in the First-Line Treatment of Locally Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00907348 | Prospective Multicenter Dose Finding Phase II Pilot Trial to Evaluate Efficacy and Safety of LR-CHOP21 for Elderly Patients With Untreated Diffuse Large B Cell Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT01666444 | VTX-2337 and Pegylated Liposomal Doxorubicin (PLD) in Patients With Recurrent or Persistent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00451178 | A Study of Participants With Lymphoma Who Take R-CHOP and Enzastaurin Compared to Participants Who Take R-CHOP Only | lymphoma | Doxorubicin (NPC261012) | |
| NCT01236716 | Nab-Paclitaxel Treatment in Advanced Squamous Cell Carcinoma of Lung | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04684381 | Pharmacokinetics and Safety of Endari (L-glutamine) in Sickle Cell Disease Patients | sickle cell anemia | Glutamine (NPC197087) | |
| NCT03175666 | QUILT-3.049: NANT Triple Negative Breast Cancer (TNBC) Vaccine: Combination Immunotherapy in Subjects With TNBC Who Have Progressed on or After Anthracycline-based Chemotherapy | breast cancer | Fluorouracil (NPC75844) | |
| NCT05092373 | Tumor Treating Fields Therapy in Combination With Chemotherapy for the Treatment of Advanced Solid Tumors Involving the Abdomen or Thorax | renal cell carcinoma;hepatocellular carcinoma;endometrial carcinoma;Fallopian Tube Carcinoma;ovarian carcinoma;breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02243007 | Phase II Study of Preoperative FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel in Patients With Resectable Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00201734 | Capecitabine, Carboplatin and Weekly Paclitaxel for Patients With Solid Tumors and Adenocarcinoma of Unknown Primary | adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03755804 | Pediatric Classical Hodgkin Lymphoma Consortium Study: cHOD17 | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00098787 | Bevacizumab and Oxaliplatin Combined With Irinotecan or Leucovorin and Fluorouracil in Treating Patients With Metastatic or Recurrent Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03485118 | RHCACD20MA (HS006) Combined With CHOP (Hi-CHOP) in Patients With Previously Untreated Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05410847 | Nab-paclitaxel Plus Camrelizumab and S-1 Conversion Therapy for Gastric Cancer With Positive Exfoliative Cancer Cells | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04216472 | Nab-paclitaxel and Alpelisib for the Treatment of Anthracycline Refractory Triple Negative Breast Cancer With PIK3CA or PTEN Alterations | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT05234684 | A Study of Orelabrutinib Plus R-CHOP in Treatment-naïve Patients With MCD Subtype Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05303792 | Testing the Combination of Inotuzumab Ozogamicin and Lower Dose Chemotherapy Compared to Usual Chemotherapy for Adults With B-Cell Acute Lymphoblastic Leukemia or B-Cell Lymphoblastic Lymphoma | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT01491204 | Clinical Trial to Determine the Maximum Tolerated Dose and to Assess the Safety and Pharmacokinetic Profile of Oral Paclitaxel in Patients With Advanced Solid Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT02472353 | Use of Metformin to Reduce Cardiac Toxicity in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003294 | Chemotherapy Given With Amifostine and Filgrastim in Treating Patients With Recurrent or Metastatic Cancer | leukemia;lymphoma | Paclitaxel (NPC208553) | |
| NCT02054884 | F16IL2 Plus Paclitaxel in Metastatic Merkel Cell Carcinoma | Merkel cell skin cancer | Paclitaxel (NPC208553) | |
| NCT01414244 | Glutamine for the Treatment of Patients With Irritable Bowel Syndrome | irritable bowel syndrome | Glutamine (NPC197087) | |
| NCT00655850 | Lower Dose Chemotherapy Given More Frequent With Avastin to Treat Advanced Non-Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03167164 | QUILT-3.045: NANT Merkel Cell Carcinoma (MCC) Vaccine: Combination Immunotherapy in Subjects With MCC Who Have Progressed on or After PD-L1 Therapy | Merkel cell skin cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00006472 | Combination Chemotherapy Plus Radiation Therapy Followed By Surgery in Treating Patients With Stage I, Stage II, or Stage III Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02251951 | Study of Afuresertib Combined With Paclitaxel in Gastric Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT02515734 | A Study of FOLFOXIRI Plus Cetuximab vs. FOLFOXIRI Plus Bevacizumab | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03281369 | A Study of Multiple Immunotherapy-Based Treatment Combinations in Patients With Locally Advanced Unresectable or Metastatic Gastric or Gastroesophageal Junction Cancer (G/GEJ) or Esophageal Cancer (Morpheus-Gastric and Esophageal Cancer) | esophageal carcinoma;gastric adenocarcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT05179239 | A Study of SHR-1701 Plus Platinum-containing Chemotherapy With or Without BP102 (Bevacizumab) as First-line Treatment in Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01551394 | Efficacy, Pharmacokinetics and Safety of Meropenem in Infants Below 90 Days With Clinical or Confirmed Late-onset Sepsis | Sepsis | Gentamicin (NPC471628) | |
| NCT00705016 | Cilengitide in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck (SCCHN) | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT01396655 | PET in Breast Cancer Receiving Neoadjuvant Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT01560104 | A Clinical Study Conducted in Multiple Centers Comparing Veliparib in Combination With Carboplatin and Paclitaxel Versus a Placebo in Combination With Carboplatin and Paclitaxel in Patients With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04997837 | Study of Adjuvant Chemotherapy With or Without PD-1 Inhibitors and Chemoradiotherapy in Resected pN3 Gastric (G) or GEJ Adenocarcinoma | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02057380 | A Rollover Study for Subjects That Have Participated in an Astellas Sponsored Linsitinib Trial | neoplasm | Paclitaxel (NPC208553) | |
| NCT05247619 | A Clinical Study to Explore the Efficacy and Safety of Tislelizumab in Combination With Bevacizumab and Chemotherapy in Patients With Persistent, Recurrent, or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00478361 | Gemcitabine, Paclitaxel, Doxorubicin in Metastatic or Unresectable Bladder Cancer With Decreased Kidney Function | urethra cancer;urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT01485744 | LDE225 With Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan for Untreated Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01696032 | Pertuzumab in Platinum-Resistant Low Human Epidermal Growth Factor Receptor 3 (HER3) Messenger Ribonucleic Acid (mRNA) Epithelial Ovarian Cancer (PENELOPE) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04831320 | Nab-Paclitaxel in Combination With Nivolumab to Treat Recurrent or Metastatic Head and Neck Squamous-Cell Carcinoma That Progressed on a PD-1 or PD-L1 Inhibitor | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01834235 | QUILT-3.010: A Study of Gemcitabine and Nab-paclitaxel With or Without NPC-1C to Treat Patients With Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03225924 | Study of Entospletinib (ENTO) in Newly Diagnosed DLBCL Patients With aaIPI>=1 Treated by Chemiotherapy | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00437749 | A Study of CBT-1 and Paclitaxel With Carboplatin in Patients With Advanced Inoperable Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04175912 | Testing the Combination of Pevonedistat With Chemotherapy for Bile Duct Cancer of the Liver | hepatocellular carcinoma;intrahepatic cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT00161278 | Pilot Study for the Determination of Tumor Response to Chemotherapy in Advanced NSCLC Through Gene Expression Profiling | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04296175 | Carboplatin Intensified Chemotherapy for TRIple NEgative Breast Cancer(CITRINE) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00169156 | A Phase II Study of Rituximab Combined With CHOP in T-cell Angio-immunoblastic Lymphoma | angioimmunoblastic T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00861627 | Phase 2 Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin for Non-Small Cell Lung Cancer With KRAS or EGFR Activation | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00006459 | Paclitaxel With or Without Gemcitabine in Treating Women With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02485548 | Cisplatin Plus Raltitrexed or 5-fluorouracil Concurrent With Radiotherapy for Head and Neck Squamous Cell Cancer | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT02207465 | A Phase I Dual Dose Escalation Study of Radiation and Nab-Paclitaxel in Patients With Unresectable and Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01593228 | Treatment Extension Study for Patients Who Have Previously Participated and Have Benefited From Iniparib in a Clinical Trial | neoplasm | Paclitaxel (NPC208553) | |
| NCT03387111 | QUILT-3.090: NANT Squamous Cell Carcinoma (SCC) Vaccine: Subjects With SCC Who Have Progressed | squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02705196 | LOAd703 Oncolytic Virus Therapy for Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00081302 | Cetuximab, Paclitaxel, Carboplatin, and Radiation Therapy in Treating Patients With Unresectable Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00023868 | Combination Chemotherapy With or Without Chemoembolization in Treating Patients With Colorectal Cancer Metastatic to the Liver (6655) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01516567 | Intergroup Trial for Children or Adolescents With Primary Mediastinal Large B-Cell Lymphoma: DA-EPOCH-Rituximab Evaluation | lymphoma | Doxorubicin (NPC261012) | |
| NCT02551991 | Study of Nanoliposomal Irinotecan (Nal-IRI)-Containing Regimens Versus Nab-paclitaxel Plus Gemcitabine in Patients With Previously Untreated, Metastatic Pancreatic Adenocarcinoma | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02272738 | A Study of Chemoradiotherapy Using Gem Plus Nab-paclitaxel for Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04705519 | Nab-paclitaxel Combined With Bevacizumab in the Treatment of Metastatic Neuroendocrine Carcinoma | neuroendocrine carcinoma | Paclitaxel (NPC208553) | |
| NCT01529164 | Efficacy and Safety Study of Recombinant Endostatin Combined With Chemotherapy to Treat Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01719835 | CHOP vs GEM-P in 1st Line Treatment of T-cell Lymphoma, Multicentre Phase II Study | anaplastic large cell lymphoma;angioimmunoblastic T-cell lymphoma;unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02500940 | Comparison the Effects of Different Neoadjuvant Chemotherapy Regimen on Acute Toxicity, Tumor Response, and Survival in Patients With Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00568451 | Paclitaxel and Carboplatin or Temozolomide in Treating Patients With Stage IV Melanoma | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT00002837 | High-Dose Combination Chemotherapy and Peripheral Stem Cell Transplantation in Treating Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02050009 | Metformin Hydrochloride, Carboplatin, and Paclitaxel in Treating Patients With Recurrent Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | ovarian serous cystadenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01310244 | MTD Study PXD-101 in Combination With Paclitaxel + Carboplatin in Chemotherapy-Naive Patients With Stage IV NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03467178 | Study on Decitabine Plus Carboplatin Versus Physician's Choice Chemotherapy in Recurrent, Platinum-resistant Ovarian Cancer. | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00660270 | Chemotherapy and Radiation Following Pancreatic Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01139359 | Safety Study of Darinaparsin in Combination With Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) to Treat Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00801736 | ERCC1 Targeted Trial | lung cancer | Paclitaxel (NPC208553) | |
| NCT03719430 | APX005M and Doxorubicin in Advanced Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00002707 | Chemotherapy in Treating Women With Breast Cancer That Can Be Surgically Removed | breast cancer | Doxorubicin (NPC261012) | |
| NCT00479128 | Bortezomib With Gemcitabine/Doxorubicin in Patients With Urothelial Cancer and Other Solid Tumors | urethra cancer | Doxorubicin (NPC261012) | |
| NCT00707707 | Phase I/II Study of AZD2281 Given in Combination With Paclitaxel in Metastatic Triple Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04502641 | Induction Chemotherapy in Locally Advanced Hypopharyngeal Carcinoma: a Randomised Phase 3 Trial | hypopharyngeal carcinoma | Fluorouracil (NPC75844) | |
| NCT04159818 | Immune Induction Strategies to Improve Response to Immune Checkpoint Blockade in Triple Negative Breast Cancer (TNBC) Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT00298415 | Chemotherapy of Elderly Patients With Non-Small-Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00131508 | Trial of Oral Glutamine in Patients With Sickle Cell Anemia | sickle cell anemia | Glutamine (NPC197087) | |
| NCT00039208 | Combination Chemotherapy in Treating Patients With Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01431794 | Gemcitabine + Nab-paclitaxel With LDE-225 (Hedgehog Inhibitor) as Neoadjuvant Therapy for Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01802749 | Phase I/Ib Study of Paclitaxel in Combination With VS-6063 in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04692051 | A Phase II Study for Nab-paclitaxel Plus Cisplatin vs Gemcitabine Plus Cispatin as First Line Chemotherapy in Advanced Biliary Tract Cancer | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT00001428 | A Phase II Study of 5-Fluorouracil Administered as a One Hour Infusion in Combination With Calcium Leucovorin and Interferon Alpha-2A in Advanced Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00484432 | Study of NGR-hTNF in Combination With Doxorubicin in Patients Affected by Advanced or Metastatic Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02677116 | A Study of Olaratumab Alone and in Combination With Standard Chemotherapies in Children With Cancer | metastasis | Doxorubicin (NPC261012) | |
| NCT02761694 | Absorption, Metabolism, Excretion and Pharmacokinetics of a Single Dose [14C]AZD2014 Followed by a Multiple Dose Phase | cancer | Paclitaxel (NPC208553) | |
| NCT00628251 | Dose-finding Study Comparing Efficacy and Safety of a PARP Inhibitor Against Doxil in BRCA+ve Advanced Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT01463072 | Nab-Paclitaxel in Treating Older Patients With Locally Advanced or Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00202774 | Safety and Efficacy Study of XELOX vs. Oxaliplatin+5-FU CI as First Line Treatment in Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01836432 | Immunotherapy Study in Borderline Resectable or Locally Advanced Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01492881 | Study of Vorinostat With Doxil and Bortezomib for Patients With Relapsed/Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT02426450 | RFA Combined With Oxaliplatin + 5-FluoroUracil/LeucoVorin (5-FU/LV) (FOLFOX4) for Recurrent HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00152191 | A Randomized Controlled Study of Postoperative Adjuvant Therapy of Uracil-tegafur (UFT) Compared With Cyclophosphamide/Methotrexate/5-fluorouracil (CMF) in Breast Cancer (NSAS-BC) | breast cancer | Fluorouracil (NPC75844) | |
| NCT00879359 | Trial of Vascular Endothelial Growth Factor (VEGF), Bevacizumab, in Combination With Cytotoxic Chemotherapy for Endometrial Cancer | endometrial carcinoma | Paclitaxel (NPC208553) | |
| NCT00614484 | Chemotherapy and Proton Radiation for the Treatment of Locally Advanced Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01953406 | The Efficacy of 5-fluorouracil/Mitomycin for the Patients With Pulmonary Metastasis of Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00784537 | High-dose Chemotherapy and Stem Cell Transplantation, in Patients PET-2 Positive, After 2 Courses of ABVD and Comparison of RT Versus no RT in PET-2 Negative Patients | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01329627 | Feasibility Study of Metronomic Chemotherapy for Locally Advanced HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00096226 | Paclitaxel, Carboplatin, and Radiation Therapy in Treating Patients Who Are Undergoing Surgery for Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00475670 | A Study of Herceptin (Trastuzumab) in Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02437812 | Study of Paclitaxel, Carboplatin and Oral Metformin in the Treatment of Advanced Stage Ovarian Carcinoma | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00785291 | Paclitaxel, Nab-paclitaxel, or Ixabepilone With or Without Bevacizumab in Treating Patients With Stage IIIC or Stage IV Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02795988 | A Study of IMU-131(HER-Vaxx) and Chemotherapy Compared to Chemotherapy Only in Patients With HER2 Positive Advanced Gastric Cancer | adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT04707118 | Ntraperitoneal Thermal Perfusion Combined With Chemotherapy Versus Chemotherapy | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00548548 | A Study of Bevacizumab in Combination With Capecitabine and Cisplatin as First-line Therapy in Patients With Advanced Gastric Cancer | adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01041404 | ToGA Study - A Study of Herceptin (Trastuzumab) in Combination With Chemotherapy Compared With Chemotherapy Alone in Patients With HER2-Positive Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT04821765 | Study of PD-1 Antibody Combined With Chemoradiotherapy in Oligometastatic Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02114359 | Chemotherapy Options for the First Line Chemotherapy in Elderly Patient With Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00680940 | A Study of Mycobacterium w in Combination With Paclitaxel Plus Cisplatin in Advanced Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004094 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Previously Untreated Advanced Cancer of the Mouth, Pharynx, or Larynx | head and neck malignant neoplasia | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03586869 | QUILT-3.080: NANT Pancreatic Cancer Vaccine | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT04213794 | Heated Intra-peritoneal Chemotherapy With Doxorubicin and Cisplatin for the Treatment of Resectable, Refractory, or Recurrent Abdominal or Pelvic Tumors in Pediatric Patients, T.O.A.S.T. I.T. Study | rhabdomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00506779 | Gleevec/Taxol for Patients With Uterine Papillary Serous Carcinoma | uterine cancer | Paclitaxel (NPC208553) | |
| NCT00789581 | A Randomized Trial of Ixempra Versus Taxol in Adjuvant Therapy of Triple Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003089 | Chemotherapy, Amifostine, and Radiation Therapy in Treating Patients With Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01644825 | A Phase IIclinical Trial of Carboplatin and Paclitaxel or Carboplatin and Gemcitabine in Platinum-sensitive, Recurrent Ovarian, Fallopian Tube, and Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01405235 | Study on Paclitaxel Plus Topotecan in Comparison With Topotecan Plus Cisplatin in Recurrent or Persistent Cervical Carcinoma | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00874107 | Efficacy/Safety of Imprime PGG® Injection With Bevacizumab and Paclitaxel/Carboplatin in Patients With Untreated Advanced Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01281150 | Veliparib in Combination With Carboplatin and Paclitaxel in Treating Patients With Locally Advanced or Metastatic Solid Tumors | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00010062 | Fluorouracil Plus Radiation Therapy Following Surgery in Treating Patients With Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00042809 | Erlotinib, Trastuzumab, and Paclitaxel in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00006276 | Micellar Paclitaxel to Treat Severe Psoriasis | psoriasis | Paclitaxel (NPC208553) | |
| NCT03167112 | A Study of Administering FOLFIRINOX Before Surgery For Potentially Curable Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01042379 | I-SPY TRIAL: Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer | angiosarcoma;triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00997009 | Study of Adding Cetuximab to Chemotherapy for the Treatment of Advanced and/or Recurrent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00404235 | Carboplatin and ABI-007 in Treating Patients With Stage IV Melanoma That Cannot Be Removed By Surgery | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT02369939 | Effects of Deep Regional Hyperthermia in Patients With Anal Carcinoma Treated by Standard Radiochemotherapy | anal carcinoma | Fluorouracil (NPC75844) | |
| NCT03386838 | An Immuno-therapy Study of Nivolumab in Combination With Experimental Medication BMS-986205 Compared to Standard of Care EXTREME Regimen in First-line Recurrent/Metastatic Squamous Cell Carcinoma of Head and Neck | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT01555853 | Phase I/II Study of Abraxane in Recurrent and Refractory Lymphoma | Hodgkins lymphoma;non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT00176293 | Randomized Phase II Trial of Doxil With or Without Dexamethasone for Metastatic Hormone Refractory Prostate Cancer | prostate cancer | Doxorubicin (NPC261012) | |
| NCT03943043 | Gemcitabine + Oxaliplatin +Nab-paclitaxel in Subjects With Advanced Biliary Tract Cancer | cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT00068393 | Doxorubicin and Gemcitabine in Treating Patients With Locally Recurrent or Metastatic Unresectable Renal Cell Carcinoma | renal cell carcinoma | Doxorubicin (NPC261012) | |
| NCT00052351 | Vaccine Therapy Plus Sargramostim and Chemotherapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01666418 | Pilot Study Investigating the Metabolic Activity and Transcriptional Profiling in Vivo in Tumor Biopsies in Melanoma Patients During Treatment With Pazopanib Alone and in Combination With Paclitaxel | melanoma | Paclitaxel (NPC208553) | |
| NCT00006015 | Combination Chemotherapy Plus Trastuzumab in Treating Patients With Advanced, Recurrent, or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01924819 | Trial of Preoperative Therapy for Gastric and Esophagogastric Junction Adenocarcinoma | gastric cancer | Fluorouracil (NPC75844) | |
| NCT04488159 | Immunoscore as Decision Guidance for Adjuvant Chemotherapy in Colon Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00174356 | PH 1 Evaluation Of Oral CI-1033 In Combination With Paclitaxel/ Carboplatin As 1st Line Chemotherapy In NSCLC Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01217060 | Trimodality Management of T1b Esophageal Cancers | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00004916 | Ifosfamide, Teniposide, and Paclitaxel in Treating Patients With Relapsed Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00005806 | Combination Chemotherapy in Treating Patients With Advanced Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003086 | Repeated Bone Marrow Transplantation in Treating Women With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00160030 | Study Comparing Radiochemotherapy With Folfox 4 Regimen or 5FU-Cisplatin in Patients With Inoperable Esophageal Cancer | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT00046514 | ABI-007 in Taxol Resistant Patients With Metastatic Breast Cancer | metastasis;breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04274114 | Treatment of Adults With Generalized Anxiety Disorder Using Glutamine | generalized anxiety disorder | Glutamine (NPC197087) | |
| NCT03328494 | Trial of ZW25 (Zanidatamab) in Patients With Advanced HER2-expressing Cancers | cancer | Paclitaxel (NPC208553) | |
| NCT00284258 | Phase II/III Trial of CPT-11/5-FU/l-LV Versus CPT-11/TS-1 as Second Line Chemotherapy of Unresectable Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00155883 | Bi-Weekly Docetaxel Plus 24-Hour Infusion of High-Dose 5-Fluorouracil / Leucovorin (HDFL) for Inoperable Advanced or Metastatic Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00003888 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Advanced Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT03879109 | Chemotherapy Followed by Pelvic Reirradiation Versus Chemotherapy Alone as Pre-operative Treatment for Locally Recurrent Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04884906 | Camrelizumab Combined With Radiotherapy and Chemotherapy for the Treatment of Recurrent or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03159897 | FIL Study on ABVD DD-DI as Upfront Therapy in HL. | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00636441 | Trial to Evaluate Genomic Expression Profiles to Direct Preoperative Chemotherapy in Early Stage Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02379416 | Combination Nilotinib and Paclitaxel in Adults With Relapsed Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01303926 | Quality of Life Comparison in Advanced Non-squamous Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00148681 | Preoperative Herceptin and Navelbine for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05171166 | Neoadjuvant HAIC of TACE Plus Donafenib in BCLC B Stage HCC: a Multi-center Randomized Controlled Trial. | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT04664972 | The TP Regimen in the Treatment of Early Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01974440 | A Study of PCI-32765 (Ibrutinib) in Combination With Either Bendamustine and Rituximab or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Participants With Previously Treated Indolent Non-Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00851877 | Nab-Paclitaxel, Cisplatin, and Cetuximab With Concurrent Radiation Therapy for Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00808639 | Dose-Dense MVAC With Pegfilgrastim Support in Subjects With Muscle-Invasive Urothelial Carcinoma | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT05493995 | A Study of Penpulimab and Anlotinib Plus Nab-paclitaxel and Gemcitabine for Metastatic Pancreatic Cancer. | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT02931890 | Multicentric Randomised Trial for Resectable Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02672917 | Study of HuMab-5B1 (MVT-5873) in Subjects With Pancreatic Cancer or Other Cancer Antigen 19-9 (CA19-9) Positive Malignancies | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03484195 | Neoadjuvant FOLFOXIRI Chemotherapy in Patients With Locally Advanced Colon Cancer | colonic neoplasm | Fluorouracil (NPC75844) | |
| NCT03939962 | Camrelizumab Combined With FOLFOX Neoadjuvant Therapy for Resectable Gastric and Gastroesophageal Junctional Adenocarcinoma | gastric cancer | Fluorouracil (NPC75844) | |
| NCT01752205 | Paclitaxel Plus Radiation With Erlotinib to Treat Esophageal Squamous Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02644863 | Autologous Tumor Tissue Antigen-sensitized DC-CIK Cells Combined With Chemotherapy for Esophageal Cancer | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT00974584 | A Study of the Safety and Pharmacology Of PI3-Kinase Inhibitor GDC-0941 In Combination With Either Paclitaxel And Carboplatin (With or Without Bevacizumab) or Pemetrexed, Cisplatin, And Bevacizumab in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05466799 | FOLFIRINOX Versus OncoSil™ in Addition to FOLFIRINOX in Patients With Locally Advanced Pancreatic Adenocarcinoma | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00003088 | Combination Chemotherapy in Treating Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00988195 | Study of Pegylated Human Recombinant Arginase for Liver Cancer | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT05360732 | Redefining FOLFIORINOX in Older Pancreatic Cancer Patients | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT04935359 | Study of Efficacy and Safety of NIS793 in Combination With Standard of Care (SOC) Chemotherapy in First-line Metastatic Pancreatic Ductal Adenocarcinoma (mPDAC) - daNIS-2 | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00047112 | Surgery With or Without Radiation Therapy and Chemotherapy in Treating Patients With Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT01653470 | Study to Evaluate Safety & Tolerability of BMS-906024 in Combination With Chemotherapy & to Define DLTs & MTD of BMS-906024 in Combination With One of the Following Chemotherapy Regimens | cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT02221999 | Weekly Paclitaxel and Cisplatin to Treat Hormone Receptor Positive and Triple Negative Breast Cancer Patients | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00702299 | Alimta® Plus Cisplatin & Paclitaxel Given Intraperitonelly | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00005831 | Trastuzumab and Combination Chemotherapy in Treating Patients With Locally Recurrent or Metastatic Urinary Tract Cancer | urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT00507585 | Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, Leucovorin, and Avastin | liver cancer | Fluorouracil (NPC75844) | |
| NCT02036528 | Safety and Efficacy of Gentamicin Topical Gel (AppliGel-G) for Treatment of Mild to Moderately Infected Diabetic Foot Ulcers | diabetic foot | Gentamicin (NPC471628) | |
| NCT03167177 | QUILT-3.046: NANT Melanoma Vaccine: Combination Immunotherapy in Subjects With Melanoma Who Have Progressed On or After Chemotherapy and PD-1/PD-L1 Therapy | melanoma | Fluorouracil (NPC75844) | |
| NCT00123318 | A Feasibility Study to Evaluate Adjuvant Chemoradiotherapy for Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00397761 | Capecitabine and Paclitaxel (Albumin-Stabilized Nanoparticle Formulation) in Treating Women Undergoing Surgery for Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01967043 | A Study to Evaluate Safety, Tolerability, Pharmacokinetics and Activity of Oraxol in Subjects With Advanced Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT01507428 | Study of Positron Emission Tomography and Computed Tomography in Guiding Radiation Therapy in Patients With Stage III Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03503136 | Nedaplatin Versus Cisplatin and Capecitabine Versus Fluorouracil in IC + CCRT for Locoregionally Advanced NPC | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00003385 | Combination Chemotherapy in Treating Patients With Untreated Ovarian, Peritoneal, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03300609 | 5-FU Based Maintenance Therapy in RAS Wild Type Metastatic Colorectal Cancer After Induction With FOLFOX Plus Panitumumab | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00014222 | Combination Chemotherapy With or Without Colony-stimulating Factors in Treating Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT05283226 | Study to Evaluate the Safety and Efficacy of Oral NRC-2694-A in Combination With Paclitaxel in Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma, Who Progressed on or After Immune Checkpoint Inhibitor Therapy | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00006110 | Multimodality Treatment for Women With Stage II, Stage III, or Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003050 | Radiation Therapy Plus Paclitaxel in Treating Patients With Stage IIB or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00034151 | Safety and Efficacy of S-8184 in Second Line Treatment of Stage III or IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01964534 | Efficacy of ABI-007 Plus Gemcitabine or sLV5FU2 as First-line Therapy in Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00929162 | A Study Evaluating Intermittent and Continuous OSI-906 and Weekly Paclitaxel in Patients With Recurrent Epithelial Ovarian Cancer (and Other Solid Tumors) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00454324 | Study of Abraxane and Carboplatin to Treat Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003877 | Peripheral Stem Cell Transplantation With or Without Stromagen Following Chemotherapy in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00467012 | Phase Ⅱ Clinical Study of R435 (Bevacizumab) in Combination With Paclitaxel in Patients With Inoperable Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00102219 | A Study of Pemetrexed Plus Doxorubicin Given to Patients With Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05233696 | Radiotherapy in Combo With Chemo and Immunotherapy in Patients With PD-L1 Positive Metastatic TNBC | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00615602 | Six vs 12 Months of Trastuzumab With Docetaxel Following FEC as Adjuvant Treatment in N+ Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00454116 | A Phase II, Double Blind Study of 2 Doses of ZACTIMA™(ZD6474) in Combination With FOLFIRI vs FOLFIRI Alone for the Treatment of Colorectal Cancer in Patients | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00513695 | Sunitinib Malate, Paclitaxel, Doxorubicin Hydrochloride, and Cyclophosphamide Before Surgery in Treating Patients With Stage IIB-IIIC Breast Cancer | inflammatory breast carcinoma;male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00003086 | Repeated Bone Marrow Transplantation in Treating Women With Advanced Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00458315 | Cisplatin/Paclitaxel/Gemcitabine +/- Avastin in Patients With Unknown Primary Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT00075712 | Timing of Surgery and Chemotherapy in Treating Patients With Newly Diagnosed Advanced Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cavity Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00671372 | A Study of Dulanermin Administered in Combination With Camptosar®/Erbitux® Chemotherapy or FOLFIRI (With or Without Bevacizumab) in Subjects With Previously Treated Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02449278 | The Palliative Benefit of Involved-site Radiotherapy for Patients With Advanced-stage Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01506973 | A Phase I/II/Pharmacodynamic Study of Hydroxychloroquine in Combination With Gemcitabine/Abraxane to Inhibit Autophagy in Pancreatic Cancer | adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00609622 | Randomized Study Of Sunitinib Plus FOLFOX Versus Bevacizumab Plus FOLFOX In Metastatic Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT01676259 | A Phase 2 Study of siG12D LODER in Combination With Chemotherapy in Patients With Locally Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00317200 | A Study of Paclitaxel Plus Bevacizumab in Patients With Chemosensitive Relapsed Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00179725 | Phase I/II Open-Label, Dose Escalation Study To Determine The Maximum Tolerated Dose And To Evaluate The Safety Profile of Lenalidomide (Revlimid® CC-5013) With Liposomal Doxorubicin In Subjects With Advanced Ovarian and Primary Peritoneal Carcinoma | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02043730 | Abraxane and Gemcitabine Versus Gemcitabine Alone in Locally Advanced Unresectable Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05378919 | Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00016978 | Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer Previously Treated With Irinotecan | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04215731 | Neoadjuvant mFOLFOXIRI Plus Bevacizumab in Patients With High-Risk Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00002496 | Radiation Therapy With or Without Chemotherapy in Treating Patients With Advanced Cancer of the Larynx | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT01479465 | Efficacy and Safety of Simtuzumab (SIM) With FOLFIRI as Second Line Treatment in Colorectal Adenocarcinoma | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT05251038 | Study of Sotorasib Combined With Chemotherapy for Second Line Treatment of Pancreas Cancer | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00554164 | Positron Emission Tomography Guided Therapy of Aggressive Non-Hodgkin's Lymphomas | lymphoma | Doxorubicin (NPC261012) | |
| NCT00020501 | Chemotherapy With or Without Isolated Hepatic Perfusion With Melphalan in Treating Patients With Colorectal Cancer That Has Spread to the Liver | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00022633 | S0028, Gemcitabine and Paclitaxel in Treating Patients With Advanced or Recurrent Cancer of the Urinary Tract | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00797485 | Bevacizumab and Combination Chemotherapy in Treating Patients With Previously Untreated Metastatic Colorectal Cancer That Cannot Be Removed By Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00653939 | A Safety and Efficacy Study of Carboplatin, Paclitaxel, Bevacizumab and CA4P in Non-Small Cell Lung Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT01858883 | Safety Study of Itacitinib (INCB039110) in Combination With Gemcitabine and Nab-Paclitaxel in Subjects With Advanced Solid Tumors | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02617485 | MabionCD20® Compared to MabThera® in Lymphoma Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02784015 | Response Based Treatment for Children With Unresectable Localized Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00002870 | High Dose Chemotherapy Plus Peripheral Stem Cell Transplantation Compared With Standard Therapy in Treating Women With Locally Recurrent or Metastatic Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01442935 | Chemotherapies Associated With Targeted Therapies on the Resection Rate of Hepatic Metastases | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00188266 | Study of Adjuvant Radiochemotherapy for Gastric Cancer | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | Fallopian Tube Carcinoma;ovarian carcinoma | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT03308552 | Radiotherapy With or Without Concurrent Chemotherapy for Limited Lymphatic Metastasis of Esophageal Cancer - 3JECROG P-02 | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT05441254 | Camrelizumab Combined With Intraperitoneal Infusion of Nab-paclitaxel, Intravenous Chemotherapy and S-1 in the Treatment of Advanced Gastric Cancer With Peritoneal Metastasis:Single-arm, Prospective Clinical Study | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00188877 | Intensity Modulated Radiation Therapy for Head and Neck Cancer | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT04592211 | Pembrolizumab, Olaparib, Recurrent/Advanced Gastric and Gastro-esophageal Junction(GEJ) Cancer With HRR Mutation and MSS | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04449549 | Rapid Analysis and Response Evaluation of Combination Anti-Neoplastic Agents in Rare Tumors (RARE CANCER) Trial: RARE 1 Nilotinib and Paclitaxel | neoplasm | Paclitaxel (NPC208553) | |
| NCT00006051 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00484601 | Ifosfamide and Doxorubicin in Patients With Refractory Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Doxorubicin (NPC261012) | |
| NCT00479856 | Lapatinib In Combination With Chemotherapy In Subjects With Relapsed Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00507091 | Phase I Irinotecan, 5-Fluorouracil and Leucovorin Combination | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03524898 | NAPAGE: NAb-PAclitaxel and GEmcitabine in Advanced Soft Tissue Sarcoma | soft tissue sarcoma | Paclitaxel (NPC208553) | |
| NCT03736720 | Liposomal Irinotecan, Fluorouracil and Leucovorin in Treating Patients With Refractory Advanced High Grade Neuroendocrine Cancer of Gastrointestinal, Unknown, or Pancreatic Origin | pancreatic endocrine carcinoma | Fluorouracil (NPC75844) | |
| NCT00034177 | Safety and Efficacy of S-8184 in Treatment of Locally Advanced, Metastatic, or Recurrent TCC of the Urothelium | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT00049348 | Chemotherapy and Radiation Therapy in Treating Patients With Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00003352 | Combination Chemotherapy in Treating Women With Stage IIIB or Stage IV Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03085056 | Trametinib in Combination With Paclitaxel in the Treatment of Anaplastic Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT01862003 | Phase I/II Trial of Antagonism of HER in GI Cancer | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00003994 | Combination Chemotherapy With or Without Amifostine in Treating Young Patients With Liver Cancer | Hepatoblastoma | Fluorouracil (NPC75844) | |
| NCT01248299 | Benefit of Chemotherapy Over Best Supportive Care in Metastatic and Squamous Cell-type Esophageal Cancer. | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00322452 | First Line IRESSA™ Versus Carboplatin/Paclitaxel in Asia | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02155088 | BYL719 in Combination With Gemcitabine and (Nab)-Paclitaxel in Locally Advanced and Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00945191 | A Study of First Line Treatment With Avastin (Bevacizumab) in Combination With Carboplatin and Weekly Paclitaxel in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00028873 | R101933 Combined With Chemotherapy in Treating Patients With Metastatic Breast Cancer That Has Not Responded to Previous Chemotherapy | breast cancer | Paclitaxel (NPC208553) | |
| NCT01327521 | Transarterial Chemoembolization vs CyberKnife for Recurrent Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT00003378 | Combination Chemotherapy in Treating Patients With Previously Untreated Stage III or Stage IV Ovarian or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04527991 | Study of Sacituzumab Govitecan-hziy (IMMU-132) Versus Treatment of Physician's Choice in Participants With Metastatic or Locally Advanced Unresectable Urothelial Cancer | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT00079274 | Comparison of Combination Chemotherapy Regimens With or Without Cetuximab in Treating Patients Who Have Undergone Surgery For Stage III Colon Cancer | colorectal adenocarcinoma;malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT01051999 | Glutamine Supplementation in Cystic Fibrosis | cystic fibrosis | Glutamine (NPC197087) | |
| NCT00055679 | Combination Chemotherapy in Treating Women With Stage I Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02834975 | Phase II: Pembrolizumab/Carboplatin/Taxol in Epithelial Ovary Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02798536 | Mesothelin-Targeted Immunotoxin LMB-100 in People With Malignant Mesothelioma | mesothelioma | Paclitaxel (NPC208553) | |
| NCT00718484 | A Study of Palifosfamide Tris Plus Doxorubicin Versus Doxorubicin in Unresectable or Metastatic Soft-tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00004097 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Advanced and/or Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01941641 | Colon Neoadjuvant FOLFOXIRI Study | rectum cancer | Fluorouracil (NPC75844) | |
| NCT05058404 | Shortened vs Standard Chemotherapy Combined With Immunotherapy for the Initial Treatment of Patients With High Tumor Burden Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT00004887 | Paclitaxel and Carboplatin Chemotherapy Compared With Standard Chemotherapy in Treating Patients With Stage III or Stage IV Non-small Cell Lung Cancer That Cannot Be Removed During Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00047099 | Combination Chemotherapy in Treating Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00063999 | Doxorubicin Hydrochloride, Cisplatin, and Paclitaxel or Carboplatin and Paclitaxel in Treating Patients With Stage III-IV or Recurrent Endometrial Cancer | uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT00585689 | Neoadjuvant ABI-007, Carboplatin and Gemcitabine in Locally Advanced Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01231802 | Study of Eniluracil + 5-Fluorouracil (5-FU) + Leucovorin Versus Capecitabine in Metastatic Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00031577 | Paclitaxel Plus Radiation Therapy in Treating Children With Newly Diagnosed Brain Stem Glioma | Central Nervous System Neoplasm | Paclitaxel (NPC208553) | |
| NCT01372202 | CHFR Methylation Status Esophageal Cancer Study | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00041470 | Navelbine, Taxol, Herceptin and Neupogen in Stage IV Breast Cancer: A Phase I - II Trial | breast cancer | Paclitaxel (NPC208553) | |
| NCT00002969 | S9714: Paclitaxel in Treating Patients With Stage IIIB or Stage IV Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02855359 | Denintuzumab Mafodotin (SGN-CD19A) Combined With RCHOP or RCHP Versus RCHOP Alone in Diffuse Large B-Cell Lymphoma or Follicular Lymphoma | diffuse large B-cell lymphoma;follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT01646034 | High Dose Chemotherapy in Oligo-metastatic Homologous Recombination Deficient Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00449657 | Phase II Trial of Pulsed Taxol With Concurrent Thoracic Radiotherapy, & Adjuvant Chemo in Stage III NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00129376 | Doxorubicin and Cyclophosphamide (AC) Followed by Weekly Docetaxel as Neoadjuvant Treatment of Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT05020860 | Correlation of Clinical Response to Pathologic Response in Patients With Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00976911 | AURELIA: A Study of Avastin (Bevacizumab) Added to Chemotherapy in Patients With Platinum-resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02903004 | Trial on Trabectedin (ET-743) vs Clinician's Choice Chemotherapy in Recurrent Ovarian, Primary Peritoneal or Fallopian Tube Cancers of BRCA Mutated or BRCAness Phenotype Patients | ovarian neoplasm | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT00520975 | Bevacizumab in Treating Patients With Metastatic Breast Cancer That Overexpresses HER-2/NEU | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01220128 | Treatment With Pazopanib for Neoadjuvant Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT01083576 | Pharmacokinetics, Safety, and Efficacy Trial of WR 279,396 (Paromomycin + Gentamicin Topical Cream) and Paromomycin Topical Cream for the Treatment of Cutaneous Leishmaniasis in Panama | Leishmaniasis | Gentamicin (NPC471628) | |
| NCT00516191 | A Phase I/II Study of Liposomal Doxorubicin (Doxil)/Melphalan/Bortezomib (Velcade) in Relapsed/Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT03760614 | A Study of Entinostat and FOLFOX in Subjects With Pancreatic Adenocarcinoma | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01006252 | A Study of Tasisulam-sodium Versus Paclitaxel as Treatment for Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT05448820 | YH001 Plus Envafolimab With or Without Doxorubicin in Patients With Advanced or Metastatic Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00010075 | Combination Chemotherapy in Treating Older Women With Metastatic Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01192165 | Safety and Tolerability Study of GSK1120212, a MEK Inhibitor, in Combination With Docetaxel, Erlotinib, Pemetrexed, Pemetrexed + Carboplatin, Pemetrexed + Cisplatin, or Nab-Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT04703101 | Short Course Radiation Therapy and Combination Chemotherapy for the Treatment of Stage II-III Rectal Cancer | rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02787239 | Clinical Study to Compare the Efficacy and Safety of Rituximab Biosimilar HLX01 and Rituximab in Combination With CHOP, in Previously Untreated Subjects With CD20+ DLBCL | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT04766359 | Paclitaxel (Albumin-bound) Combined With Radiotherapy for the Treatment of Early Stage Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00238160 | Surgery With or Without Hepatic Arterial Chemotherapy in Treating Patients With Liver Cancer | liver cancer | Fluorouracil (NPC75844) | |
| NCT02446574 | Postoperative Chemoradiation in Patients With Node-positive Esophageal Squamous Cell Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT01569204 | Targeted BEACOPP Variants in Patients With Newly Diagnosed Advanced Classical Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02697838 | Clinical Trial of Apatinib Reverses Chemotherapy-Resistance of Patients With Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02017015 | Safety and Efficacy Study of Nab-paclitaxel Plus Gemcitabine in Chinese Patients With Metastatic Pancreatic Cancer | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT04546620 | Acalabrutinib in Combination With R-CHOP for Previously Untreated Diffuse Large B-cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01090128 | Study of Neoadjuvant Chemotherapy With Nanoparticle Albumin Bound Paclitaxel, Doxorubicin and Cyclophosphamide (NAC) in Patients With Stages II-III Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00193882 | Advanced Oesophageal Cancer Study to Compare Quality of Life and Palliation of Dysphagia. | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT05201547 | Endometrial Cancer Patientes MMR Deficient Comparing Chimiotherapy vs Dostarlimab in First Line | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02398240 | Brentuximab for Newly Diagnosed Hodgkin Disease | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00931918 | Study to Assess the Effectiveness of RCHOP With or Without VELCADE in Previously Untreated Non-Germinal Center B-Cell-like Diffuse Large B-Cell Lymphoma Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02889523 | Study of Tazemetostat in Newly Diagnosed Diffuse Large B Cell and Follicular Lymphoma Patients Treated by Chemiotherapy | diffuse large B-cell lymphoma;follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT00815945 | Multicenter Trial With PegLiposomal Doxorubicin and Carboplatin Combination Chemotherapy in Gynecological Sarcomas and Mixed Epithelial-Mesenchymal Tumors | carcinosarcoma;leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00016900 | PNU-93914 in Treating Patients With Locally Advanced or Metastatic Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00262951 | Chemoradiation in Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00471965 | Oxaliplatin + 5-FluoroUracil/LeucoVorin (5-FU/LV) (FOLFOX4) Versus Doxorubicin as Palliative Chemotherapy in Advanced Hepatocellular Carcinoma Patients | hepatocellular carcinoma | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT03257033 | Intra-arterial Gemcitabine vs. IV Gemcitabine and Nab-Paclitaxel Following Radiotherapy for LAPC | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00002953 | Epirubicin and Cyclophosphamide Compared With Epirubicin and Paclitaxel in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00337532 | A Phase II Trial Neoadjuvant Paclitaxel and Cisplatin in Patients With Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00144053 | A Phase Ⅲ Randomized Study of Mitomycin/Vindesine/Cisplatin Versus Irinotecan/Carboplatin Versus Paclitaxel/Carboplatin With Concurrent Thoracic Radiotherapy for Unresectable Stage Ⅲ Non-Small-Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00574587 | Trial for Locally Advanced Breast Cancer Using Vorinostat Plus Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT00002897 | Surgery With or Without Chemotherapy in Treating Patients With Stage II or Stage III Cancer of the Esophagus | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT04884035 | Study of Safety and Efficacy of Iberdomide (CC-220) and CC-99282 Combined With R-CHOP to Treat Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01269216 | A Study of Neoadjuvant Chemoradiotherapy With 5-FU/Leucovorin (FL) vs. TS-1/Irinotecan in Patients With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03412643 | Study of Neoadjuvant Chemotherapy Plus Trastuzumab and Pertuzumab in HER2-Negative Breast Cancer Patients With Abnormal HER2 Signaling | breast cancer | Doxorubicin (NPC261012) | |
| NCT00434135 | Alimta and Gemcitabine in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04423965 | A Trial of Neoadjuvant mFOLFOXIRI Versus CRT in the EMVI Positive LARC | rectum cancer | Fluorouracil (NPC75844) | |
| NCT05357846 | PD-1 Inhibitor Combined With Neoadjuvant Chemoradiotherapy Plus Surgery for Locally Advanced ESCC | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT04172259 | ACH-TH vs EC-TH as Neoadjuvant Therapy for HER2-positive EBC | breast cancer | Doxorubicin (NPC261012) | |
| NCT02921256 | Veliparib, Pembrolizumab, and Combination Chemotherapy in Treating Patient With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00278889 | Second Line ColoRectal Cancer Therapy in Combination With Combination of FOL- Folinic Acid(Leucovorin), F - Fluorouracil and OX - Oxaliplatin (FOLFOX) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04164368 | Lenalidomide Combined With R-CHOP(R2-CHOP) in Newly Diagnosed Double-expressor Diffuse Large B-Cell Lymphoma Patients | lymphoma | Doxorubicin (NPC261012) | |
| NCT04548440 | Efficacy and Safety of Preoperative Sintilimab Plus Nab-paclitaxel and Cisplatin in BR-ESCC Patients | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01622543 | Reolysin in Combination With FOLFOX6 and Bevacizumab or FOLFOX6 and Bevacizumab Alone in Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01797900 | The Role of Induction Chemotherapy for High-risk Locally Advanced Nasopharyngeal Carcinoma in the Era of IMRT | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT01969955 | Nab-paclitaxel as Second-line Therapy in Locally Advanced or Metastatic Squamous Lung Cancer | squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04569032 | A Study of Brentuximab Vedotin and CHP in Frontline Treatment of PTCL With Less Than 10% CD30 Expression | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00493025 | Paclitaxel, Cisplatin, Gefitinib, and Radiation Therapy Followed by Surgery and Gefitinib in Treating Patients With Locally Advanced Cancer of the Esophagus or Gastroesophageal Junction That Can Be Removed By Surgery | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03912415 | Efficacy and Safety of BCD-100 (Anti-PD-1) in Combination With Platinum-Based Chemotherapy With and Without Bevacizumab as First-Line Treatment of Subjects With Advanced Cervical Cancer (FERMATA) | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02132949 | A Study Evaluating Pertuzumab (Perjeta) Combined With Trastuzumab (Herceptin) and Standard Anthracycline-based Chemotherapy in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Locally Advanced, Inflammatory, or Early-stage Breast Cancer | breast cancer | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT01820858 | The Efficacy and Safety of the Postoperative Adjuvant Treatment in Patients With High-risk Stage I Endometrial Carcinoma | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT01174238 | A Two Arm Trial of Axitinib and Carboplatin/Paclitaxel in Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT04962958 | Hepatic Artery Infusion Chemotherapy Plus Donafenib in Patients With Hepatocellular Carcinoma After Surgery | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00006094 | Oxaliplatin, Fluorouracil, and External-Beam Radiation Therapy Followed by Surgery in Treating Patients With Locally Advanced Cancer of the Rectum | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00100789 | S0329, Gemcitabine and Paclitaxel in Treating Patients With Persistent, Recurrent, or Metastatic Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT03719924 | Nal-iri/lv5-fu Versus Paclitaxel as Second Line Therapy in Patients With Metastatic Oesophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00585052 | A Phase II Study of Interaction of Lovastatin and Paclitaxel For Patients With Refractory or Relapsed Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00002826 | Drug Resistance Inhibition in Treating Women With Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00103103 | Bortezomib, Fluorouracil, and Leucovorin in Treating Patients With Metastatic or Unresectable Stomach Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00838149 | Effect of Glutamine on Intestinal Permeability in Crohn's Disease | Crohn's disease | Glutamine (NPC197087) | |
| NCT00377273 | An Open-Label 18-Month Safety Study of Fluorouracil Cream 0.5% for the Treatment of Actinic Keratoses | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT01498588 | Trial of Eribulin Followed by Doxorubicin & Cyclophosphamide for Her2-negative, Locally Advanced Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT02981498 | Phase II Trial of Sorafenib Combined With Concurrent HAIC for Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT03029611 | Pembrolizumab, Paclitaxel, and Carboplatin in Patients With Advanced Stage Epithelial Ovarian Cancer (EOC). | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00002679 | Adjuvant High-Dose, Sequential Chemotherapy in Treating Patients With Resected Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02891083 | Adjuvant Therapies or Surgery Alone for High Risk pN0 Esophageal Cancer | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT00759226 | Chemotherapeutic Trial With Gemcitabine, Cisplatin, 5-FU and Folinic Acid in Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT05281003 | Pembrolizumab Plus Chemo in Neoadjuvant Treatment of Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04839731 | Combination 5-FU / Calcipotriene Cream for SCCIS/SCC | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00061815 | Study of Cetuximab, Oxaliplatin, 5-FU/LV Versus Oxaliplatin, 5-FU/LV in Patients With Previously Treated Metastatic, EGFR-Positive Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00445458 | A Phase 1/2 Study of HKI-272 (Neratinib) in Combination With Paclitaxel (Taxol) in Subjects With Solid Tumors and Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02560051 | Hormone Therapy Plus Chemotherapy as Initial Treatment for Local Failures or Advanced Prostate Cancer | prostate adenocarcinoma | Doxorubicin (NPC261012) | |
| NCT00526110 | Docetaxel, 5-Fluorouracil and Oxaliplatin in Adenocarcinoma of the Stomach or Gastroesophageal Junction Patients | gastrointestinal disease | Fluorouracil (NPC75844) | |
| NCT02529852 | A Phase I/II Study of Lenalidomide and Obinutuzumab With CHOP for Diffuse Large B Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00525642 | Pilot Study of the Safety & Efficacy of Two Docetaxel-Based Regimens Plus Bevacizumab for the Adjuvant Treatment of Subjects With Node Positive or High Risk Node Negative Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT02355119 | Italian Multicenter Study Comparing FOLFOXIRI Versus Gemcitabine as Adjuvant Treatment for Resected Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03884101 | Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) Versus Chemotherapy for Endometrial Carcinoma (ENGOT-en9 / MK-7902-001) | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT02106546 | Study Comparing Veliparib Plus Carboplatin and Paclitaxel Versus Placebo Plus Carboplatin and Paclitaxel in Previously Untreated Advanced or Metastatic Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01258192 | Neoadjuvant Chemotherapy With Nab-paclitaxel Plus Cisplatin for Stage Ⅱ-Ⅲ Esophageal Cancer Patients | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00002984 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Advanced or Metastatic Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00265031 | HD12 for Advanced Stages | lymphoma | Doxorubicin (NPC261012) | |
| NCT00336791 | Randomized Clinical Trial to Evaluate the Predictive Accuracy of a Gene Expression for Stage I-II Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00675259 | Phase II NCT (Neoadjuvant Chemotherapy) w/ Weekly Abraxane in Combination With Carboplatin & Bevacizumab in Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00023660 | Radiation Therapy Plus Celecoxib, Fluorouracil, and Cisplatin in Patients With Locally Advanced Cervical Cancer | cervical cancer | Fluorouracil (NPC75844) | |
| NCT00049257 | Paclitaxel and Carboplatin in Treating Patients With Metastatic Prostate Cancer That Has Not Responded to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00833261 | Nab-Paclitaxel, Cetuximab, Cisplatin, and Radiation Therapy in Treating Patients With Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00006484 | Combination Chemotherapy With or Without Tirapazamine in Treating Patients With Stage IIIB or Stage IV Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01620190 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Previously Treated Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01263171 | Oxaliplatin, Leucovorin, and Fluorouracil Before and After Radiation Therapy and Surgery in Treating Patients With Rectal Cancer That Can Be Removed by Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03036488 | Safety and Efficacy Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy as Neoadjuvant Treatment for Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-173/KEYNOTE-173) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00208936 | Phase II Study of Pre-Operative Chemotherapy in Patients With Resectable Local-Regional Carcinoma of Esophagus | gastrointestinal disease | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT02044601 | Biomarker-Integrated Approach of Targeted Therapy for Lung Cancer Elimination Plus External Beam Radiation Therapy (BATTLE-XRT) | lung cancer | Paclitaxel (NPC208553) | |
| NCT04342910 | Study to Evaluate the Efficacy and Safety of Camrelizumab and Apatinib in Patients With GC/GEJC | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00940303 | OPAL Study: A Study of Avastin (Bevacizumab) in Combination With FOLFOXIRI in Patients With Previously Untreated Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01593020 | Neoadjuvant Study of Sequential Eribulin Followed by FAC Compared to Sequential Paclitaxel Followed by FEC in Early Stage Breast Cancer Not Overexpressing HER-2 | breast cancer | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT00976911 | Paclitaxel and Cisplatin as First-Line Treatment for Patients With Stage I, Stage II, Stage III, or Stage IV Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00381706 | Combination Chemotherapy and Cetuximab in Treating Patients With Metastatic Esophageal Cancer or Gastroesophageal Junction Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00002839 | Combination Chemotherapy Plus Radiation Therapy To Preserve the Larynx in Patients With Cancer of the Hypopharynx or Larynx | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT04958785 | Study of Magrolimab Combination Therapy in Patients With Non-Surgically Removable Locally Advanced or Metastatic Triple-Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT01333423 | Maximum Tolerated Dose (MTD) of Liposomal Doxorubicin in Combination With Seliciclib for Patients With Metastatic Triple Negative Breast Cancer (TNBC) | breast cancer | Doxorubicin (NPC261012) | |
| NCT04579224 | Comparing the New Anti-cancer Drug Eribulin With or Without Chemotherapy Against the Usual Chemotherapy Alone in Metastatic Urothelial Cancer | urethra cancer | Paclitaxel (NPC208553) | |
| NCT01548573 | Tandem Auto Transplantation in Myeloma Patients With <12 Months of Prior Treatment | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00025337 | Combination Chemotherapy With or Without Bevacizumab Compared With Bevacizumab Alone in Treating Patients With Advanced or Metastatic Colorectal Cancer That Has Been Previously Treated | colorectal adenocarcinoma;malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT00270543 | An Effective and Well-Tolerated Regimen of Docetaxel Plus High-Dose 5-Fluorouracil and Leucovorin(HDFL)to Treat Inoperable Advanced or Metastatic Gastric Cancer | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT00068692 | Comparison of Adjuvant Chemotherapy Regimens in Treating Stage II/III Rectal Cancer | mucinous carcinoma;rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02272790 | Metformin Hydrochloride, Carboplatin, and Paclitaxel in Treating Patients With Recurrent Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04821284 | Sonoporation and Chemotherapy for the Treatment of Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00009750 | Monoclonal Antibody Therapy Plus Chemotherapy Followed by Peripheral Stem Cell Transplantation in Treating Patients With Metastatic Prostate Cancer That Has Not Responded to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT02574663 | TGR-1202 Alone and in Combination With Either Nab-paclitaxel + Gemcitabine or With FOLFOX in Patients With Select Relapsed or Refractory Solid Tumors | gastrointestinal stromal tumor;rectum cancer | Fluorouracil (NPC75844) | |
| NCT04243616 | Cemiplimab in High Risk or Locally Advanced Hormone Receptor Positive HER2 Negative or Triple-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00721916 | Randomized Phase II Study of SOL for Untreated Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01000285 | EPOCH Chemotherapy and Bortezomib for Associated T-Cell Leukemia Lymphoma | T-cell acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00881166 | Safety of MP470 in Combination With Standard-of-Care Chemotherapy Regimens to Treat Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT04679064 | Efficacy and Safety of Paclitaxel (Albumin-bound) Combination With Carboplatin in Ovarian Cancer. | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05145569 | A Clinical Study of TQB2450 Injection Combined With Anlotinib Hydrochloride Capsules Versus Paclitaxel as Weekly Treatment of Relapsed Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00525785 | Preoperative Chemo and Chemoradiotherapy for Adenocarcinoma of the Stomach and Gastroesophageal Junction (GEJ) | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00861094 | Radiation Therapy + Combination Chemotherapy as 1st-Line Therapy for Patients With Inoperable Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT02312661 | Lower Dose Decitabine (DAC)-Primed TC (Carboplatin-Paclitaxel) Regimen in Ovary Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04293393 | Neoadjuvant Study Chemotherapy vs Letrozole + Abemaciclib in HR+/HER2- High/Intermediate Risk Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT03940001 | A Study of Sintilimab Plus Chemoradiation Before Surgery for Esophageal Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02570711 | Phase 2, Nab Paclitaxel/Gemcitabine Alone and in Combination With ACP-196 in Subjects With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00063258 | Tarceva Surgery for Resectable Stage IIIA(N2) and IIIB (T4 N2) Non-Small-Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003558 | Chemotherapy in Treating Patients With Cancer of Unknown Primary Origin | carcinoma | Paclitaxel (NPC208553) | |
| NCT00006037 | Chemotherapy Plus IM-862 in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00003998 | Carboplatin Plus Paclitaxel or Docetaxel in Treating Patients With Ovarian Epithelial Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00828009 | BLP25 Liposome Vaccine and Bevacizumab After Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00886717 | Imatinib Mesylate (Gleevec) and Paclitaxel in Recurrent Patients of Ovarian and Other Cancers of Mullerian Origin | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02270814 | Cisplatin, Nab-Paclitaxel, and Cetuximab (CACTUX) in Patients With Incurable Head and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02834975 | Adavosertib Plus Chemotherapy in Platinum-Resistant Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01077739 | A Study of Avastin (Bevacizumab) With XELOX or FOLFOX in Patients With Metastatic Colorectal Cancer and Disease Progression Under First-line FOLFIRI and Avastin | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT05235542 | A Phase Ib/II Study of AK112 in Combination With AK117 in Advanced Malignant Tumors | cancer | Fluorouracil (NPC75844) | |
| NCT00039546 | Combination Chemotherapy in Treating Women With Breast Cancer Who Have Undergone Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT03129828 | Ibrutinib, Bortezomib and Rituximab-CHOP for the Treatment of Elderly Patients With CD20+ DLBCL, IPI ≥ 2 | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01150045 | Oxaliplatin, Leucovorin Calcium, and Fluorouracil With or Without Celecoxib in Treating Patients With Stage III Colon Cancer Previously Treated With Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03285607 | MCS110 Combined With Neoadjuvant Doxorubicin, Cyclophosphamide, and Weekly Paclitaxel in Patients With Hormone-Receptor Positive and HER2- Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03892018 | The Effect of Food on the Pharmacokinetics of Paclitaxel Administered Orally as Oraxol | neoplasm | Paclitaxel (NPC208553) | |
| NCT00422864 | A Single Arm Trial of Oxaliplatin and 5FU With Concurrent Radiation in Patients With Metastatic Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03047265 | Concurrent Chemoradiotherapy in Patients With Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00002828 | Chemotherapy With Raltitrexed and Fluorouracil in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01198392 | Trial of S-1 Plus Cisplatin in Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00577993 | Fludarabine, Mitoxantrone, and Dexamethasone (FND) Plus Rituximab for Lymphoma Patients | lymphoma | Doxorubicin (NPC261012) | |
| NCT01980810 | First Line Chemotherapy for Advanced Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04807673 | Pembrolizumab Plus Neoadjuvant Chemotherapy vs. Neoadjuvant Chemoradiotherapy for Locally Advanced ESCC (KEYSTONE-002) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05275777 | A Phase Ib Safety lead-in, Followed by Phase II Trial of ADG106 in Combination With Neoadjuvant Chemotherapy in HER2 Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01924533 | Efficacy and Safety Study of Olaparib in Combination With Paclitaxel to Treat Advanced Gastric Cancer. | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02434614 | Induction Chemotherapy Followed by IMRT With or Without Concurrent Chemotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT04155671 | POF Versus FOLFOX Plus IP Paclitaxel in AGC | gastric cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00054392 | Combination Chemotherapy in Treating Patients With Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04104672 | A Study to Evaluate the Safety and Tolerability of AB680 in Participants With Gastrointestinal Malignancies | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03639246 | Efficacy and Safety Study of AVB-S6-500 in Patients With Platinum-Resistant Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00535119 | Veliparib, Carboplatin, and Paclitaxel in Treating Patients With Advanced Solid Cancer | Hereditary breast and ovarian cancer syndrome | Paclitaxel (NPC208553) | |
| NCT02589145 | Lenalidomide Combined With Vorinostat/Gemcitabine/Busulfan/Melphalan With Autologous Stem-Cell Transplantation in Diffuse Large B-Cell Lymphoma of the ABC Subtype | lymphoma | Glutamine (NPC197087) | |
| NCT05498259 | Orelabrutinib With R-CHOP-like Regimen for Patients With Newly Diagnosed Untreated Non-GCB DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00942266 | Vorinostat, Fluorouracil, and Leucovorin Calcium in Treating Patients With Metastatic Colorectal Cancer That Has Not Responded to Previous Treatment | colorectal adenocarcinoma;malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT03443661 | Neoadjuvant Triplet Chemotherapy Regimen in Patients With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00139633 | Selective Dose Escalation for Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003054 | Paclitaxel in Treating Patients With Recurrent or Persistent Cancer of the Uterus | sarcoma | Paclitaxel (NPC208553) | |
| NCT02109445 | Study Of PF-03084014 In Combination With Gemcitabine And Nab-Paclitaxel In Patients With Metastatic Pancreatic Adenocarcinoma Not Previously Treated With Anticancer Therapies | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01246063 | Carfilzomib, Pegylated Liposomal Doxorubicin Hydrochloride, and Dexamethasone in Treating Patients With Relapsed or Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT04358341 | Pegliposomal Doxorubicin and 5-fluorouracil as Second Line Therapy for Metastatic Gastric Cancer | gastric cancer | Doxorubicin (NPC261012) | |
| NCT00005060 | Combination Chemotherapy and Surgery in Treating Patients With Locally Advanced Stomach Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT01527864 | Paclitaxel-Carboplatin Alone or With M2ES for Non-Small-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05453500 | Chemotherapy (DA-EPOCH+/-R) and Targeted Therapy (Tafasitamab) for the Treatment of Newly-Diagnosed Philadelphia Chromosome Negative B Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00001569 | Phase I Trial of Continuous Hyperthermic Peritoneal Perfusion (CHPP) With Cisplatin Plus Early Postoperative Intraperitoneal Paclitaxel and 5-FU for Peritoneal Carcinomatosis | peritoneal neoplasm;carcinoma | Fluorouracil (NPC75844) | |
| NCT01953536 | Effect of Trastuzumab on Disease Free Survival in Early Stage HER2-Negative Breast Cancer Patients With ERBB2 Expressing Disseminated Tumor Cells | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00944047 | Evaluate Trastuzumab Plus Standard Chemotherapy Given Before Surgery in Breast Cancer Patients With Low HER 2 Expression | breast cancer | Paclitaxel (NPC208553) | |
| NCT00466960 | Sargramostim and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer That Did Not Respond to Previous Chemotherapy | ovarian serous cystadenocarcinoma;endometrioid carcinoma;fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00305084 | Study of NGR-hTNF in Combination With Doxorubicin in Solid Tumors | cancer | Doxorubicin (NPC261012) | |
| NCT00225173 | Combination Chemotherapy +/- Radiation in High Risk Hodgkin's Disease | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00002593 | S9415 Chemotherapy in Patients With Stage II or III Colon Cancer That Has Been Surgically Removed | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01867892 | A Phase II Study of Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01739894 | Feasibility Study of Intraperitoneal Paclitaxel | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01470417 | Gemcitabine With Abraxane and Other Investigational Therapies in Neoadjuvant Treatment of Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05412082 | SMART TNT for the Conservative Management of Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03635489 | Study on MK-3475 Plus Chemotherapy Versus Chemotherapy Alone in Recurrent, Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01283204 | Trial of 4-regimen (SP, FL/Tax, FL/Doc, FOLFOX) in Patients With Recurrent or Metastatic Gastric Cancer | gastric cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00003287 | Combination Chemotherapy in Treating Patients With Recurrent or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00003331 | Combination Chemotherapy in Treating Patients With Advanced Cancer | chronic myeloproliferative disorder;multiple myeloma;myelodysplastic syndrome | Fluorouracil (NPC75844) | |
| NCT03786783 | Dinutuximab, Sargramostim, and Combination Chemotherapy in Treating Patients With Newly Diagnosed High-Risk Neuroblastoma | ganglioneuroblastoma;neuroblastoma | Doxorubicin (NPC261012) | |
| NCT01091428 | Alisertib (MLN8237) in Participants With Ovarian, Fallopian Tube or Peritoneal Cancer Preceded by Phase 1 Study of MLN8237 Plus Paclitaxel Treatment of Ovary or Breast Cancer | ovarian carcinoma;breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03866993 | A Study of Anti-PD-1 AK105 in Patients With Metastatic Squamous Non-small Cell Lung Cancer | squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00032032 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03740165 | A Study of the Efficacy and Safety of Bevacizumab in Chinese Women With Newly Diagnosed, Previously Untreated Stage III or Stage IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00793897 | Multiple Dose Study In Cancer Patients: Safety and Tolerability of BMS-754807 in Combination With Paclitaxel and Carboplatin in Patients With Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04337632 | NUVOLA TRIAL Open-label Multicentre Study | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01649336 | A Phase IIclinical Trial of Carboplatin and Paclitaxel or Carboplatin and Gemcitabine in Platinum-sensitive, Recurrent Ovarian, Fallopian Tube, and Primary Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT03384654 | A Study to Evaluate the Efficacy and Safety of Daratumumab in Pediatric and Young Adult Participants Greater Than or Equal to (>=)1 and Less Than or Equal to (<=) 30 Years of Age With Relapsed/Refractory Precursor B-cell or T-cell Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01661088 | A Study of Neoadjuvant FOLFIRINOX and FDR-Gemcitabine With Concurrent IMRT in Patients | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02158520 | Nab-Paclitaxel and Bevacizumab or Ipilimumab as First-Line Therapy in Treating Patients With Stage IV Melanoma That Cannot Be Removed by Surgery | cutaneous melanoma;metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT05270057 | Loncastuximab Tesirine in Combination With DA-EPOCH-R in Patients With Previously Untreated Aggressive B-cell Lymphoid Malignancies | Burkitts lymphoma | Doxorubicin (NPC261012) | |
| NCT00068744 | Radiation Therapy, Mitomycin, and Either Fluorouracil or Cisplatin in Treating Patients With Locally Advanced Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT00009737 | A Study of Xeloda (Capecitabine) Compared With 5-Fluorouracil in Combination With Low-Dose Leucovorin in Patients Who Have Undergone Surgery for Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00262847 | Carboplatin and Paclitaxel With or Without Bevacizumab in Treating Patients With Stage III or Stage IV Ovarian Epithelial, Primary Peritoneal, or Fallopian Tube Cancer | endometrioid carcinoma | Paclitaxel (NPC208553) | |
| NCT01851941 | A Phase II Trial of Perioperative Chemotherapy With Oxaliplatin, 5-Fluorouracil, Leucovorin(MODIFIED FOLFOX6) in Patients With Locally Advanced Operable Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00706953 | A Study of Bortezomib and Pegylated Liposomal Doxorubicin in Patients With Relapsed Multiple Myeloma Previously Treated With Bortezomib | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00542451 | Adjuvant Paclitaxel and Trastuzumab for Node-Negative HER2-Positive Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00085501 | S0342: Paclitaxel, Carboplatin, and Cetuximab in Treating Patients With Stage IIIB or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05319639 | Phase I Study of the Combination of Irinotecan and POF (POFI) and Tislelizumab | gastric cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00005979 | Combination Chemotherapy With or Without Irinotecan in Treating Patients With Stage III Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00212082 | Gene Expression Profiles in Predicting Chemotherapy Response in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00256243 | Neoadjuvant Biweekly Treatment Followed by Weekly Treatment of Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02461043 | Adjuvant Chemotherapy With Paclitaxel and Cisplatin in Lymph Node-Positive Adjuvant Chemotherapy in the Treatment of the Lymph Node Positive Thoracic Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003440 | Paclitaxel With or Without Trastuzumab in Treating Patients With or Without HER-2/Neu Breast Cancer That is Inoperable, Recurrent, or Metastatic | breast cancer | Paclitaxel (NPC208553) | |
| NCT03420014 | Treatment of Metastatic Soft Tissue Sarcoma (STS) Patients (FIBROSARC USA) | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00686322 | Concurrent Chemoradiotherapy (CCRT) With Paclitaxel Plus Cisplatin in LA Non-small-cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02414685 | First Line TIP in Poor Prognosis TGCTs. | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT00563784 | TARCEVA (Erlotinib) in Combination With Chemoradiation in Patients With Stage IIIA/B Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00031876 | Capecitabine and Paclitaxel in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00176241 | Phase I Trial of Biweekly Gemcitabine & Paclitaxel & Low-Dose Radiation for Metastatic or Recurrent Head & Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04874311 | Bintrafusp Alfa and Doxorubicin Hydrochloride in Treating Patients With Advanced Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT04919226 | Lutetium 177Lu-Edotreotide Versus Best Standard of Care in Well-differentiated Aggressive Grade-2 and Grade-3 GastroEnteroPancreatic NeuroEndocrine Tumors (GEP-NETs) - COMPOSE | neuroendocrine neoplasm | Fluorouracil (NPC75844) | |
| NCT02715531 | A Study of the Safety and Efficacy of Atezolizumab Administered in Combination With Bevacizumab and/or Other Treatments in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00388700 | A New Agent GM-CT-01 in Combination With 5-FU, Avastin and Leucovorin in Subjects With Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00016406 | S0012 Doxorubicin, Cyclophosphamide, and Paclitaxel With or Without Filgrastim in Treating Women With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01732640 | A Phase I/II Study Afatinib/Carboplatin/Paclitaxel Induction Chemotherapy In HPV-Negative HNSCC. | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02053350 | Alanyl-glutamine Supplementation of Standard Treatment for C. Difficile Infection | clostridium difficile infection | Glutamine (NPC197087) | |
| NCT00064181 | Combination Chemotherapy With or Without Celecoxib in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00463840 | Chemoradiation With Oxaliplatin and Fluorouracil (5FU) for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00866749 | Augmented Berlin-Frankfurt-Munster (BFM) Therapy for Adolescent/Young Adults With Acute Lymphoblastic Leukemia or Acute Lymphoblastic Lymphoma | lymphoid leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT03512834 | Paclitaxel-Avelumab for Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT04807140 | Neoadjuvant Toripalimab or Toripalimab in Combination With Carboplatin and Nab-paclitaxel in Untreated HNSCC (HNSCC-002) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00093119 | Trial of ABI-007 in Previously Treated Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT03997123 | A Study Comparing Two Standard of Care Adjuvant Chemotherapy Regimens for Lower Risk HER-2 Positive Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00878254 | Rituximab and Combination Chemotherapy in Treating Patients With Previously Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04120701 | CIRCULATING TUMOR DNA BASED DECISION FOR ADJUVANT TREATMENT IN COLON CANCER STAGE II | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT01912625 | Trametinib, Combination Chemotherapy, and Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Removed by Surgery | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003111 | Combination Chemotherapy Followed by Surgery in Treating Patients With Stage IIIA Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02033993 | Nab-Paclitaxel to Paclitaxel in Advanced Urothelial Cancer Progressing on or After Platinum Containing Regimen. | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT03400306 | A Study Evaluating the Bioavailability and Food Effect of Veliparib Tablets Followed by an Extension in Subjects With Ovarian Cancer | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT01933061 | Study to Determine Efficacy and Safety of CC-486 With Nab-Paclitaxel Versus Nab-Paclitaxel in Patients With Chemotherapy naïve Metastatic Melanoma | metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT04607421 | A Study of Encorafenib Plus Cetuximab With or Without Chemotherapy in People With Previously Untreated Metastatic Colorectal Cancer | neoplasm | Fluorouracil (NPC75844) | |
| NCT00637897 | Paricalcitol and Chemotherapy in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02876302 | Study Of Ruxolitinib (INCB018424) With Preoperative Chemotherapy For Triple Negative Inflammatory Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT05158062 | Pembrolizumab and Bevacizumab With Chemotherapy Followed by Pembrolizumab, Bevacizumab and Olaparib in Recurrent Ovarian Cancer | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT01253681 | Study of Intraperitoneal Carboplatin With IV Paclitaxel and Bevacizumab in Untreated Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT04909905 | Glutamine Supplementation and Short-term Mortality in Covid-19 | COVID-19 | Glutamine (NPC197087) | |
| NCT00006256 | Paclitaxel Plus Radiation Therapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00004897 | Combination Chemotherapy and Interferon Alfa Followed by Surgery and/or Radiation Therapy in Treating Patients With Stage I, Stage II, or Stage III Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT01116635 | Clinical Study Examining the Safety and Efficacy of Doxorubicin Drug Eluting Microspheres Transarterial Embolization in the Setting of Hepatocellular Carcinoma (HCC) | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT03991962 | Phase II Study to Evaluate Modified Folfirinox and Stereotactic Body Radiation Therapy in Non-metastatic Unresectable Pancreatic Adenocarcinoma | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02640755 | Study of AZD6738, DNA Damage Repair/Novel Anti-cancer Agent, in Combination With Paclitaxel, in Refractory Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT00012311 | Chemotherapy Plus Peripheral Stem Cell Transplantation Compared With Chemotherapy Alone in Treating Women With Stage IV Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00626405 | Bevacizumab and Temozolomide or Bevacizumab and Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients With Stage IV Malignant Melanoma That Cannot Be Removed by Surgery | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT01024062 | Phase II Study of Weekly Paclitaxel (BMS-181339) in Patient With Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04854499 | Study of Magrolimab Combination Therapy in Patients With Head and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT02040454 | Paclitaxel for the Treatment of Distal Radial Artery Arteriovenous Access Fistula Stenosis (PaciFIST-2) | stricture | Paclitaxel (NPC208553) | |
| NCT00499525 | Phase 2b Study of Taxol Plus Sorafenib or Placebo in Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04895358 | Capivasertib+Paclitaxel as First Line Treatment for Patients With Locally Advanced or Metastatic TNBC | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00307736 | Bevacizumab, Erlotinib and 5-Fluorouracil With External Beam Radiation Therapy in Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04478292 | A Multi-institutional Study for Treatment of Children With Newly Diagnosed Hepatoblastoma Using a Modified PHITT Strategy | Hepatoblastoma | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT02830139 | Radical Colorectal Resection and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Locally Advanced Colorectal Cancer | colorectal cancer | Fluorouracil (NPC75844) | |
| NCT00002793 | Chemotherapy Via an Implantable Pump Compared With a Subcutaneous Port for Unresectable Liver Metastases in Patients With Resected Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01332656 | Study of AMG 386 in Combination With Paclitaxel and Carboplatin in Subjects With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003193 | Paclitaxel and Radiation Therapy Plus Chemoprotection With Amifostine in Treating Patients With Stage III or Stage IV Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00622466 | Sorafenib and Paclitaxel in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00502203 | Paclitaxel and Carboplatin in Women With Malignant Mixed Mullerian Tumors (MMMT) of the Uterus | mixed neoplasm | Paclitaxel (NPC208553) | |
| NCT00626704 | Phase 1b/2 Study of AMG 655 With Doxorubicin for the First-Line Treatment of Unresectable Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00576225 | CT-2103/Carboplatin vs Paclitaxel/Carboplatin for NSCLC in Women With Estradiol > 25 pg/mL | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00811993 | A Study of R1507 in Combination With Multiple Standard Chemotherapy Treatments in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00550771 | Lapatinib +/- Trastuzumab In Addition To Standard Neoadjuvant Breast Cancer Therapy. | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01736904 | wXELIRI Versus FOLFIRI Regimen in the Treatment of Advanced Colorectal Cancer Patients | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04625543 | Neoadjuvant Immunotherapy to ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02055820 | A Study Evaluating the Safety, Efficacy and Pharmacokinetics of Venetoclax Combined With Chemotherapy in Participants With B-Cell Non-Hodgkin's Lymphoma (NHL) and DLBCL | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00448266 | Intensified IAA With PBPC Support in Breast Tumors With Evidence of a HRD | breast cancer | Doxorubicin (NPC261012) | |
| NCT00006049 | ZD 1839 Plus Chemotherapy in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003175 | Fluorouracil in Treating Patients With Recurrent or Metastatic Bladder Cancer | urethra cancer;urinary bladder cancer | Fluorouracil (NPC75844) | |
| NCT01525602 | Safety Study of PLX3397 and Paclitaxel in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00629278 | Combination Chemotherapy and Trastuzumab in Treating Women With Stage I, Stage II, or Stage III HER2-Positive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02039674 | A Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy or Immunotherapy in Participants With Non-small Cell Lung Cancer (MK-3475-021/KEYNOTE-021) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01611857 | Phase I/II Trial of Tivantinib With FOLFOX for the Treatment of Advanced Solid Tumors and Previously Untreated Metastatic Adenocarcinoma of the Distal Esophagus, Gastroesophageal Junction or Stomach | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT03817320 | PO Ixazomib in Combination With Chemotherapy for Childhood Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT01276379 | Study Evaluating Biomarkers in Patients With Colorectal Cancer and Native KRAS Treated With Chemotherapy + Cetuximab | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00672295 | PH I SRC Kinase, Dasatinib Combo Paclitaxel & Carboplatin in Pts w Ovarian, Peritoneal, & Tubal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02403895 | AZD2014 and Weekly Paclitaxel in Squamous NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00956930 | Chemoembolization Versus Radioembolization in Treating Patients With Liver Cancer That Cannot Be Treated With Radiofrequency Ablation Or Surgery | liver cancer | Doxorubicin (NPC261012) | |
| NCT00433433 | Fludeoxyglucose F 18 PET Scan-Guided Therapy or Standard Therapy in Treating Patients With Previously Untreated Stage I or Stage II Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03329378 | Neoadjuvant Dose-Dense For Early Her2Neu Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00128856 | Gemcitabine, Doxorubicin and Paclitaxel (GAT) as Neoadjuvant Treatment of Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT04780464 | A 3 Arm Randomized Study on Health-related QoL of Elderly Patients With Advanced Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01367002 | Evaluation of Carboplatin/Paclitaxel With and Without Trastuzumab (Herceptin) in Uterine Serous Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT04261465 | Adoptive T Cell Therapy in Patients With Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02810418 | Mesothelin-Targeted Immunotoxin LMB-100 Alone or in Combination With Nab-Paclitaxel in People With Previously Treated Metastatic and/or Locally Advanced Pancreatic Ductal Adenocarcinoma and Mesothelin Expressing Solid Tumors | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00003578 | High Dose Chemotherapy With or Without Bone Marrow Transplantation in Treating Patients With Intermediate- or High-Grade Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00009763 | Monoclonal Antibody Therapy, Cyclosporine, and Paclitaxel in Treating Patients With Recurrent or Refractory Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04001569 | AZD8186 and Paclitaxel in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00046423 | A Trial of ABI-007 in Patients With Advanced Non-Hematologic Malignancies | metastasis;neoplasm | Paclitaxel (NPC208553) | |
| NCT00004865 | Cetuximab Plus Cisplatin in Treating Patients With Metastatic or Recurrent Cancer of the Head and Neck That Has Not Responded to Cisplatin Chemotherapy | head and neck malignant neoplasia | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00069953 | Combination Chemotherapy Followed By Chemoradiotherapy, With or Without Surgery, in Treating Patients With Resectable Locally Advanced Cancer of the Esophagus or Gastroesophageal Junction | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03192644 | The Impact on Recurrence Risk of Adjuvant Transarterial Chemoinfusion (TAI) Versus Adjuvant Transarterial Chemoembolization (TACE) for Patients With Hepatocellular Carcinoma And Portal Vein Tumor Thrombosis (PVTT) After Hepatectomy : A Random, Controlled, Stage III Clinical Trial. | hepatocellular carcinoma | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT03329248 | QUILT-3.060: NANT Pancreatic Cancer Vaccine: Molecularly Informed Integrated Immunotherapy in Subjects With Pancreatic Cancer Who Have Progressed on or After Standard-of-care Therapy | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03086369 | A Study of Nab-Paclitaxel and Gemcitabine With or Without Olaratumab (LY3012207) in Participants With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03056833 | A Study of Atezolizumab Versus Placebo in Combination With Paclitaxel, Carboplatin, and Bevacizumab in Participants With Newly-Diagnosed Stage III or Stage IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04077905 | Pegylated Liposomal Doxorubicin as a Induction Therapy for Lymphoma Induced Hemophagocytic Lymphohistiocytosis. | hemophagocytic syndrome | Doxorubicin (NPC261012) | |
| NCT00082095 | To Compare Treatment With Doxorubicin or Capecitabine for Metastatic Breast Cancer in Women 60 Years and Older | breast cancer | Doxorubicin (NPC261012) | |
| NCT02049905 | Phase 3 Study to Treat Patients With Soft Tissue Sarcomas | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00602602 | Bevacizumab, Combination Chemotherapy, and Radiation Therapy in Treating Patients Undergoing Surgery For Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00884767 | Biomarkers in Predicting Neurotoxicity in Patients With Colorectal Cancer Receiving Oxaliplatin | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02794571 | Safety and Pharmacokinetics (PK) of Escalating Doses of Tiragolumab as a Single Agent and in Combination With Atezolizumab and/or Other Anti-Cancer Therapies in Locally Advanced or Metastatic Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00021372 | Paclitaxel and Estramustine in Treating Patients With Relapsed or Refractory Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00481078 | Vorinostat, Carboplatin, and Paclitaxel in Treating Patients With Advanced or Metastatic Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03556839 | Platinum Chemotherapy Plus Paclitaxel With Bevacizumab and Atezolizumab in Metastatic Carcinoma of the Cervix | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT02762981 | Study to Evaluate Relacorilant (CORT125134) in Combination With Nab-paclitaxel in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00024388 | Chemotherapy in Treating Patients With Metastatic Kidney Cancer | kidney cancer | Paclitaxel (NPC208553) | |
| NCT01779050 | Evaluation of an Anti-cancer Immunotherapy Combined With Standard Neoadjuvant Treatment in Patients With WT1-positive Primary Invasive Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00027937 | Combination Chemotherapy, Peripheral Stem Cell Transplantation, and Biological Therapy in Treating Patients With Solid Tumors or Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00625092 | Hyperthermic Intraperitoneal Oxaliplatin for Peritoneal Malignancies | peritoneum cancer | Fluorouracil (NPC75844) | |
| NCT03726879 | A Study To Evaluate the Efficacy and Safety Of Atezolizumab or Placebo in Combination With Neoadjuvant Doxorubicin + Cyclophosphamide Followed By Paclitaxel + Trastuzumab + Pertuzumab In Early Her2-Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00007878 | Bortezomib, Fluorouracil, and Leucovorin Calcium in Treating Patients With Solid Tumors That Are Metastatic or Cannot Be Removed By Surgery | neoplasm | Fluorouracil (NPC75844) | |
| NCT03699449 | An uMbrella Study of BIomarker-driven Targeted Therapy In Patients With Platinum-resistant Recurrent OvariaN Cancer(AMBITION) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03928314 | Study of ORIC-101 in Combination With Anticancer Therapy | neoplasm | Paclitaxel (NPC208553) | |
| NCT02713386 | Ruxolitinib Phosphate, Paclitaxel, and Carboplatin in Treating Patients With Stage III-IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | endometrioid carcinoma | Paclitaxel (NPC208553) | |
| NCT03263741 | Exploratory Study on the Paclitaxel + S-1 + Oxaliplatin (PSOX) for Locally Advanced or Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03964753 | Neoadjuvant Therapy With Nab-paclitaxel and Cisplatin for Locally Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01952847 | Randomized Trial of Glutamine in Patients With Mucositis or Esophagitis | cancer | Glutamine (NPC197087) | |
| NCT02252796 | Phase I Hypofractionated Stereotactic Boost (Radiotherapy) for Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03188198 | Risk Adapted Therapy in Diffuse Large B Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01730222 | A Phase I-II Study of PAXG in Stage III-IV Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00551213 | A Study to Determine the Activity of Robatumumab (SCH 717454, MK-7454) in Participants With Relapsed or Recurrent Colorectal Cancer (P04721, MK-7454-003) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00176267 | Paclitaxel, Carboplatin And Low Dose Radiation As Induction Therapy In Locally Advanced Head And Neck Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05074966 | The Efficacy and Safety of Modified XELOX(mXELOX) Plus Cetuximab vs FOLFOX Plus Cetuximab in RAS and BRAF WT mCRC Pts | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04694183 | The Conversion Therapy of Chemotherapy Plus Camrelizumab in Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00004253 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Stage II or Stage III Non-small Cell Lung Cancer That Cannot Be Removed By Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00111007 | A Treatment Combination for Patients With Unresectable Stage III or Stage IV Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT05013697 | TQB2450 Solution for Injection (TQB2450)+Paclitaxel+Cisplatin ± Anlotinib in the Treatment of Esophageal Cancer. | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT02275598 | Brentuximab Vedotin Followed by ABVD in Patients With Previously Untreated Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01848132 | Efficacy/Safety Study of R-CHOP vs Bortezomib-R-CAP for Young Patients With Diffuse Large B-cell Lymphoma With Poor IPI. | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00002632 | Paclitaxel in Treating Patients With Metastatic or Recurrent Salivary Gland Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00095875 | Combination Chemotherapy and Radiation in Treating Patients With Stage III or IV Head and Neck Cancer (Paradigm Trial) | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT03000374 | Induction Therapy With Panitumumab + mFOLFOX-6 in Rectal Cancer and Quadruple Wild-type Mutation Before Surgery | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00303758 | Combination Chemotherapy in Treating Patients With Metastatic Pancreatic Cancer That Cannot Be Removed By Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT04517435 | ME-401 and R-CHOP in Newly Diagnosed Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00085839 | Erlotinib vs. Standard Chemotherapy in Patients With Advanced Non-small Cell Lung Cancer (NSCLC) and Eastern Cooperative Oncology Group (ECOG)Performance Status (PS) 2 | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00793377 | Neoadjuvant Doxorubicin/Cyclophosphamide Followed by Docetaxel (AC-Doc) Versus Dose-Dense Doxorubicin and Docetaxel (ADoc) in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00303108 | Doxil & Carboplatin Plus HER2+ in Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01604863 | A Study of HGS1036 in Combination With Chemotherapy in Subjects With Advanced Solid Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00308516 | 5-Fluorouracil, Bevacizumab, and Radiation Followed by Modified FOLFOX6 and Bevacizumab in Stage II/III Rectal Cancer | rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00610792 | Phase 2 Study of Twice Weekly VELCADE and CAELYX in Patients With Ovarian Cancer Failing Platinum Containing Regimens | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT04723030 | The Transformation of Locally Advanced Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05456022 | Therapeutic Efficacy of Quercetin Versus Its Encapsulated Nanoparticle on Tongue Squamous Cell Carcinoma Cell Line | mouth neoplasm | Doxorubicin (NPC261012) | |
| NCT01362374 | Safety and Clinical Pharmacology of GDC-0068 in Combination With Docetaxel, Fluoropyrimidine Plus Oxaliplatin, Paclitaxel, or Enzalutamide in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT03038100 | A Study of Atezolizumab Versus Placebo in Combination With Paclitaxel, Carboplatin, and Bevacizumab in Participants With Newly-Diagnosed Stage III or Stage IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT01375816 | Liposome-encapsulated Irinotecan Hydrochloride PEP02 or Irinotecan Hydrochloride, Leucovorin Calcium, and Fluorouracil as Second-Line Therapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00046995 | Combination Chemotherapy in Treating Patients With Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04838496 | Induction Chemotherapy for Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03025477 | Safety and Efficacy of Quisinostat, a Histone Deacetylase Inhibitor, in Combination With Chemotherapy | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03603834 | Neoadjuvant mFOLFOXIRI for Potentially Resectable Cholangiocarcinoma | cholangiocarcinoma | Fluorouracil (NPC75844) | |
| NCT03213249 | Bacterial Biofilms in Reconstructive Breast Prostheses Following Mastectomy | infection | Gentamicin (NPC471628) | |
| NCT02008656 | Trial Evaluating 3-year Disease Free Survival in Patients With Locally Advanced Rectal Cancer Treated With Chemoradiation Plus Induction or Consolidation Chemotherapy and Total Mesorectal Excision or Non-operative Management | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00003397 | Peripheral Stem Cell Transplantation Plus Combination Chemotherapy and Monoclonal Antibody Therapy in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT01750073 | Paclitaxel and Cyclophosphamide With or Without Trastuzumab Before Surgery in Treating Patients With Previously Untreated Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00301730 | Cellular Adoptive Immunotherapy in Treating a Patient Who Has Undergone a Donor Stem Cell Transplant for Breast Cancer That Has Spread to the Lung | breast cancer | Paclitaxel (NPC208553) | |
| NCT00086996 | S0356 Oxaliplatin, 5-FU, Radiation Therapy (RT), Surgery for Pts With Stage II or III Cancer of Esophagus or Gastroesophageal (GE) Junction | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00220649 | Safety Study of Combination Chemotherapy in Patients With Metastatic Solid Tumors or Adenocarcinoma of the Pancreas | pancreatic neoplasm | Fluorouracil (NPC75844) | |
| NCT00848718 | A Phase I Study of MK-2206 in Combination With Standard Chemotherapy in Participants With Locally Advanced or Metastatic Solid Tumors (MK-2206-003) | neoplasm | Paclitaxel (NPC208553) | |
| NCT05009069 | A Study of Atezolizumab With or Without Tiragolumab Following Neoadjuvant Chemoradiotherapy in Participants With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03279237 | A Pilot Study of FOLFIRINOX in Combination With Neoadjuvant Radiation for Gastric and GE Junction Cancers | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00003627 | Radiation Therapy With or Without Combination Chemotherapy in Treating Patients With Advanced Cancer of the Oropharynx or Hypopharynx | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT04329949 | Study of Relacorilant in Combination With Nab-Paclitaxel in Patients With Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05145218 | Biomarker-driven Targeted Therapy in Patients With Recurrent Platinum-resistant Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00030381 | Iododoxorubicin in Treating Patients With Primary Systemic Amyloidosis | primary systemic amyloidosis | Doxorubicin (NPC261012) | |
| NCT03649321 | Clinical Trial of Chemotherapy and Bemcentinib for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00203905 | A Study of Chemoradiotherapy for Intermediate Stage/Selected Stage IV Cancers of the Head and Neck | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT01812369 | Perioperative Chemotherapy for Patients With Locally Advanced Bladder Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT00911183 | Diffuse Large B Cell Non-Hodgkin's Lymphoma in the Vulnerable/Frail Elderly. A Multicentric Randomized Phase II Trial | lymphoma | Doxorubicin (NPC261012) | |
| NCT00128466 | Treatment and Diagnosis of Plague | Yersinia pestis infectious disease | Gentamicin (NPC471628) | |
| NCT00877071 | LC Drug Eluting Bead for Treatment of Liver Cancer Which Cannot be Surgically Removed | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00893516 | CD4 in Combination With CHOP in Treating Non-cutaneous Peripheral TCell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02341456 | Phase Ib Study AZD1775 in Combination With Carboplatin and Paclitaxel in Adult Asian Patients With Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT00040794 | Combination Chemotherapy, Radiation Therapy, and Gefitinib in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03484221 | Totally Neoadjuvant FOLFOXIRI + Short-course Radiation + XELOX in Patients With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT02353260 | Efficacy and Safety of Ultrasound Hyperthermia Combined With Chemotherapy on Oral and Maxillofacial-Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00003596 | Chemotherapy Plus Radiation Therapy in Treating Patients With Stage II or Stage III Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT00014573 | Chemotherapy and Vaccine Therapy Followed by Bone Marrow or Peripheral Stem Cell Transplantation and Interleukin-2 in Treating Patients With Recurrent or Refractory Brain Cancer | Central Nervous System Neoplasm | Paclitaxel (NPC208553) | |
| NCT02756013 | Weight-based Dosing for Dense Weekly Paclitaxel and Carboplatin in Overweight Patients w/ Body Surface Area (BSA) > 2.0 | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT00635050 | Therapy for Locally Advanced Breast Cancer Using Doxil, Paclitaxel, and Cyclophosphamide With Avastin | breast cancer | Paclitaxel (NPC208553) | |
| NCT00801580 | My-HyperCVAD in the Treatment of Relapsed Refractory Adult Acute Lymphoid Leukemia | lymphoid leukemia | Doxorubicin (NPC261012) | |
| NCT00738699 | First Line Tx Stage III Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04158635 | Gemcitabine, Nab-Paclitaxel, and Bosentan for the Treatment of Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02138617 | Genotype-Directed Study Of Irinotecan Dosing In FOLFIRI + BevacizumabTreated Metastatic Colorectal Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00387387 | Study On Pazopanib When Given With FOLFOX6 (Fluorouracil, Oxaliplatin, Leucovorin) Or CapeOx (Capecitabine, Oxaliplatin) | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT02958163 | Clinical Trial Comparing TACE With TACE + SABR in Stage BCLC B HCC (HepSTAR) | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT02178709 | A Phase II Study of Neoadjuvant FOLFIRINOX | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03678883 | Lapatinib Plus Chemotherapy Versus Trastuzumab Plus Chemotherapy in HER2-positive Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00661167 | Phase II Study of ABI-007 for Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03577704 | The Safety,Efficacy of Anti-EGFR Humanized Monoclonal Antibody Combined With Chemotherapy in Advanced Solid Tumors. | neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT05371184 | Glutamine Role in Preventing Vaso-occlusive Crisis Among SCD Patients | sickle cell anemia | Glutamine (NPC197087) | |
| NCT02138383 | Enzalutamide in Combination With Gemcitabine and Nab-Paclitaxel for the Treatment of Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00814541 | PAD. ICORG 05-01, V11 | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00206466 | Biologic Correlative Taxotere/AC | breast cancer | Doxorubicin (NPC261012) | |
| NCT00194753 | Adjuvant Therapy for High-Risk Breast Cancer With Wkly Adriamycin & Oral Cytoxan With G-CSF for 12 Wks | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04498689 | Efficacy and Safety of Camrelizumab Combined With Nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01412229 | Induction Chemotherapy for Locally Advanced Squamous Cell Carcinoma of the Head and Neck | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00028769 | S0032, Combination Chemotherapy Plus Hormone Therapy in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT01788137 | A Study of Improving the Efficacy of Treatment in High Risk T Cell Lymphoma Patients | T-cell acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00645879 | Anaplerotic Therapy in Propionic Acidemia | propionic acidemia | Glutamine (NPC197087) | |
| NCT00003284 | High-Dose Combination Chemotherapy Followed by Peripheral Stem Cell Transplantation in Treating Patients With Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00600925 | A Study of an Antibiotic Implant in General Surgical Subjects at Higher Risk for Surgical Wound Infection | infection | Gentamicin (NPC471628) | |
| NCT00003159 | Surgery With or Without Preoperative Chemotherapy in Treating Patients With Resectable Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00903630 | Lenalidomide and Doxorubicin Hydrochloride Liposome in Recurrent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Doxorubicin (NPC261012) | |
| NCT03850769 | Neoadjuvant Nab-Paclitaxel and S-1 in Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01441349 | Irinotecan/Cisplatin With or Without Simvastatin in Chemo-naive Patients With Extensive Disease-small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00786643 | Study of Gamma Interfereon in Metastatic Colorectal Carcinoma | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00010634 | Complementary Naturopathic Medicine for Periodontitis | periodontitis | Glutamine (NPC197087) | |
| NCT03125902 | A Study of Atezolizumab and Paclitaxel Versus Placebo and Paclitaxel in Participants With Previously Untreated Locally Advanced or Metastatic Triple Negative Breast Cancer (TNBC) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT05200312 | A Phase II Study of Zanubrutinib, Lenalidomide Plus R-CHOP as the First-line Treatment for Diffused Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00230451 | Pre-operative Chemotherapy and Radiation Therapy for Esophageal Carcinoma | esophageal cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01756170 | Postoperative Chemoradiation v.s Radiotherapy for Lymph Node Negative Cervical Cancer Patients | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00426127 | Docetaxel and Liposomal Doxorubicin Chemotherapy With Enoxaparin in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Doxorubicin (NPC261012) | |
| NCT05159193 | Neoadjuvant Treatment Pegylated Liposomal Doxorubicin Plus Cyclophosphamide Sequential Docetaxel Plus Trastuzumab and Pertuzumab Versus Docetaxel Plus Carboplatin Combined With Trastuzumab and Pertuzumab in HER-2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00518284 | Prevention of Restenosis Following Revascularization | peripheral vascular disease | Paclitaxel (NPC208553) | |
| NCT01069328 | Dose Escalating Study With BAY43-9006 With Carboplatin, Paclitaxel and Bevacizumab in Untreated Stage IIIb Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03026881 | A Study of Fluzoparib Given in Combination With Apatinib and Paclitaxel in Gastric Cancer Patients | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00541112 | Radiation Therapy, Chemotherapy, and Cetuximab Followed by Surgery, Chemotherapy, and Cetuximab in Treating Patients With Locally Advanced or Metastatic Rectal Cancer That Can Be Removed by Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03678883 | 9-ING-41 in Patients With Advanced Cancers | lung neoplasm;metastasis;kidney cancer | Doxorubicin (NPC261012) | |
| NCT01581476 | Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial | type 1 diabetes mellitus | Paclitaxel (NPC208553) | |
| NCT03004287 | 2015-12: A Study Exploring the Use of Early and Late Consolidation/Maintenance Therapy | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00584909 | A Phase II Study of Paclitaxel and Carboplatin in Patients With an Elevated-Risk Cancer of the Uterus | uterine cancer | Paclitaxel (NPC208553) | |
| NCT04834206 | Nedaplatin in Treatment for Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT01046825 | Mature B-Cell Lymphoma And Leukemia Study III | lymphoma | Doxorubicin (NPC261012) | |
| NCT00093795 | Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00002696 | Combination Chemotherapy in Treating Women With Stage III Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02259621 | Neoadjuvant Nivolumab, or Nivolumab in Combination With Ipilimumab, in Resectable NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02366143 | A Study of Atezolizumab in Combination With Carboplatin Plus (+) Paclitaxel With or Without Bevacizumab Compared With Carboplatin+Paclitaxel+Bevacizumab in Participants With Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01930552 | A Phase I Study of Aflibercept Plus FOLFIRI (Irinotecan, 5-Fluorouracil, and Leucovorin) in Chinese Patients With Advanced Solid Malignancies | neoplasm | Fluorouracil (NPC75844) | |
| NCT02272699 | Neoadjuvant Treatment of Nimotuzumab With Chemotherapy or Radiotherapy in Resectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00589238 | Paclitaxel, Doxorubicin, and Cyclophosphamide With Or Without Carboplatin in Treating Women With Locally Advanced Breast Cancer That Can Be Removed by Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT00470301 | Tipifarnib and Combination Chemotherapy in Treating Patients With Stage II or Stage III Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03092895 | A Study of SHR-1210 in Combination With Apatinib or Chemotherapy in Subjects With Advanced PLC or BTC | carcinoma;liver cancer | Fluorouracil (NPC75844) | |
| NCT03018080 | Pilot Study of Paclitaxel Plus Pembrolizumab in Metastatic HER2-Negative Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01039844 | Study of Weekly LOC-paclitaxel Injection for Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00001426 | A Multi-Institutional Phase II Study of Cyclophosphamide, Paclitaxel, Cisplatin With G-CSF for Patients With Newly Diagnosed Advanced Stage Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00226590 | Induction Gemcitabine & Carboplatin Followed by Paclitaxel & Carboplatin +XRT in NSCLC | lung cancer | Paclitaxel (NPC208553) | |
| NCT00250874 | Myocet, Docetaxel & Trastuzumab as 1st Line Treatment of Patients With HER-2/Neu Positive Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00005032 | Bcl-2 Antisense Oligodeoxynucleotide G3139 and Paclitaxel in Treating Patients With Recurrent Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003644 | Carboplatin Plus Paclitaxel With or Without Continued Low-Dose Paclitaxel in Treating Patients With Early-Stage Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00044785 | Phase I Study of PN401, Fluorouracil, Leucovorin and CPT-11 in Patients With Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT04217954 | HAIC With Oxaliplatin, 5-FU and Bevacizumab Plus Intravenous Toripalimab for Advanced BTC | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT03635567 | Efficacy and Safety Study of First-line Treatment With Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy in Women With Persistent, Recurrent, or Metastatic Cervical Cancer (MK-3475-826/KEYNOTE-826) | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03517137 | Very Early PET-response Adapted Targeted Therapy for Advanced Hodgkin Lymphoma: a Single -Arm Phase II Study | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00003105 | Combination Chemotherapy in Treating Patients With Metastatic or Locally Advanced Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00500292 | A Phase II Study of 2 Doses of ZD6474 (Vandetanib) in Combination With FOLFOX vs FOLFOX Alone for the Treatment of Colorectal Cancer | cancer | Fluorouracil (NPC75844) | |
| NCT01994993 | Antibiotic Safety (SCAMP) | infection | Gentamicin (NPC471628) | |
| NCT03574649 | QUILT-2.024: Phase 2 Neoadjuvant, Consolidation, and Adjuvant Combination NANT Immunotherapy Versus Standard of Care in Subjects With Resectable Non-small Cell Lung Cancer | non-small cell lung carcinoma | Fluorouracil (NPC75844) | |
| NCT00578149 | Bevacizumab and Carboplatin/Paclitaxel and Radiation in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00145639 | Osteosarcoma1999-A Study Of Intensive Chemotherapy for Osteosarcoma | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT04161755 | Study of Personalized Tumor Vaccines (PCVs) and a PD-L1 Blocker in Patients With Pancreatic Cancer That Can be Treated With Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01286818 | A Study of Irinotecan, Levofolinate, and 5-Fluorouracil (FOLFIRI) Plus Ramucirumab (IMC-1121B) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00513292 | Combination Chemotherapy and Paclitaxel Plus Trastuzumab in Treating Women With Palpable Breast Cancer That Can Be Removed by Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT00577629 | Chemotherapy With Monoclonal Antibody and Radioimmunotherapy for High-Risk B-Cell Non-Hodgkins Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00513786 | Evaluation of Carboplatin/Paclitaxel/Bevacizumab in the Treatment of Advanced Stage Endometrial Carcinoma | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00816634 | Efficacy Comparison Study of Combination Regimens to Treat Advanced Esophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03248427 | Neadjuvant Multi-agent Chemotherapy or Letrozole Plus Ribociclib in Luminal B/HER2-negative Breast Cancer. | breast cancer | Doxorubicin (NPC261012) | |
| NCT00089297 | Cetuximab, Chemotherapy, and Radiation Therapy for Operable Stage III or IV Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02270606 | Phase I Study of Neoadjuvant Radiotherapy With 5-Fluorouracil for Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03410784 | A Study of VB-111 With Paclitaxel vs Paclitaxel for Treatment of Recurrent Platinum-Resistant Ovarian Cancer (OVAL) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02215083 | L-glutamine Supplementation to Alleviate Symptoms of Taxane-Induced Neuropathy in Patients With Breast Cancer | peripheral neuropathy | Glutamine (NPC197087) | |
| NCT02858206 | Simultaneous Integrated Boost Radiotherapy and Concurrent Nimotuzumab or Chemotherapy for Esophageal Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT01593306 | Concurrent Chemoradiotherapy With Weekly Cisplatin Versus Concurrent Chemoradiotherapy With Weekly Cisplatin and Paclitaxel in Locally Advanced Carcinoma Cervix | carcinoma | Paclitaxel (NPC208553) | |
| NCT03853044 | Study Evaluating the Safety and Efficacy of C-CHOP in Untreated Subjects With Angioimmunoblastic T Cell Lymphoma | angioimmunoblastic T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00732836 | Hepatic Arterial Infusion (HAI) of Abraxane | liver cancer | Paclitaxel (NPC208553) | |
| NCT00017407 | Carboplatin and Paclitaxel With or Without ISIS 3521 in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00133562 | HIAS II - Study of Nutritional Supplementation in Hospitalized Children With Persistent Diarrhea or Malnutrition | malnutrition | Glutamine (NPC197087) | |
| NCT03737643 | An uMbrella Study of BIomarker-driven Targeted Therapy In Patients With Platinum-resistant Recurrent OvariaN Cancer(AMBITION) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01176799 | Randomized Study of Doxorubicin and Cyclophosphamide With or Without Intermittent Sunitinib in the First-line Treatment of Locally Advanced or Metastatic Breast Cancer Patients With Measurable Primary Breast Tumor | breast cancer | Doxorubicin (NPC261012) | |
| NCT00113399 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Recurrent Head and Neck Cancer That Cannot Be Removed By Surgery | head and neck malignant neoplasia | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT01285466 | A Trial of Oral BEZ235 and BKM120 in Combination With Paclitaxel With or Without Trastuzumab | neoplasm | Paclitaxel (NPC208553) | |
| NCT02291289 | A Study of Biomarker-Driven Therapy in Metastatic Colorectal Cancer (mCRC) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01822496 | Erlotinib Hydrochloride or Crizotinib and Chemoradiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04550260 | Study of Durvalumab Versus Placebo in Combination With Definitive Chemoradiation Therapy in Patient With ESCC | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT01417494 | 1st Line Chemotherapy Alone or With Bevacizumab in Treating Older Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00002568 | Combination Chemotherapy With or Without Surgery in Treating Patients With Stage III Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00750815 | Cyclophosphamide, VELCADE, DOXIL, and Dexamethasone, (CVDD) in Newly Diagnosed Patients With Multiple Myeloma (MM) | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01954355 | Clinical Study in Treatment of Malignant Ascites of Ovarian Cancer With Intraperitoneal Injection Bevacizumab Combined With Intraperitoneal Hyperthermic Perfusion Chemotherapy | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04967859 | Evaluation of the Efficacy of Prophylactic Topical Gentamicin in Tunnelled Catheters for Hemodialysis | infection | Gentamicin (NPC471628) | |
| NCT00090025 | XL119 Versus 5-Fluorouracil (5-FU) Plus Leucovorin (LV) in Subjects With Advanced Biliary Tumors | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT00004934 | Paclitaxel and Carboplatin With or Without Epirubicin in Treating Patients With Stage IIB, Stage III, or Stage IV Invasive Ovarian Epithelial, Fallopian Tube, or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01992653 | A Study of Polatuzumab Vedotin in Combination With Rituximab or Obinutuzumab, Cyclophosphamide, Doxorubicin, and Prednisone in Participants With B-Cell Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02012192 | A Study of MEK162 and Paclitaxel in Patients With Epithelial Ovarian, Fallopian Tube or Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01090830 | Safety and Efficacy of Belinostat When Used With Standard of Care Chemotherapy for Untreated Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03818282 | Trial Comparing Niraparib-bevacizumab-Dostarlimab and Niraparib-bevacizumab to Standard of Care in Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04286711 | Clinical Study of Albumin-paclitaxel Combined With Apatinib and Camrelizumab in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00060346 | Rituximab and Combination Chemotherapy in Treating Patients With Newly Diagnosed Waldenstrom's Macroglobulinemia | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003327 | Paclitaxel in Treating Patients With Recurrent or Refractory Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT03650933 | A Study Comparing the Efficacy and Safety of G-CHOP Versus R-CHOP in Untreated Diffuse Large B-cell Lymphoma Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01424982 | Combination Chemotherapy and Ponatinib Hydrochloride in Treating Patients With Acute Lymphoblastic Leukemia | Blast Phase Chronic Myelogenous Leukemia, BCR-ABL1 Positive | Doxorubicin (NPC261012) | |
| NCT00816959 | Study Evaluating the Effect of R-mabHDI in Lymphocytic Predominant Hodgkin's Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02603679 | Neoadjuvant Response-guided Treatment of Luminal B-type Tumors and Luminal A-type Tumors With Node Metastases | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00584857 | A Phase II Study of Therapy With Paclitaxel, Carboplatin and Megesterol Acetate for the Management of Uterine Cancer | uterine cancer | Paclitaxel (NPC208553) | |
| NCT02220894 | Study of Pembrolizumab (MK-3475) Versus Platinum-Based Chemotherapy for Participants With Programmed Cell Death-Ligand 1 (PD-L1)-Positive Advanced or Metastatic Non-Small Cell Lung Cancer (MK-3475-042/KEYNOTE-042) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02994251 | A Trial of Systemic Chemotherapy in Combination With Conventional Transarterial Chemoembolization in Patients With Advanced Intra-Hepatic Cholangiocarcinoma | cholangiocarcinoma | Doxorubicin (NPC261012) | |
| NCT00595972 | Epirubicin Cisplatin and Fluorouracil (FU) Combined With Endostar in Patients With Advanced or Metastatic Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00310011 | Gemcitabine, Paclitaxel, and Cisplatin in Treating Patients With Advanced Cancer of the Urothelium | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00154804 | CCRT With Twice Weekly Paclitaxel and Cisplatin Followed by Surgery for Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00618657 | Carboplatin+Nab-paclitaxel, Plus Trastuzumab (HER2+) or Bevacizumab (HER2-) in the Neoadjuvant Setting | breast cancer | Paclitaxel (NPC208553) | |
| NCT05257993 | Study to Assess the Safety, Tolerability of JPI-547 in Combination With Modified FOLFIRINOX or Gemcitabine-nab-paclitaxel in Patients With Locally Advanced and Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00085358 | Carboplatin and Paclitaxel With or Without Bevacizumab Compared to Docetaxel, Carboplatin, and Paclitaxel in Treating Patients With Stage II, Stage III, or Stage IV Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cavity Carcinoma (Cancer) | ovarian serous cystadenocarcinoma;endometrioid carcinoma;fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00004873 | Combination Chemotherapy in Treating Patients With Advanced Stomach Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02376452 | Phase II Study of 2-weekly RAILIRI Versus FOLFIRI as Second-line Treatment in Advanced Colorectal Cancer Patients | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00368875 | Phase I-II Study of Vorinostat, Paclitaxel, and Bevacizumab in Metastatic Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00273507 | Neoadjuvant Chemotherapy in Non Small Cell Lung Cancer (NSCLC) Stages IB, IIA, IIB, and IIIA/T3 | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01238965 | Panobinostat and Fluorouracil Followed By Leucovorin Calcium in Treating Patients With Stage IV Colorectal Cancer Who Did Not Respond to Previous Fluorouracil-Based Chemotherapy | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT02005315 | A Study of Vantictumab (OMP-18R5) in Combination With Nab-Paclitaxel and Gemcitabine in Previously Untreated Stage IV Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003402 | Peripheral Stem Cell Transplantation Plus Combination Chemotherapy in Treating Patients With Low-Grade Non-Hodgkin's Lymphoma or Chronic Lymphocytic Leukemia | leukemia;lymphoma | Paclitaxel (NPC208553) | |
| NCT00431795 | Randomized Phase II Study of Epirubicin vs Caelyx in Pretreated Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00136539 | Neoadjuvant Therapy With Herceptin and Taxol for Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04437329 | Nedaplatin Versus Cisplatin in Treatment for Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00913835 | A Study of Liposomal Doxorubicin With or Without Olaratumab (IMC-3G3) in Platinum-Refractory or Resistant Advanced Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT01802645 | Cetuximab/FOLFIRI With or Without Oxaliplatin and FOLFOXIRI With or Without Bevacizumab in Neoadjuvant Treatment of Non-resectable Colorectal Liver Metastases | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02514278 | Optimisation of Response for Organ Preservation in Rectal Cancer : Neoadjuvant Chemotherapy and Radiochemotherapy vs. Radiochemotherapy | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00133978 | Trial of Glutamine and Antioxidant Supplementation in Critically Ill Patients | Multiple Organ Failure;Sepsis | Glutamine (NPC197087) | |
| NCT01326000 | A Study of RO5083945 in Combination With FOLFIRI Versus FOLFIRI Plus Cetuximab or FOLFIRI Alone as Second Line Treatment in Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00532285 | Primary Paclitaxel Plus Gemcitabine in Patients With Stage II and III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02590133 | A Phase II Clinical Trial of Chemotherapy With or Without Endostar® Continuous Intravenous Infusion in Refractory NPC | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT04634877 | Study of Pembrolizumab (MK-3475) in Combination With Adjuvant Chemotherapy With or Without Radiotherapy in Participants With Newly Diagnosed Endometrial Cancer After Surgery With Curative Intent (MK-3475-B21 / KEYNOTE-B21 / ENGOT-en11 / GOG-3053) | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT00193362 | Study of Paclitaxel, Carboplatin, and Gemcitabine Versus Gemcitabine and Vinorelbine for Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03020030 | Treatment of Newly Diagnosed Acute Lymphoblastic Leukemia in Children and Adolescents | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00930930 | Cisplatin and Paclitaxel With or Without Everolimus in Treating Patients With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00358163 | Trial of PTK787/ZK 222584 Plus Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT00135135 | Therapy for Children With Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT00003008 | Paclitaxel in Treating Patients With AIDS-Related Kaposi's Sarcoma | sarcoma | Paclitaxel (NPC208553) | |
| NCT03143153 | A Study of the Safety and Tolerability of ABBV-621 in Participants With Previously-Treated Solid Tumors and Hematologic Malignancies | cancer | Fluorouracil (NPC75844) | |
| NCT01490047 | Single Dose of Intravenous rhTNF-α and Liposomal Doxorubicin in Patients With Advanced Solid Tumors or Lymphomas | lymphoma | Doxorubicin (NPC261012) | |
| NCT00075595 | Fluorouracil, Leucovorin, and Irinotecan in Treating Patients With Recurrent or Refractory Metastatic Unresectable Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04528680 | Ultrasound-based Blood-brain Barrier Opening and Albumin-bound Paclitaxel and Carboplatin for Recurrent Glioblastoma | gliosarcoma | Paclitaxel (NPC208553) | |
| NCT01100931 | A Phase I/II Study of Paclitaxel, Carboplatin and YM155 (Survivin Suppressor) in Subjects With Solid Tumors (Phase I) and Advanced Non-Small Cell Lung Carcinoma (Phase II) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00002716 | Combination Chemotherapy in Treating Patients With Liver Metastases From Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00290537 | Phase II Study of ZD6474 in Advanced NSCLC | lung cancer | Paclitaxel (NPC208553) | |
| NCT00753038 | Phase 2 Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin in Patients With Head and Neck Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00483509 | Study of NGR-hTNF in Combination With Doxorubicin in Patients Affected by Metastatic Small Cell Lung Carcinoma | small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT03067792 | FOLFIRI Versus Docetaxel and Cisplatin as a Second-line Chemotherapy After Failure of First-line Chemotherapy in Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT04393298 | A Study to Assess the Safety, Pharmacokinetics and Antitumor Activity of UCB6114 Administered Intravenously to Participants With Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT03398655 | Atezolizumab With Neoadjuvant Chemotherapy for Patients With Newly-Diagnosed Advanced-Stage Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00140140 | A Phase I/II Study of ABI-007 (Abraxane®, Nab®-Paclitaxel)and Vinorelbine in Patients With Stage IV (Metastatic) Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00054210 | Polyglutamate Paclitaxel Plus Carboplatin Compared With Paclitaxel Plus Carboplatin in Treating Patients With Advanced or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00480831 | A Study of PRO95780 in Patients With Previously Untreated, Advanced-Stage Non-Small Cell Lung Cancer (APM4074g) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01026116 | Treatment With Pazopanib for Neoadjuvant Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT05489848 | Chemotherapy vs Chemoradiotherapy for Post-operative Endometrial Cancer Patients With P53-mutation | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT05108428 | Adaptive Radiation for Locally Advanced Rectal Adenocarcinoma | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00337389 | Phase III Randomized Study of 5-FU, CoFactor, and Avastin vs. 5-FU, LV and Avastin for First-Line Colorectal Cancer. | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT02036164 | Adjuvant Chemotherapy for Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03274492 | A Study Comparing the Efficacy and Safety of Polatuzumab Vedotin With Rituximab-Cyclophosphamide, Doxorubicin, and Prednisone (R-CHP) Versus Rituximab-Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Participants With Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03110926 | Induction Chemotherapy for Locally Advanced Esophageal Cancer | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT02465593 | A Study of PEP503(Radio-enhancer) With Radiotherapy and Chemotherapy for Patients With Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01969032 | Induction Preoperative Chemotherapy for Patients With Locally Advanced Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00662311 | Vorinostat, Paclitaxel, and Radiation Therapy in Treating Patients Unable to Tolerate Cisplatin With Stage III Non-Small Lung Cancer That Cannot Be Removed By Surgery | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05244993 | Study of Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy for HR+/HER2- Locally Recurrent Inoperable or Metastatic Breast Cancer (MK-3475-B49/KEYNOTE-B49) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00507429 | Study of Combretastatin and Paclitaxel/Carboplatin in the Treatment of Anaplastic Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT00508326 | Paclitaxel Administered by HAI to Patients With Advanced Cancer and Dominant Liver Involvement | cancer | Paclitaxel (NPC208553) | |
| NCT01225302 | A Study of Linifanib (ABT-869) in Combination With Carboplatin/Paclitaxel in Japanese Subjects With Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01324076 | Transarterial Chemoembolization Using Doxorubicin Beads With or Without Sorafenib Tosylate in Treating Patients With Liver Cancer That Cannot Be Removed By Surgery | liver cancer | Doxorubicin (NPC261012) | |
| NCT02215876 | Neoadjuvant Doxorubicin/Cyclophosphamide Followed by Eribulin Chemotherapy (ACE) in Operable HER2-negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01494688 | A Study of RO5509554 as Monotherapy and in Combination With Paclitaxel in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04752696 | Onvansertib in Combination With Nanoliposomal Irinotecan, Leucovorin, and Fluorouracil for Second-Line Treatment of Participants With Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00033410 | Chemotherapy, Tirapazamine, and Radiation Therapy in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03975205 | To Study the Concentration Level of, Doxil, and Doxorubicin at Various Time Frames | leukemia;lymphoma | Doxorubicin (NPC261012) | |
| NCT00781612 | A Safety Extension Study of Trastuzumab Emtansine in Participants Previously Treated With Trastuzumab Emtansine Alone or in Combination With Other Anti-Cancer Therapy in One of the Parent Studies | metastasis | Paclitaxel (NPC208553) | |
| NCT03291899 | Trial of Infusional FOLFIRINOX in First Line Treatment of Advanced Biliary Tract Cancers | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT03575520 | Safety and Efficacy of Pegteograstim in Korean Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT01868022 | Study to Evaluate GSK3052230 in Combination With Paclitaxel and Carboplatin, or Docetaxel or as Single Agent in Subjects With Solid Malignancies and Deregulated Fibroblast Growth Factor (FGF) Pathway Signaling | neoplasm | Paclitaxel (NPC208553) | |
| NCT01528618 | Effect and Safety Study of GP/FP Regimens in Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT03179904 | FASN Inhibitor TVB-2640 and Trastuzumab in Combination With Paclitaxel or Endocrine Therapy for the Treatment of HER2 Positive Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00002884 | Chemotherapy and Radiation Therapy in Treating Patients With Cancer of the Esophagus | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT03632109 | Gent for Pharyngeal Gonorrhea (GC) | infection | Gentamicin (NPC471628) | |
| NCT04243434 | PK Study on Ready-to-Use Injection (VSLI-RTU) 1 Vial & 3 Vial Formulation Marqibo® in Hematological Malignant Patients | Abnormality of blood and blood-forming tissues | Doxorubicin (NPC261012) | |
| NCT05420636 | Iadademstat in Combination With Paclitaxel in Relapsed/Refractory SCLC and Extrapulmonary High Grade NET | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00001272 | A Phase I Study of Taxol, Cisplatin, Cyclophosphamide and Granulocyte Colony-Stimulating Factor (G-CSF) in Previously Nontreated Ovarian Cancer Patients | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT02419755 | Bortezomib and Vorinostat in Younger Patients With Refractory or Relapsed MLL Rearranged Hematologic Malignancies | acute leukemia of ambiguous lineage;acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01185964 | A Study of Olaratumab in Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT01068132 | Prognostic Factors for Patients With Advanced Colorectal Cancer Treated With Cetuximab. | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03585062 | Trial of Neoadjuvant Chemotherapy With S1 Plus Paclitaxel-albumin on Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00006018 | Paclitaxel and BMS-214662 in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02779855 | Talimogene Laherparepvec in Combination With Neoadjuvant Chemotherapy in Triple Negative Breast Cancer | breast ductal carcinoma in situ | Paclitaxel (NPC208553) | |
| NCT02937272 | A Study of LY3200882 in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00661193 | S0709: Erlotinib With or Without Carboplatin and Paclitaxel in Stage IIIB or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03340896 | Trial of Laryngeal Preservation Comparing Induced CT Followed by RT vs CT Concomitant to RT | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT04135781 | Nab-paclitaxel Combined With S-1 as Adjuvant Chemotherapy for Stage Ⅲ Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01086826 | Treatment of Patients With Locally Advanced Squamous Cell Carcinoma of the Head and Neck | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT05481645 | Efficacy and Safety of TQB2450 Combined With Chemotherapy ± Anlotinib for Advanced Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01078441 | Bortezomib, Liposomal Doxorubicin Hydrochloride, Dexamethasone, and Cyclophosphamide in Treating Patients With Multiple Myeloma That Relapsed After Autologous Stem Cell Transplant | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT03944304 | Paclitaxel in Patients With GIST With Low P-glycoprotein Expression After Failure of at Least Imatinib and Sunitinib, and Regorafenib. | gastrointestinal stromal tumor | Paclitaxel (NPC208553) | |
| NCT03259035 | NEO: Neoadjuvant Chemotherapy, Excision and Observation for Early Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00391118 | Comparing Two Treatments for Ovarian Cancer: Standard Chemotherapy Plus Enzastaurin, or Placebo ("Sugar Pill") | Fallopian Tube Carcinoma;ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT02186847 | Chemotherapy and Radiation Therapy With or Without Metformin Hydrochloride in Treating Patients With Stage III Non-small Cell Lung Cancer | bronchoalveolar adenocarcinoma;large cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00001339 | A Study of Combination Chemotherapy and Surgical Resection in the Treatment of Adrenocortical Carcinoma: Continuous Infusion Doxorubicin, Vincristine and Etoposide With Daily Mitotane Before and After Surgical Resection | carcinoma | Doxorubicin (NPC261012) | |
| NCT01031212 | ASA404 in Combination With Carboplatin/Paclitaxel/Cetuximab in Treating Patients With Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01496742 | A Study of Onartuzumab (MetMAb) in Combination With Bevacizumab (Avastin) Plus Platinum And Paclitaxel or With Pemetrexed Plus Platinum in Patients With Non-Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00149214 | Preoperative Treatment of Breast Cancer With Two Different Sequential Treatment Regimens | breast cancer | Doxorubicin (NPC261012) | |
| NCT01279681 | Combination Chemotherapy Plus Bevacizumab With or Without Oxaliplatin in Treating Older Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03742986 | Trial of Nivolumab With Chemotherapy as Neoadjuvant Treatment in Inflammatory Breast Cancer (IBC) | breast cancer | Doxorubicin (NPC261012) | |
| NCT04922567 | Efficacy and Safety of Lenalidomide Plus CHOP vs CHOP in Patients With Untreated Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00176774 | Irinotecan, 5-Fluorouracil, and Leucovorin in Colorectal Carcinoma | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02020707 | Nab-Paclitaxel and Bevacizumab in Treating Patients With Unresectable Stage IV Melanoma or Gynecological Cancers | cervical adenocarcinoma;cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT01177956 | A Trial to Determine the Safety and Anti-tumor Activity Profile of the Combination of Cetuximab and Concomitant Cisplatin Plus 5-Fluorouracil (5-FU) in Subjects With Recurrent and/or Metastatic Squamous Cell Carcinoma in Head and Neck | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00524810 | Liposomal Doxorubicin and Docetaxel in Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01667211 | Clinical Study of Albumin-bound Paclitaxel Plus Nedaplatin in Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02072317 | Paclitaxel Plus Raltitrexed Plug Compare With Taxol Second-line Treatment for Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02985333 | Paclitaxel,Cyclophosphamide and Dexamethasone for Relapsed or Refractory Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT00028743 | Combination Chemotherapy Regimens in Ovarian Epithelial Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00004233 | S9915 Triacetyluridine, Fluorouracil, and Leucovorin in Treating Patients With Unresectable, Locally Advanced, or Metastatic Cancer of the Esophagus or Stomach | esophageal cancer;gastric cancer | Fluorouracil (NPC75844) | |
| NCT00004931 | Fluorouracil Plus Leucovorin With or Without Oxaliplatin in Treating Patients With Stage II or Stage III Colon Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00503906 | Abraxane, Avastin, and Gemcitabine as First-Line Therapy for Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00121992 | Docetaxel, Doxorubicin (A), Cyclophosphamide (C) (TAC) vs 5-Fluorouracil, A, C (5FAC) Breast Cancer Adjuvant Treatment | breast neoplasm | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT04865289 | Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) Versus Chemotherapy for Endometrial Carcinoma (ENGOT-en9 / MK-7902-001) - China Extension Study | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT00093756 | Bortezomib, Paclitaxel, Carboplatin and Radiation Therapy for Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00976573 | Carboplatin, Paclitaxel, and Bevacizumab With or Without Everolimus in Treating Patients With Metastatic Malignant Melanoma | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT01160601 | Study of Paclitaxel/Carboplatin With or Without Bavituximab in Previously Untreated Non Small-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00034164 | Safety and Efficacy of S-8184 in Second Line Treatment of Relapsed Stage IIIB or IV Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05179889 | Adjuvant FOLFOXIRI for High-risk Stage III Colon Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00038610 | Study of Hyper-CVAD Plus Imatinib Mesylate for Philadelphia-Positive Acute Lymphocytic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT05083247 | Preoperative mFOLFIRINOX (or Gem-Nab-P) +/- Isotoxic High-dose SBRT for Borderline Resectable Pancreatic Adenocarcinoma | pancreatic neoplasm | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00193284 | Chemotherapy Plus Gefitinib Followed by Chemotherapy, Radiation Therapy, and Gefitinib For Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00021320 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT02450058 | Adjuvant FEC Versus EP in Breast Cancer (MIG5) | breast cancer | Fluorouracil (NPC75844) | |
| NCT00006118 | Cisplatin, Paclitaxel, and Gemcitabine in Treating Patients With Progressive Unresectable Regional or Metastatic Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01490866 | A Trial of Single Agent Axitinib as Maintenance Therapy for Patients With First Line Metastatic Colorectal Cancer (mCRC) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00943137 | The Optimization of 5-Fluorouracil Dose by Pharmacokinetic Monitoring in Asian Patients With Advanced Stage Cancer | cancer | Fluorouracil (NPC75844) | |
| NCT01199055 | CS-7017 in Combination With Carboplatin/Paclitaxel in Subjects With Stage IIIb/IV Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003130 | Paclitaxel in Treating Women With Recurrent Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01548924 | Determination of Dose of Antiangiogenic Multitargeted DOVITINIB (TKI258) Plus Paclitaxel in Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03812770 | Phase 2 Study of Sorafenib Plus HAIC of FOLFOX vs. Sorafenib Plus HAIC of OXA for Advanced HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02494713 | Hormonal Therapy and Chemotherapy Followed by Prostatectomy in Patients With Prostate Cancer | prostate cancer | Doxorubicin (NPC261012) | |
| NCT01083537 | Intraperitoneal vs Intravenous Chemotherapy Following Neoadjuvant Chemotherapy in Ovarian Cancer | fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02358161 | Phase I/II Study of LDE225 With Gemcitabine and Nab-paclitaxel in Patients With Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01111604 | A Study of Ramucirumab or Icrucumab in Colorectal Cancer | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT00550771 | German Preoperative Adriamycin Docetaxel Study | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT02468557 | Study of Single Agent Idelalisib Followed by Idelalisib in Combination With Chemotherapy in Adults With Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT02351219 | FOLFOXIRI as Primary Treatment for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00801281 | First-line R-CVP vs R-CHOP Induction Immunochemotherapy for Indolent Lymphoma and R Maintenance. | chronic lymphocytic leukemia;follicular lymphoma;lymphoplasmacytic lymphoma | Doxorubicin (NPC261012) | |
| NCT00349076 | Neoadjuvant Chemoradiotherapy and Adjuvant Chemotherapy With 5-Fluorouracil and Oxaliplatin Versus 5-Fluorouracil Alone in Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00002476 | Radiation Therapy With or Without Chemotherapy in Treating Patients With Advanced Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00720512 | Second-Line Combination Chemotherapy With or Without Bevacizumab in Treating Patients With Metastatic Colorectal Cancer Who Have Received First-Line Chemotherapy and Bevacizumab | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01578551 | Study of Metformin Plus Paclitaxel/Carboplatin/Bevacizumab in Patients With Adenocarcinoma. | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00536939 | Trial of Paclitaxel, Bevacizumab, and Enzastaurin Versus Paclitaxel, Bevacizumab and Placebo for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01352689 | CKD-828(80/2.5mg) Pharmacokinetic Study_2nd | hypertension | Paclitaxel (NPC208553) | |
| NCT00491855 | Oxaliplatin and Paclitaxel Plus Bevacizumab in Advanced Peritoneal Carcinomatosis | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01488552 | Gemcitabine+Nab-paclitaxel and FOLFIRINOX and Molecular Profiling for Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT02569242 | Study of Nivolumab in Unresectable Advanced or Recurrent Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00004885 | Fluorouracil and Leucovorin With or Without Irinotecan in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00077220 | Paclitaxel, Carboplatin, and Radiation Therapy With or Without Adjuvant Paclitaxel and Carboplatin in Treating Patients With Stage II or Stage III Unresectable Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01839773 | A Phase 3 Study to Compare Efficacy and Safety of DHP107 Versus Taxol® in Patients With Metastatic or Recurrent Gastric Cancer After Failure of First-line Chemotherapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01253525 | Study of Weekly Paclitaxel With Ramucirumab in Participants With Advanced Gastric Adenocarcinomas | adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00520013 | Avastin +/- Erlotinib Consolidation Chemotherapy After Carboplatin, Paclitaxel, and Avastin (CTA) Induction Therapy for Advanced Ovarian, Fallopian Tube, Primary Peritoneal Cancer & Papillary Serous or Clear Cell Mullerian Tumors | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00936702 | Carboplatin, Paclitaxel, and Everolimus in Treating Patients With Previously Untreated Cancer of Unknown Primary | carcinoma | Paclitaxel (NPC208553) | |
| NCT03918278 | A Study of MK-0482 as Monotherapy and in Combination With Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-0482-001) | neoplasm | Paclitaxel (NPC208553) | |
| NCT01211210 | Neoadjuvant FOLFOX6 Chemotherapy With or Without Radiation in Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT02054559 | R-CHOP Alone vs. R-CHOP Plus Radiotherapy for Localized CD20+ DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04806945 | A Phase III Study to Evaluate Efficacy and Safety of First-Line Treatment With HLX10 + Chemotherapy in Patients With Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04354662 | Toripalimab Combined With FLOT Neoadjuvant Chemotherapy in Patients With Resectable Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT01281943 | Study of Pegylated Liposomal Doxorubicin and Temsirolimus in Patients With Advanced Hepatocellular Cancer | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00429299 | Neoadjuvant Study With Chemotherapy, Lapatinib And Trastuzumab In Breast Cancer | breast neoplasm | Fluorouracil (NPC75844) | |
| NCT00069121 | A Study of Xeloda (Capecitabine) Plus Oxaliplatin in Patients With Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00556088 | LBH589, Paclitaxel, Carboplatin +/- Bevacizumab for Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01386385 | Veliparib With or Without Radiation Therapy, Carboplatin, and Paclitaxel in Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Removed by Surgery | squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05035381 | PD-1 Inhibitor Combined With Bevacizumab and FOLFIRI Regimen in the Second-line Treatment of Advanced Colorectal Cancer | colorectal cancer | Fluorouracil (NPC75844) | |
| NCT02107378 | Efficacy of DCVAC/OvCa Plus Standard of Care in Relapsed Platinum Resistant Epithelial Ovarian Carcinoma | ovarian carcinoma | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT03763123 | Study of Chemotherapy With Pembrolizumab (MK-3475) Followed by Maintenance With Olaparib (MK-7339) for the First-Line Treatment of Women With BRCA Non-mutated Advanced Epithelial Ovarian Cancer (EOC) (MK-7339-001/KEYLYNK-001/ENGOT-ov43/GOG-3036) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00085163 | Celecoxib Combined With Fluorouracil and Leucovorin in Treating Patients With Resected Stage III Adenocarcinoma (Cancer) of the Colon | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00264953 | HD11 for Intermediate Stages | lymphoma | Doxorubicin (NPC261012) | |
| NCT00189566 | Taxol® in Monotherapy or in Combination With Topotecan or Carboplatin in Patients With Epithelial Ovarian Cancer in Early Relapse | fallopian tube cancer;ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00350948 | Phase 3 Randomized Study of Telcyta + Doxorubicin Versus Doxorubicin in Platinum Refractory or Resistant Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT00002628 | Addition of Paclitaxel to High-Dose Combination Chemotherapy in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00434356 | A Study of Sunitinib in Combination With Bevacizumab and Paclitaxel in Previously Untreated Patients With Metastatic Breast Cancer (SABRE-B) | breast cancer | Paclitaxel (NPC208553) | |
| NCT04897854 | Timing of Start of systemIc Treatment for Asymptomatic Metastasized Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT05491694 | To Evaluate the Efficacy of Toripalimab Combined With Chemotherapy After HIFU Induction in the Treatment of Early Triple-negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00023673 | Radiation Therapy Combined With Paclitaxel and Carboplatin in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00021398 | Radiation Therapy and Chemotherapy Followed by Surgery and Combination Chemotherapy in Treating Patients With Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04390399 | Combination Immunotherapy Plus Standard-of-Care Chemotherapy Versus Standard-of-Care Chemotherapy for the Treatment of Locally Advanced or Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00016276 | Combination Chemotherapy, Surgery, and Radiation Therapy With or Without Dexrazoxane and Trastuzumab in Treating Women With Stage III or Stage IV Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04263584 | Copanlisib in Combination With Rituximab and CHOP Chemotherapy in Patients With Previously Untreated DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01075464 | A Study of the Safety and Pharmacology of MEGF0444A in Combination With Bevacizumab With or Without Paclitaxel in Patients With Locally Advanced or Metastatic Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT03933319 | Phase Ⅱ Study of Pegylated Liposomal Doxorubicin(PLD)Plus Trastuzumab in HER-2 Positive Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003013 | Chemotherapy Plus Surgery in Treating Women With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00429299 | Wkly Taxol x 12 | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00632333 | Combination of Chemoradiation Therapy and Epitope Peptide Vaccine Therapy in Treating Patients With Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT05390710 | PhI to Solid Tumors and PhII to Locally Advanced or mTNBC | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00290732 | Liposomal Doxorubicin Before Mastectomy in Treating Women With Invasive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00209703 | Feasibility Study of mFOLFOX6 in Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00069108 | A Study of Xeloda (Capecitabine) in Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02771743 | Response-based Treatment of High-risk Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT00770224 | S0801 Iodine I 131 Tositumomab, Rituximab, and Combination Chemotherapy in Previously Untreated Stage II, Stage III, or Stage IV Follicular Non-Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02997358 | Study Comparing Efficacy of Doxorubicin With Trabectedin Followed by Trabectedin Versus Doxorubicine in Patients With Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00026130 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00295893 | Combination Chemotherapy With or Without Trastuzumab in Treating Patients With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00592501 | Proton Radiotherapy With Chemotherapy for Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT02186834 | Selinexor (KPT-330) and Liposomal Doxorubicin For Relapsed and Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00193297 | Topotecan Plus Paclitaxel and Carboplatin in the Initial Treatment of Advanced Ovarian and Primary Peritoneal Carcinoma | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02581501 | Prospective Phase I Study of GAX for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00005612 | Combination Chemotherapy Followed by Peripheral Stem Cell Transplantation in Treating Patients With Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02012088 | Clinical Trial to Evaluate R-COMP Versus R-CHOP in Newly Diagnosed Patients With Non-localised Diffuse Large B-cell Lymphoma (DLBCL)/Follicular Lymphoma Grade IIIb | lymphoma | Doxorubicin (NPC261012) | |
| NCT04937673 | The Neoadjuvant Treatment of Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01978184 | Randomized Phase II Trial of Pre-Operative Gemcitabine and Nab Paclitacel With or With Out Hydroxychloroquine | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00400179 | A Safety and Efficacy Study in Patients With Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02116530 | Antiemetic Therapy With or Without Olanzapine in Preventing Chemotherapy-Induced Nausea and Vomiting in Patients With Cancer Receiving Highly Emetogenic Chemotherapy | Nausea and vomiting | Doxorubicin (NPC261012) | |
| NCT00165087 | Treatment of Childhood Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00887575 | Neoadjuvant Sunitinib With Paclitaxel/Carboplatin in Patients With Triple-Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00394082 | ABI-007 In Combination With Bevacizumab in Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00477412 | Bortezomib, Rituximab and Combination Chemotherapy in Treating Participants With Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00593567 | Safety and Efficacy of an Antibiotic Sponge in Diabetic Patients With a Mild Infection of a Foot Ulcer | diabetic foot | Gentamicin (NPC471628) | |
| NCT00401674 | MITO-5: Weekly Carboplatin and Paclitaxel as First Line Chemotherapy for Elderly Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00786006 | Randomized Phase II Study of FOLFOX Versus FOLFIRI.3 in Gemcitabine-refractory Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03391843 | Neoadjuvant Chemotherapy Combined With Cetuximab for EGFR Wild Type Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00334763 | Radiation Therapy, Chemotherapy, and Bevacizumab in Treating Patients With Recurrent, Unresectable or Stage III or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02000050 | Phase II Study of Up-front Chemotherapy and Neo-adjuvant Short-course Radiotherapy for Resectable Rectal Carcinoma (COLORE) | rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00006120 | Docetaxel or Paclitaxel in Treating Women With Unresectable Locally Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00786552 | Pemetrexed + Paclitaxel in Patients With Recurrent/Advanced Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT02883062 | Carboplatin and Paclitaxel With or Without Atezolizumab Before Surgery in Treating Patients With Newly Diagnosed, Stage II-III Triple-Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03946969 | Sintilimab in Combination With Chemotherapy in Neoadjuvant Treatment of Potentially Resectable Esophageal Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00001384 | A Pilot Trial of AC (Adriamycin, Cyclophosphamide) Chemotherapy With G-CSF (Granulocyte Colony-Stimulating Factor) Followed by Infusional Taxol (Paclitaxel) as Adjuvant Treatment for High Risk Stage II and Stage III Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT01288261 | Paclitaxel and Bavituximab in Treating Patients With HER2-Negative Metastatic Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04164238 | Neoadjuvant Anti-PD-1 Antibody (Toripalimab) or Combined With Chemotherapy in HNSCC Patients | head and neck squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00198367 | Three Modalities of Treatment in Operable and Resectable Stage IIIA (T1-3, N2) Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00006248 | S0007 - Combination Chemotherapy in Treating Patients With Metastatic or Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT04379596 | Ph1b/2 Study of the Safety and Efficacy of T-DXd Combinations in Advanced HER2+ Gastric Cancer (DESTINY-Gastric03) | gastric cancer | Fluorouracil (NPC75844) | |
| NCT01862328 | Dose Escalation, Multi-arm Study of MLN4924 Plus Docetaxel, Gemcitabine, or Combination of Carboplatin and Paclitaxel in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04529772 | A Combination of Acalabrutinib With R-CHOP in Subjects With Previously Untreated Non-GCB DLBCL (ACE-LY-312) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00003732 | Combination Chemotherapy in Treating Patients With Stage II, Stage III, or Stage IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04227990 | Plinabulin iv Solution in Prevention of TAC Induced Neutropenia | neutropenia | Doxorubicin (NPC261012) | |
| NCT00003696 | Combination Chemotherapy in Treating Patients Who Have Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01287741 | A Study of Obinutuzumab in Combination With CHOP Chemotherapy Versus Rituximab With CHOP in Participants With CD20-Positive Diffuse Large B-Cell Lymphoma (GOYA) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04138719 | Nab-paclitaxel Plus Carboplatin Versus Nab-paclitaxel Plus Epirubicin in the Neoadjuvant Therapy for Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03410030 | Trial of Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02937116 | A Dose Escalation Study of MM-398 Plus Irinotecan in Patients With Unresectable Advanced Cancer | cancer | Fluorouracil (NPC75844) | |
| NCT00352105 | Cisplatin, Fluorouracil, Iressa, and Radiation Therapy Patients With Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT03539328 | Study on Decitabine Plus Carboplatin Versus Physician's Choice Chemotherapy in Recurrent, Platinum-resistant Ovarian Cancer. | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02704520 | Magnetic Resonance Tumour Regression Grade as Biomarker for Stratified Management of Rectal Cancer Patients | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00716534 | Study of Carboplatin/Paclitaxel in Combination With ABT-869 in Subjects With Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00259675 | Alternating Cycles of Carboplatin/Gemcitabine and Carboplatin/Taxol for Advanced Stage NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00731380 | A Phase I Trial of Abraxane, Cisplatin and 5-Fluorouracil Along With Chemoradiotherapy in Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00486668 | A Study of AC Followed by a Combination of Paclitaxel Plus Trastuzumab or Lapatinib or Both Given Before Surgery to Patients With Operable HER2 Positive Invasive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00881101 | Clinical Study of Liposomal Paclitaxel in Chinese Patients | neoplasm | Paclitaxel (NPC208553) | |
| NCT02024191 | The Role of Glutamine for Preventing Oxaliplatin-Induced Peripheral Neuropathy | peripheral neuropathy | Glutamine (NPC197087) | |
| NCT02308553 | Efficacy and Safety of Nintedanib Combined With Paclitaxel Chemotherapy for Patients With BRAF wt Metastatic Melanoma | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT00900627 | Phase I/II AZD8931/Paclitaxel in Treatment of Advanced Solid Tumours (Phase I) and Advanced Breast Cancer (Phase II) | breast cancer | Paclitaxel (NPC208553) | |
| NCT04672928 | A Phase Ib/III Clinical Study to Evaluate the Efficacy and Safety of IBI318 in Combination With Paclitaxel Versus Placebo in Combination With Paclitaxel in Patients With Small Cell Lung Cancer Who Have Failed First-line or Above Chemotherapies | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04996004 | A Study to Learn About the Study Medicine (Called Ontorpacept or TTI-621) Given Alone and in Combination With Doxorubicin in People With Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT02452853 | HR Combined With FOLFOX4 for HCC With PVTT | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00189371 | Reinduction Chemotherapy Containing Carboplatin and Paclitaxel With or Without Epoetin Alpha in Recurrent Platinum Sensitive Ovarian Cancer, Cancer of the Fallopian Tube or Peritoneum | anemia (phenotype);ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00574587 | Trial for Locally Advanced Breast Cancer Using Vorinostat Plus Chemotherapy | breast cancer | Paclitaxel (NPC208553) | |
| NCT01324141 | Topical MTS-01 for Dermatitis During Radiation and Chemotherapy for Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT00324467 | Tailoring Treatment for B Cell Non-hodgkin's Lymphoma Based on PET Scan Results Mid Treatment | neoplasm of mature B-cells;non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03698227 | OlaReDo - Olaratumab and Rechallenge With Doxorubicin in Soft Tissue Sarcoma Patients | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00004913 | Combination Chemotherapy in Treating Patients With Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT01107626 | Bevacizumab or Pemetrexed Disodium Alone or In Combination After Induction Therapy in Treating Patients With Advanced Non-Squamous Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01679327 | Study of Bevacizumab Plus Chemotherapy in Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00822120 | S0816 Fludeoxyglucose F 18-PET/CT Imaging and Combination Chemotherapy With or Without Additional Chemotherapy and G-CSF in Treating Patients With Stage III or Stage IV Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00265850 | Cetuximab and/or Bevacizumab Combined With Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00349336 | A Study of Avastin (Bevacizumab) in Combination With XELOX or FOLFOX-4 in Patients With Metastatic Colorectal Cancer. | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00574236 | Phase II Trial of Bortezomib and Doxorubicin in Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02817113 | Study of NC-6004 in Combination With 5-FU and Cetuximab in Patients With Head and Neck Cancer | upper aerodigestive tract neoplasm | Fluorouracil (NPC75844) | |
| NCT04325425 | Chemotherapy For Metastatic Grade 3 Poorly Differentiated NEuroendocrine Carcinoma Of GastroEnteroPancreatic And Unknown Primary | neuroendocrine carcinoma | Fluorouracil (NPC75844) | |
| NCT00052312 | Doxorubicin and Cisplatin With or Without Paclitaxel in Treating Patients With Locally Advanced, Metastatic, and/or Relapsed Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00960297 | Preoperative Chemotherapy and Bevacizumab in Patients With Stage IB (>4 cm), II, or Select Stage III NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00450385 | Genes in Predicting Outcome of Patients With DLBCL Treated With Rituximab and Combination Chemotherapy (R-CHOP) | lymphoma | Doxorubicin (NPC261012) | |
| NCT03502343 | Efficacy and Safety of Modified Nab-Paclitaxel Plus Gemcitabine Chemotherapy for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03197571 | QUILT-3.048: NANT Urothelial Cancer Vaccine: Combination Immunotherapy in Subjects With Urothelial Cancer Who Have Progressed on or After Chemotherapy and PD-1/PD-L1 Therapy | urothelial carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00779714 | Comparative Study of Individualized Sensitivity-Directed Chemotherapy Versus DTIC | melanoma | Paclitaxel (NPC208553) | |
| NCT04506138 | Camrelizumab in Combination With Neoadjuvant Chemotherapy for Resectable Thoracic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00329641 | Sorafenib, Carboplatin, and Paclitaxel in Treating Patients With Stage IV Melanoma of the Eye | melanoma | Paclitaxel (NPC208553) | |
| NCT00540514 | Albumin-bound Paclitaxel (ABI-007) for Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00553358 | Phase II Trial to Compare the Safety of Two Chemotherapy Plus Trastuzumab Regimens as Adjuvant Therapy for HER2-positive Breast Cancer (Study P05048) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00088088 | STA-4783 in Combination With Paclitaxel and Carboplatin for the Treatment of Chemotherapy Naive Patients With Stage IIIB/IV Non Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00486668 | A Study of AC Followed by a Combination of Paclitaxel Plus Trastuzumab or Lapatinib or Both Given Before Surgery to Patients With Operable HER2 Positive Invasive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00235898 | Clinical Trial in Patients With Metastatic Colorectal Cancer | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT00002854 | High-Dose Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Advanced Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT00849472 | Phase II Lapatinib Plus Nab-Paclitaxel As First And Second Line Therapy In her2+ MBC | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT05217069 | A Multicenter Prospective Phase II Study of Modified FOLFIRINOX for 1st Line Treatment for Advanced Urachus Cancer | cancer | Fluorouracil (NPC75844) | |
| NCT05262335 | Anlotinib Plus Chemotherapy as First-line Therapy for Gastrointestinal Tumor Patients With Unresectable Liver Metastasis (ALTER-G-001) | neoplasm | Paclitaxel (NPC208553) | |
| NCT00062062 | Gefitinib With or Without Carboplatin and Paclitaxel in Treating Older Patients With Unresectable or Metastatic Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00072215 | Cisplatin and Ifosfamide Combined With Either Paclitaxel or Vinblastine in Treating Men With Progressive or Recurrent Metastatic Germ Cell Tumors | testicular neoplasm | Paclitaxel (NPC208553) | |
| NCT01493505 | A Study of MM-121 With Paclitaxel in Platinum Resistant/ Refractory Advanced Ovarian Cancers | fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00715208 | Phase 2 Study of VELCADE (Bortezomib) in Patients With Relapsed Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02429622 | Simultaneous Integrated Boost Radiotherapy and Concurrent Chemotherapy for Locally Advanced Esophageal Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT00619541 | Study of Sorafenib and Infusional 5-Fluorouracil in Advanced Hepatocellular Carcinoma (HCC) | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00054587 | Combination Chemotherapy With or Without Trastuzumab in Treating Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00986674 | Carboplatin and Paclitaxel Combined With Cetuximab and/or IMC-A12 in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02292979 | Brentuximab Vedotin Associated With Chemotherapy in Untreated Patients With Hodgkin Lymphoma. | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01622361 | Premenopausal Patient With Hormone Responsive, HER2 Negative, Lymph Node Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01822613 | Study of Safety & Efficacy of the Combination of LJM716 & BYL719 in Patients With Previously Treated Esophageal Squamous Cell Carcinoma (ESCC) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01655693 | Efficacy and Safety Doxorubicin Transdrug Study in Patients Suffering From Advanced Hepatocellular Carcinoma | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT04794699 | Study of IDE397 in Participants With Solid Tumors Harboring MTAP Deletion | neoplasm | Paclitaxel (NPC208553) | |
| NCT00251472 | A Phase II Trial of Abraxane™ Given Weekly as a Single Agent in First-line Treatment of Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05462496 | Modulation of the Gut Microbiome With Pembrolizumab Following Chemotherapy in Resectable Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01055743 | The Effect of Fluorouracil Implants Regional Chemotherapy During the Surgical Treatment for Early Stage Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT01481194 | ACVDL Treatment for Patients With Newly Diagnosed Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00581360 | Phase II Trial of Doxorubicin and Bortezomib in Patients With Incurable Adenoid Cystic Carcinoma of the Head and Neck | adenoid cystic carcinoma | Doxorubicin (NPC261012) | |
| NCT02142322 | Perioperative mFOLFOX-6 in Locally Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00006374 | Combination Chemotherapy in Treating Patients With Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01279291 | Study of Anti-HB-EGF Antibody KHK2866 in Subjects With Advanced Solid Tumors and Ovarian Cancer | Fallopian Tube Carcinoma;peritoneal neoplasm | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT02767557 | Study of Nab-Paclitaxel and Gemcitabine With or Without Tocilizumab in Pancreatic Cancer Patients | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01862081 | A Dose-escalation Study to Assess the Safety, Tolerability, and Pharmacokinetics of GDC-0032 in Combination With Docetaxel or With Paclitaxel in Patients With HER2-negative Locally Recurrent or Metastatic Breast Cancer or Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05455918 | Paclitaxel/Ifosfamide/Cisplatin Chemotherapy for High Risk Pediatric Germ Cell Tumor | childhood germ cell tumor | Paclitaxel (NPC208553) | |
| NCT01148446 | R-CHOP Versus R-mini-CEOP in Elderly Patients(>65)With DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04974996 | A Study to Evaluate the Tolerability, Safety, Pharmacokinetics, and Antitumor Activity of Loncastuximab Tesirine in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Participants With Previously Untreated Diffuse Large B-cell Lymphoma (LOTIS-8) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03126812 | Ribociclib (Ribociclib (LEE-011)) With Platinum-based Chemotherapy in Recurrent Platinum Sensitive Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT02701673 | Belinostat Combined With Azacitidine/Gemcitabine/Busulfan/Melphalan With Autologous Stem-Cell Transplantation in Refractory or Relapsed Lymphoma | lymphoma | Glutamine (NPC197087) | |
| NCT02420717 | Ruxolitinib Phosphate or Dasatinib With Chemotherapy in Treating Patients With Relapsed or Refractory Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00616031 | Paclitaxel and Carboplatin With or Without Nitroglycerin in Treating Patients With Previously Untreated Stage III or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00861120 | Panitumumab and Pegylated Liposomal Doxorubicin for Platinum-Resistant Epithelial Ovarian Cancer With KRAS Wild-type | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00041262 | Combination Chemotherapy in Treating Patients With Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT03246074 | QUILT-3.051: NANT Ovarian Cancer Vaccine: Combination Immunotherapy in Subjects With Epithelial Ovarian Cancer Who Have Progressed on or After Standard-of-care (SoC) Therapy | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00307255 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Gemcitabine in Treating Patients With Advanced Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02762474 | Trial of Concurrent Chemoradiotherapy for Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01457846 | Efficacy and Safety of AZD4547 Versus Paclitaxel in Patients With Advanced Gastric or Gastro-oesophageal Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01998347 | Paclitaxel Liposome and Cisplatin as First-line Chemotherapy in Patients With Metastatic Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03328234 | Radiotherapy With or Without Concurrent Chemotherapy for Extensive Lymphatic Metastasis of Esophageal Cancer - 3JECROG P-03 | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00287989 | Erlotinib, Paclitaxel, and Carboplatin in Treating Patients With Stage III, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00033553 | Combination Chemotherapy and Computer-Planned Radiation Therapy in Treating Patients With Unresectable Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03033914 | A(B)VD Followed by Nivolumab as Frontline Therapy for Higher Risk Patients With Classical Hodgkin Lymphoma (HL) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03003520 | A Study of Durvalumab in Combination With R-CHOP or Lenalidomide Plus R-CHOP in Previously Untreated High-Risk Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01712490 | A Frontline Therapy Trial in Participants With Advanced Classical Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00201435 | Wkly Taxol x 12 | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03109158 | NC-6004 With 5-FU and Cetuximab for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT02732015 | Rolapitant Hydrochloride in Preventing Nausea/Vomiting in Patients With Sarcoma Receiving Chemotherapy | sarcoma | Doxorubicin (NPC261012) | |
| NCT01763671 | Paclitaxel-bevacizumab in Advanced Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00971945 | Rollover Study of Weekly Paclitaxel (BMS-181339) in Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05329766 | A Phase Ib/II Study of AK104 and AK117 in Combination With or Without Chemotherapy in Advanced Malignant Tumors | cancer | Fluorouracil (NPC75844) | |
| NCT05008224 | Study of Safety and Efficacy of Pembrolizumab and Chemotherapy in Participants With Newly Diagnosed Classical Hodgkin Lymphoma (cHL) (MK-3475-C11/KEYNOTE-C11) | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00003387 | Carboplatin, Paclitaxel, and Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Removed During Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT03168607 | Study to Evaluate the Efficiency of FOLFIRI as Seconde-line Chemotherapy in Neuroendocrine Carcinoma | neuroendocrine carcinoma | Fluorouracil (NPC75844) | |
| NCT00686959 | Chemotherapy and Radiation in Treating Participants With Stage 3 Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00008021 | Monoclonal Antibody Therapy, Chemotherapy, and Peripheral Stem Cell Transplantation in Treating Patients With Refractory Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00596830 | Carboplatin And Paclitaxel With Or Without CP-751, 871 (An IGF-1R Inhibitor) For Advanced NSCLC Of Squamous, Large Cell And Adenosquamous Carcinoma Histology | large cell lung carcinoma;squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04138212 | Neoadjuvant Chemotherapy Versus Neoadjuvant Chemoradiotherapy for Resectable Locally Advanced Esophageal Cancer (HCHTOG1903) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00613457 | Combination Chemotherapy Based on Risk of Relapse in Treating Young Patients With Acute Lymphoblastic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT00813137 | FOLFOX4 Combined With Endostar in Patients With Advanced Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT01688791 | Weekly Paclitaxel, 5FU+Irinotecan or Carboplatin+Paclitaxel in Subjects With Advanced / Metastatic Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT02262897 | The Efficacy and Safety of Nab-paclitaxel in Pretreated Patients With Extensive Disease of Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01008150 | Phase II Randomized Trial Evaluating Neoadjuvant Therapy With Neratinib and/or Trastuzumab Followed by Postoperative Trastuzumab in Women With Locally Advanced HER2-positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00006004 | Comparison of Two Combination Chemotherapy Regimens in Treating Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003035 | Doxorubicin and Paclitaxel in Treating Women With Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00482391 | Doxorubicin and Cyclophosphamide Followed by Paclitaxel, Trastuzumab, and Lapatinib in Treating Patients With HER2/Neu-Overexpressed Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04001829 | Docetaxel or Paclitaxel in Reducing Chemotherapy-Induced Peripheral Neuropathy in African American Patients With Stage I-III Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03883802 | Foxy-5 as Neo-Adjuvant Therapy in Subjects With Wnt-5a Low Colon Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00479817 | Phase 2 AMG 386 in Comb. Paclitaxel for Subjects With Advanced Recurrent Epithelial Ovarian or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00147680 | Uterine Papillary Serous Cancer (UPSC) Trial | uterine cancer | Paclitaxel (NPC208553) | |
| NCT02897050 | Trial of Neoadjuvant Docetaxel ± Metronomic Capecitabine/CTX Followed by FEC in Women With Operable Triple Negative Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT03013218 | A Study of Evorpacept (ALX148) in Patients With Advanced Solid Tumors and Lymphoma (ASPEN-01) | non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT00733408 | Nab-Paclitaxel and Bevacizumab Followed By Bevacizumab and Erlotinib in Metastatic Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00646607 | FOLFOX-4 3months Versus 6 Months and Bevacizumab as Adjuvant Therapy for Patients With Stage II/III Colon Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00003733 | Sequential Chemotherapy in Treating Patients With Residual Disease Following Surgery for Stage IIB, Stage III, or Stage IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02117024 | A Phase 2 Study of Viagenpumatucel-L (HS-110) in Patients With Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05166772 | Donafenib Combine With Sintilimab and HAIC (Hepatic Artery Infusion Chemotherapy,HAIC)in the First-line Treatment of Unresectable Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00570531 | Phase II Trial for Patients With Loco-Regional Esophageal Carcinoma | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00146549 | Trastuzumab in Combination With Vinorelbine or Taxane-Based Chemotherapy in Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03732508 | SHR-1316 in Combination With Chemotherapy in Patients With Esophageal Squamous Cell Cancer | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00212589 | Patients Preference for Oral or i.v. Therapy | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00009997 | Trastuzumab and Chemotherapy Followed by Surgery and Combination Chemotherapy in Treating Women With Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00849264 | Comprehensive Treatment for Different Types of Tumor Thrombi in the Portal Vein for Hepatocellular Carcinoma Patients | thrombotic disease;hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02631109 | L-DEP Regimen as a Salvage Therapy for Refractory Epstein Barr Virus-induced Hemophagocytic Lymphohistiocytosis | hemophagocytic syndrome | Doxorubicin (NPC261012) | |
| NCT02225652 | A Phase II Study of Dose Density Regimen With Fluorouracil, Epirubicin and Cyclophosphamide at Days 1, 4 Every 14 Days With Filgrastim Support Followed by Weekly Paclitaxel in Women With Primary Breast Cancer. | breast cancer | Fluorouracil (NPC75844) | |
| NCT03994107 | Pegylated Liposomal Doxorubicin Plus Albumin-Bound Paclitaxel and Trastuzumab in HER-2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00258960 | Caelyx, Cyclophosphamide and Herceptin in Patients With Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00009828 | Paclitaxel Combined With Fluorouracil-Uracil and Leucovorin in Treating Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01354522 | TAC Versus TCX as Adjuvant Treatment for Node-Positive Her2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04247126 | A Study of SY 5609, a Selective CDK7 Inhibitor, in Advanced Solid Tumors | small cell lung carcinoma;pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00311636 | Triptorelin in Preventing Early Menopause in Premenopausal Women Who Are Receiving Chemotherapy for Stage I, Stage II, or Stage III Breast Cancer That Has Been Removed By Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT00874848 | Efficacy/Safety of Imprime PGG With Cetuximab & Paclitaxel/Carboplatin Therapy in Pts With Untreated Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01493843 | Safety and Efficacy of Carboplatin/Paclitaxel and Carboplatin/Paclitaxel/Bevacizumab With and Without Pictilisib in Previously Untreated Advanced or Recurrent Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01110603 | A Study of MK-4827 in Combination With Standard Chemotherapy in Participants With Advanced Solid Tumors (MK-4827-008 AM1) | cancer | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT03971409 | Avelumab With Binimetinib, Sacituzumab Govitecan, or Liposomal Doxorubicin in Treating Patients With Stage IV or Unresectable, Recurrent Triple Negative Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT00955305 | Paclitaxel, Carboplatin, and Bevacizumab With or Without Cixutumumab in Treating Patients With Stage IV or Recurrent Non-small Cell Lung Cancer | large cell lung carcinoma;lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04047004 | Adjuvant PIPAC in Gastric Cancer Patients | gastric adenocarcinoma | Doxorubicin (NPC261012) | |
| NCT05095467 | HIPEC-AS in Patients With Peritoneal Metastasis of the Stomach or Esophagogastric Junction | stomach neoplasm | Paclitaxel (NPC208553) | |
| NCT01851733 | MRI-Guided Laser Surgery and Doxorubicin Hydrochloride in Treating Patients With Recurrent Glioblastoma Multiforme | glioblastoma multiforme | Doxorubicin (NPC261012) | |
| NCT01871766 | Risk-Adapted Focal Proton Beam Radiation and/or Surgery in Patients With Low, Intermediate and High Risk Rhabdomyosarcoma Receiving Standard or Intensified Chemotherapy | rhabdomyosarcoma | Doxorubicin (NPC261012) | |
| NCT02961816 | Panobinostat Combined With High-Dose Gemcitabine/Busulfan/Melphalan With Autologous Stem Cell Transplant for Patients With Refractory/Relapsed Lymphoma | lymphoma | Glutamine (NPC197087) | |
| NCT02560038 | Trial of Paclitaxel Plus Gemcitabine and Cisplatin in Bladder Cancer | bladder tumor | Paclitaxel (NPC208553) | |
| NCT03387592 | CAPTEM or FOLFIRI as SEcond-line Therapy in NEuroendocrine CArcinomas | neuroendocrine carcinoma | Fluorouracil (NPC75844) | |
| NCT05033522 | Immunotherapy for Advanced Liver Cancer | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT05051891 | A Randomized, Open-label, Multi-center, Phase III Study of Orelabrutinib in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) vs. R-CHOP Alone in Patients With Treatment-naїve Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02250326 | Safety and Efficacy Study of Nab®-Paclitaxel With CC-486 or Nab®-Paclitaxel With Durvalumab, and Nab®-Paclitaxel Monotherapy as Second/Third-line Treatment for Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00228319 | Treatment of Newly Diagnosed Ovarian Cancer With Antioxidants | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00110695 | Therapy With Abraxane and 5-Fluorouracil, Epirubicin, Cyclophosphamide (FEC) for Patients With Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04047862 | Study of Ociperlimab (BGB-A1217) in Combination With Tislelizumab in Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00951665 | A Study of Trastuzumab Emtansine, Paclitaxel, and Pertuzumab in Patients With HER2-Positive, Locally Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00533949 | High-Dose or Standard-Dose Radiation Therapy and Chemotherapy With or Without Cetuximab in Treating Patients With Newly Diagnosed Stage III Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00497497 | A Study of PRO95780 in Combination With Cetuximab and Irinotecan Chemotherapy or the FOLFIRI Regimen With Bevacizumab in Patients With Previously Treated Metastatic Colorectal Cancer (APM4187g) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02570893 | A Phase III Trail of Adjuvant Chemoradiotherapy Versus Adjuvant Radiotherapy in Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04781413 | Nab-PTX Plus S-1 and Sintilimab as Adjuvant Therapy in Patients With Stage IIIC Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00000758 | A Phase III Randomized Trial of Topical Vaginal Fluorouracil (5-Fluorouracil, 5-FU) Maintenance Therapy Versus Observation After Standard Treatment for High-Grade Cervical Dysplasia in HIV-Infected Women | dysplasia of cervix | Fluorouracil (NPC75844) | |
| NCT00404066 | Phase 2 Neoadjuvant Doxorubicin and Cyclophosphamide -> Docetaxel With Lapatinib in Stage II/III Her2Neu+ Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01572038 | LUX-Breast 2 | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02182245 | Dose Escalation Study of BIBF 1120 in Patients With Advanced Gynaecological Malignancies | Genital neoplasm, female | Paclitaxel (NPC208553) | |
| NCT02711137 | Open-Label Safety and Tolerability Study of INCB057643 in Subjects With Advanced Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT00007904 | Adjuvant Stage 2-3A Breast Cancer With Positive Lymph Nodes | breast cancer | Paclitaxel (NPC208553) | |
| NCT05075460 | Tucidinostat, Azacitidine Combined With CHOP Versus CHOP in Patients With Untreated Peripheral T-cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003298 | Chemotherapy, Surgery, and Radiation Therapy in Treating Patients With Gastric Cancer | gastric cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT01605344 | Crossover Evaluation of Effect of Atorvastatin on PK of Irinotecan in CRC Patients Receiving FOLFIRI | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT04711161 | First-in-Human Evaluation of GRN-300 in Subjects With Recurrent Ovarian, Primary Peritoneal, and Fallopian Tube Cancers. | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT02350517 | Endostar Combined With Paclitaxel and Nedaplatin in Treating Patients With Recurrent or Metastatic Esophageal Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00001498 | A Pilot Trial of Sequential Chemotherapy With Antimetabolite Induction, High-Dose Alkylating Agent Consolidation With Peripheral Blood Progenitor Cell Support, and Intensification With Paclitaxel and Doxorubicin for Patients With High-Risk Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02670317 | Phase II Study About Combination CHOP-21, Obinutuzumab and Ibrutinib in Untreated Young High Risk DLBCL Patients. | lymphoma | Doxorubicin (NPC261012) | |
| NCT01478594 | A Study Combining mFOLFOX6 With Tivozanib or Bevacizumab in Patients With Metastatic Colorectal Cancer as First Line Therapy | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01328236 | Bortezomib in Combination With Liposomal Doxorubicin and Dexamethasone to Treat Plasma Cell Leukemia | plasma cell leukemia;multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00184002 | Doxorubicin (Doxil) Combined With Rituxan, Cyclophosphamide, Vincristine and Prednisone in Newly Diagnosed Aggressive Non-Hodgkin's Lymphomas | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00017095 | Biomarker (p53 Gene) Analysis and Combination Chemotherapy Followed by Radiation Therapy and Surgery in Treating Women With Large Operable or Locally Advanced or Inflammatory Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT05272696 | Pembrolizumab and Induction Chemotherapy in Locally Advanced HNSCC | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00531687 | Trial of Paclitaxel, Gemcitabine and Cisplatin in Patients With Relapsing Germ Cell Cancer | testicular carcinoma | Paclitaxel (NPC208553) | |
| NCT00881816 | Dose Escalation Study of Liposomal Paclitaxel Plus Capecitabine in Chinese Patients With Advanced Gastric Carcinoma | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT01755897 | A Multicenter, Prospective, Randomized Trial of Adjuvant Chemotherapy for Early-Stage Cervical Cancer Patients | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00154726 | Paclitaxel-HDFL for Locally Advanced and Recurrent/Metastatic Gastric Cancers | gastric cancer | Paclitaxel (NPC208553) | |
| NCT05170438 | Lenvatinib Plus Paclitaxel for Patients With Advanced Biliary Tract Cancer Who Failed to Gemcitabine-based Treatment | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT02924909 | Phase II Trial of Neoadjuvant Treatment and Minimal Invasive Surgery for Esophageal and GEJ Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04043494 | International Cooperative Treatment Protocol for Children and Adolescents With Lymphoblastic Lymphoma | lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00394251 | Study of Dose-dense Adriamycin Plus Cytoxan (AC) Followed by Either ABI-007 (Abraxane) or Taxol With Bevacizumab as Adjuvant Therapy for Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01454180 | Study of Individualized Selection of Chemotherapy in Patients With Advanced Pancreatic Carcinoma According to Therapeutic Targets | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01704287 | Study of Pembrolizumab (MK-3475) Versus Chemotherapy in Participants With Advanced Melanoma (MK-3475-002/P08719/KEYNOTE-002) | melanoma | Paclitaxel (NPC208553) | |
| NCT04647344 | A Study of AK104 in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05239741 | Study of Pembrolizumab (MK-3475) Versus Chemotherapy in Chinese Participants With Stage IV Colorectal Cancer (MK-3475-C66) | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00193596 | Gemcitabine/Irinotecan/ZD1839 vs Paclitaxel/Carboplatin/Etoposide/ZD1839 in Carcinoma of Unknown Primary Site | neoplasm | Paclitaxel (NPC208553) | |
| NCT00140595 | ACVBP Plus Rituximab Versus CHOP Plus Rituximab in Patients With Diffuse Large B-cell Lymphoma and Age-adjusted IPI of 1 | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02751918 | Phase Ib Study of Anetumab Ravtansine in Combination With Pegylated Liposomal Doxorubicin in Patients With Recurrent Mesothelin-expressing Platinum-resistant Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT00098982 | Bortezomib, Fluorouracil, Leucovorin, and Oxaliplatin in Treating Patients With Advanced or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02486601 | NAB-PACLITAXEL Plus FOLFOX as Perioperative Chemotherapy in Patients With Operable Oesogastric Adenocarcinoma | stomach neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01179217 | A Phase III Safety and Efficacy Study of L-Glutamine to Treat Sickle Cell Disease or Sickle βo-thalassemia | sickle cell anemia | Glutamine (NPC197087) | |
| NCT02766582 | Avelumab in Previously Untreated Patients With Epithelial Ovarian Cancer (JAVELIN OVARIAN 100) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05025033 | Chemotherapy Combined With Apatinib and PD-1 Antibody | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02689427 | Enzalutamide and Paclitaxel Before Surgery in Treating Patients With Stage I-III Androgen Receptor-Positive Triple-Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00146562 | Pegfilgrastim and Darbepoetin Alfa in Support of Adjuvant Chemotherapy for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00125788 | L-Glutamine Therapy for Sickle Cell Anemia and Sickle ß0 Thalassemia | Thalassemia;sickle cell anemia | Glutamine (NPC197087) | |
| NCT05081609 | A Study Evaluating Targeted Therapies in Participants Who Have Advanced Solid Tumors With Genomic Alterations or Protein Expression Patterns Predictive of Response | cancer | Paclitaxel (NPC208553) | |
| NCT04251533 | A Study of Novel Anti-cancer Agents in Patients With Metastatic Triple Negative Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04961970 | HAIC With FOLFOX Versus Systemic Chemotherapy With GP for Unresectable ICC | intrahepatic cholangiocarcinoma | Fluorouracil (NPC75844) | |
| NCT05371301 | Effect of Albumin-bound Paclitaxel Plus Carboplatin Compared With Paclitaxel Plus Carboplatin in Patients Receiving Neoadjuvant Chemotherapy for Advanced Ovarian, Fallopian Tube or Peritoneal Cancer: A Phase 2 Single Center Clinical Trial | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02661503 | HD21 for Advanced Stages | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00530101 | The Magnetic Resonance Imaging Evaluation of Doxorubicin Cardiotoxicity | breast cancer | Doxorubicin (NPC261012) | |
| NCT03272477 | Study of Cirmtuzumab and Paclitaxel for Metastatic or Locally Advanced, Unresectable Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04908566 | Immune Checkpoint Inhibitor PD-1 Antibody Combined With Chemotherapy in the Perioperative Treatment of Locally Advanced Resectable Gastric or Gastroesophageal Junction Adenocarcinoma | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01447706 | Pazopanib Hydrochloride, Paclitaxel, and Carboplatin in Treating Patients With Refractory or Resistant Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04858009 | Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Pancreatic Cancer and Peritoneal Metastasis | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05042128 | The ASCEND Study: Gemcitabine and Nab-Paclitaxel With CEND-1 or Placebo in Patients With Untreated Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02459457 | A Comparison of Paclitaxel-based Three Regimens Concurrent With Radiotherapy for Patients With Local Advanced Esophageal Cancer | squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00004054 | Hormone Therapy Plus Radiation Therapy With or Without Combination Chemotherapy in Treating Patients With Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00611962 | Phase II Study of Oxaliplatine-Paclitaxel in Patients With Metastatic Germ Cell Tumor Refractory to Cisplatin | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT03571321 | Ruxolitinib and Chemotherapy in Adolescents and Young Adults With Ph-like Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00719199 | Study of FOLFIRI Plus Cetuximab Plus IMO-2055 in Patients With Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04046016 | A Study Using Whole-body PET After Oral Microdose of 18F-labeled Liporaxel in Patients With Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT04879368 | RegoNivo vs Standard of Care Chemotherapy in AGOC | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02684227 | Enzalutamide, Carboplatin, and Paclitaxel in Treating Patients With Stage III-IV or Recurrent Endometrioid Endometrial Cancer | uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT00575406 | Multicentre Study to Determine the Cardiotoxicity of R-CHOP Compared to R-COMP in Patients With Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00261703 | Docetaxel in Head and Neck Cancer | upper aerodigestive tract neoplasm | Fluorouracil (NPC75844) | |
| NCT05007145 | PD-1 Inhibitor Combined With Neoadjuvant Chemotherapy in Subjects With Resectable Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03766607 | Trastuzumab Beyond Progression in HER2 Positive Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03861702 | Liposomal Irinotecan in Combination With Oxaliplatin, Leucovorin, and 5-fluorouracil for Patients With Locally Advanced Pancreatic Carcinoma: | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00046527 | Study of ABI-007 and Taxol in Patients With Metastatic Breast Cancer | metastasis;breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02001272 | LDE225 and Paclitaxel in Solid Tumors | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00002542 | Tamoxifen in Treating Women With High-Risk Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01481545 | Bevacizumab With Pelvic Radiotherapy And Primary Chemotherapy in Patients With Poor-Risk Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01704430 | Glutamine to Improve Outcomes in Cardiac Surgery | nosocomial infection | Glutamine (NPC197087) | |
| NCT00003943 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Metastatic Cancer. | lung cancer;carcinoma | Paclitaxel (NPC208553) | |
| NCT03635489 | Carboplatin-Paclitaxel-Bevacizumab vs Carbo-Pacli-Beva-Rucaparib vs Carbo-Pacli-Ruca, Selected According to HRD Status, in Patients With Advanced Ovarian, Primary Peritoneal and Fallopian Tube Cancer, Preceded by a Phase I Dose Escalation Study on Ruca-Beva Combination | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT05400915 | Two-Part, Phase Ib/II, Open Label, Single-Arm, Multi-center Study to Evaluate the Safety and Efficacy of Varlitinib in Combination With Weekly Paclitaxel in EGFR/HER2 Co-expressing Advanced or Metastatic Gastric Cancer Patients | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00820170 | Dasatinib In Combination With Weekly Paclitaxel For Patients With Metastatic Breast Carcinoma CA 180 194 | breast cancer | Paclitaxel (NPC208553) | |
| NCT04339738 | Testing the Addition of Nivolumab to Chemotherapy in Treatment of Soft Tissue Sarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT00449137 | Arsenic Trioxide, Fluorouracil, and Leucovorin in Treating Patients With Stage IV Colorectal Cancer That Has Relapsed or Not Responded to Treatment | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00450801 | R-MACLO-IVAM and Thalidomide in Untreated Mantle Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00373620 | A Safety and Efficacy of CCRT With Paclitaxel as Adjuvant Therapy to Post-Operative Advanced Endometrial Cancer Patients | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00404404 | ABI-007 and Bevacizumab in Treating Women With Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00854568 | Comparison Study of Doxorubicin Versus Epirubicin-induced Cardiotoxicity in Patients With DLBCL | lymphoma | Doxorubicin (NPC261012) | |
| NCT00002735 | SWOG-9451, Combination Chemo & RT For Patients With Stage III/Stage IV Cancer of the Hypopharynx or Tongue | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT02474173 | Onalespib and Paclitaxel in Treating Patients With Advanced Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00251095 | Study Of SU011248 Versus Chemotherapy For Patients With Previously Treated Triple Receptor Negative Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT05097248 | Camrelizumab in Combination With PLD and Losartan in Patients With TNBC Who Have Received ≦ 1 Line of Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT02428751 | R-CHOP Versus R-CDOP as First-line Treatment for Elderly Patients With Diffuse Large-B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02028806 | mFOLFIRINOX as First-Line Chemotherapy in Treating Chinese Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01239732 | Study of Intraperitoneal Carboplatin With IV Paclitaxel and Bevacizumab in Untreated Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00999700 | Induction Chemotherapy Followed by Cetuximab Plus Definitive Radiotherapy Versus Radiation Plus Cisplatin | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT02767375 | Hepatic Arterial Infusion Chemotherapy(HAIC) for Hepatoma After Resection | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02059499 | Imiquimod, Fluorouracil, or Observation in Treating HIV-Positive Patients With High-Grade Anal Squamous Skin Lesions | anal neoplasm;squamous cell intraepithelial neoplasia | Fluorouracil (NPC75844) | |
| NCT02476955 | Open-label Phase 1b Study of ARQ 092 in Combination With Anastrozole | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT03748134 | Sintilimab or Placebo With Chemotherapy in Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01652261 | Very Early FDG-PET/CT-response Adapted Therapy for Advanced Hodgkin Lymphoma (H11) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00319839 | Study of Albumin Bound-Paclitaxel for Treatment of Recurrent or Metastatic Head and Neck Cancer With Cetuximab | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02312804 | Ph Ib/BGJ398/Cervix and Other Solid Tumors | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT00005649 | Paclitaxel and Capecitabine in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00856492 | S0800, Nab-Paclitaxel, Doxorubicin, Cyclophosphamide, and Pegfilgrastim With or Without Bevacizumab in Treating Women With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00006786 | Combination Chemotherapy Plus Bevacizumab in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT05255471 | Antitumor Activity of Neoadjuvant Chemotherapy With or Without BINTRAFUSP ALFA in Patients With Metastatic Advanced Stage Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00063401 | Phase II Study in Patients With Epidermal Growth Factor Receptor (EGFR) + Advanced Stage Ovarian, Primary Peritoneal and Fallopian Tube Cancer | Fallopian Tube Carcinoma;ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00730925 | Single-arm Trial of BIBW 2992 (Afatinib) in Demographically and Genotypically Selected NSCLC Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00816855 | Phase Ⅱ Study of Neoadjuvant Chemotherapy Followed by Concurrent Chemoradiation for Stage Ⅲ Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00002631 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Cancer of the Esophagus | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT02033538 | Neoadjuvant Chemotherapy of Nanoparticle Albumin-bound Paclitaxel in Squamous Cell Carcinoma of Esophagus | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00142350 | A Study of 5-FUci Versus CPT-11 Plus CDDP Versus S-1 Alone in Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02753647 | Chidamide Plus R-CHOP in Elderly DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02298283 | Brentuximab Vedotin as Consolidation Treatment in Patients With Stage I/II HL and PET Positivity After 2 Cycles of ABVD | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00968253 | RAD001 Study in Treatment of Relapsed or Refractory Acute Lymphocytic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT04238988 | Carboplatin-Paclitaxel-Pembrolizumab in Neoadjuvant Treatment of Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02534337 | Gemcitabine Plus Oxaliplatin Versus Oxaliplatin Plus Fluorouracil/Leucovorin for Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00003446 | Chemotherapy in Treating Patients With Recurrent or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03161132 | Resistant Ovarian Cancer, Olaparib and Liposomal Doxorubicin | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00735696 | A Study of Ramucirumab (IMC-1121B) With Paclitaxel and Carboplatin in Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04594005 | CDK4/6 Tumor, Abemaciclib, Paclitaxel | neoplasm | Paclitaxel (NPC208553) | |
| NCT00001499 | Phase II Neoadjuvant Trial of a Continuous Infusion of Paclitaxel Plus Cisplatin Followed by Chest Radiotherapy for Patients With Stage III Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01769508 | Study of 5-FU, Oxaliplatin, & Lapatinib Combined With Radiation Therapy to Treat HER2 Positive Esophagogastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02064491 | Erlotinib Treatment Beyond Progression in EGFR Mutant NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00918203 | A Study of Paclitaxel/Carboplatin With or Without Olaratumab (IMC-3G3) in Previously Untreated Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00278109 | Partial Breast Irradiation With Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT01519804 | A Study of Onartuzumab (MetMAb) Versus Placebo in Combination With Paclitaxel Plus Platinum in Patients With Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02861222 | Myocet® in Children With Relapsed or Refractory Non-brainstem Malignant Glioma | malignant glioma | Doxorubicin (NPC261012) | |
| NCT00724386 | Concomitant Chemoradiotherapy With Weekly Paclitaxel and Vinorelbine and Granulocyte Colony Stimulating Factor (GCSF) Support in Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01336062 | Trial of ABI-007 Plus S-1 as Second-line Chemotherapy in Advanced Gastric Cancer Patients | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03367871 | Combination Pembrolizumab, Chemotherapy and Bevacizumab in Patients With Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04908787 | A Phase III Study of BD0801 Combined With Chemotherapy in Recurrent, Platinum-resistant Epithelial Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT04634539 | Trial of First-line L-glutamine With Gemcitabine and Nab-paclitaxel in Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05348811 | HAIC Combined With Donafenib and Sintilimab for Unresectable ICC | cholangiocarcinoma | Fluorouracil (NPC75844) | |
| NCT00642577 | A Study of Avastin (Bevacizumab) in Combination With Chemotherapy in Chinese Patients With Metastatic Colorectal Cancer. | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00003342 | Combination Chemotherapy in Treating Patients With Advanced Bladder or Kidney Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00003717 | Paclitaxel Plus Estramustine in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT05433597 | A Two-blinded, Multicentre, Phase II/III RCT of Concurrent Chemo-radiotherapy Combined or Not Combined With TNF as the Therapy for LA-NPC | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00139269 | Study of Docetaxel in Combination With Cisplatin and 5-Fluorouracil in Locally Advanced Squamous Cell Carcinoma of the Head and Neck | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00084565 | Paclitaxel, Topotecan, and Estramustine in Treating Patients With Metastatic Hormone-Refractory Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT04617067 | Paricalcitol Trial: Phase II, Open Label Clinical Trial of Paricalcitol in Combination With Gemcitabine/ Nab-Paclitaxel Therapy in Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00955240 | Radiation Therapy, Cisplatin, Fluorouracil, and Cetuximab in Treating Patients With Locally Advanced Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT04856137 | A Phase I/II Study of Diffuse Large B-cell Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT02364999 | A Comparative Study Of PF-06439535 Plus Paclitaxel-Carboplatin And Bevacizumab Plus Paclitaxel-Carboplatin Patients With Advanced Non-Squamous NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01008150 | Phase II Randomized Trial Evaluating Neoadjuvant Therapy With Neratinib and/or Trastuzumab Followed by Postoperative Trastuzumab in Women With Locally Advanced HER2-positive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003541 | Combination Chemotherapy, Radiation Therapy, and Peripheral Stem Cell Transplantation in Treating Patients With Stage III or Stage IV Mantle Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00268970 | Satraplatin and Paclitaxel in Patients With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04332822 | A Randomized, Multicenter, Phase III Trial Comparing Treatment With R-mini-CHOP With R-mini-CHP + Polatuzumab Vedotin in Patients With Diffuse Large Cell B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00758732 | Docetaxel/Carboplatin Versus Docetaxel/Caelyx in Pretreated Patients With Ovarian Carcinoma | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02508077 | FOLFIRI and Panitumumab in Treating Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00069901 | Phase II CT-2103/Carboplatin in Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00768859 | Trastuzumab in a Neo-adjuvant Regimen for HER2+ Breast Cancer - the TRAIN Study | breast cancer | Paclitaxel (NPC208553) | |
| NCT05015621 | A Phase 3 Study to Evaluate Surufatinib Plus Toripalimab in the Treatment of Advanced Neuroendocrine Carcinoma | neuroendocrine carcinoma | Fluorouracil (NPC75844) | |
| NCT00003087 | Combination Chemotherapy, Radiation Therapy and Surgery in Treating Patients With Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01047293 | RAD001, FOLFOX and Bevacizumab in Treatment of Colorectal Carcinoma | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00126243 | Efficacy of Rituximab and Cyclophosphamide, Doxorubicin, Vincristine, Prednisone (CHOP) in Patients With HIV Associated Non-Hodgkin's Lymphoma | Lymphoma, AIDS-Related | Doxorubicin (NPC261012) | |
| NCT00003777 | Surgery, Radiation Therapy, and Combination Chemotherapy in Treating Patients With Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT04921527 | A Phase III Study of BD0801 Combined With Chemotherapy in Recurrent, Platinum-resistant Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01489865 | ABT-888 With Modified FOLFOX6 in Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02499367 | Nivolumab After Induction Treatment in Triple-negative Breast Cancer (TNBC) Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT04852367 | PanDox: Targeted Doxorubicin in Pancreatic Tumours | pancreatic ductal adenocarcinoma | Doxorubicin (NPC261012) | |
| NCT00083057 | Gefitinib, Paclitaxel, and Radiation Therapy in Treating Patients With Advanced or Recurrent Squamous Cell Carcinoma of the Head and Neck | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00979082 | Glutathione in Preventing Peripheral Neuropathy Caused by Paclitaxel and Carboplatin in Patients With Ovarian Cancer, Fallopian Tube Cancer, and/or Primary Peritoneal Cancer | peripheral neuropathy | Paclitaxel (NPC208553) | |
| NCT01459887 | Study of Recombinant Human-Mouse Chimeric Anti-CD20 Monoclonal Antibody to Treat Non-hodgkin's Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00003379 | Radiation Therapy Plus Paclitaxel and Cisplatin in Treating Patients With Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03989310 | An Open-label, Phase I/II Study of the Pan-immunotherapy in Patients With Local Advanced/Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03192618 | The Impact on Recurrence Risk of Adjuvant Transarterial Chemoinfusion (TAI) for Patients With Hepatocellular Carcinoma And Microvascular Invasion (MVI) After Hepatectomy : A Random, Controlled, Stage III Clinical Trial. | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00146562 | Pegfilgrastim and Darbepoetin Alfa in Support of Adjuvant Chemotherapy for Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00001498 | A Pilot Trial of Sequential Chemotherapy With Antimetabolite Induction, High-Dose Alkylating Agent Consolidation With Peripheral Blood Progenitor Cell Support, and Intensification With Paclitaxel and Doxorubicin for Patients With High-Risk Breast Cancer | breast cancer | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT00080951 | Irinotecan, Fluorouracil, Leucovorin, and Oxaliplatin as First-Line Therapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00832299 | Neo-Adjuvant FOLFOX for Rectal Carcinoma | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00083538 | Study of Tumor Antigen-Pulsed Autologous Dendritic Cell Vaccination Administrated Subcutaneously or Intranodally | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00825201 | First Line Tx Stage III Ovarian Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00055822 | Combination Chemotherapy and Oblimersen in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00411138 | Randomized Trial of Radiation Therapy With or Without Chemotherapy for Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00749450 | Combination Chemotherapy After Surgery in Treating Patients With High-Risk Stage II or Stage III Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04481009 | A Study to Evaluate YH003 in Combination With Toripalimab (Anti-PD-1 mAb) in Subjects With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03316599 | Study of Gemcitabine, Nab-paclitaxel, and Ficlatuzumab (AV-299) in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00809341 | R-ICE and High-Dose Cyclophosphamide With PET/CT for Diffuse Large B-Cell Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04938583 | A Study Evaluating the Efficacy and Safety of Biomarker-Driven Therapies in Patients With Persistent or Recurrent Rare Epithelial Ovarian Tumors | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00988897 | Colorectal Cancer RECHALLENGE | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00617409 | To Immunize Patients With Extensive Stage SCLC Combined With Chemo With or Without All Trans Retinoic Acid | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00616967 | Carboplatin and Nab-Paclitaxel With or Without Vorinostat in Treating Women With Newly Diagnosed Operable Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02449655 | Trial of AZD5363 Plus Paclitaxel /AZD2014 Plus Paclitaxel in Biomarker Negative (PIK3CA/MEK/RAS/TP53/MET) Gastric Adenocarcinoma Patients as Second-line Chemotherapy | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00149201 | A Study of 5-FU Versus MTX+5-FU in Gastric Cancer With Peritoneal Metastasis | gastric cancer;metastasis | Fluorouracil (NPC75844) | |
| NCT00796120 | An Efficacy and Safety Study of Trabectedin Versus Doxorubicin-Based Chemotherapy in Participants With Translocation-Related Sarcomas (TRS) | sarcoma | Doxorubicin (NPC261012) | |
| NCT01839097 | Phase 1 Dose Finding Study of Belinostat for Treatment of Patients With Peripheral T-cell Lymphoma (PTCL) | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00416858 | Radiation Therapy and Combination Chemotherapy With or Without Surgery in Treating Patients With Locally Advanced Esophageal Cancer That Can Be Removed By Surgery | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00016133 | Vaccine Therapy in Treating Patients With Stage II or Stage III Colon Cancer That Has Been Removed During Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00521781 | Study of Abraxane Plus Hormonal Therapy as Initial Treatment of Unresectable or Metastatic Prostate Cancer | prostate adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02616601 | Multicenter, Double-blind, Placebo Controlled Comparing Test Fluorouracil Cream to Carac Cream in Actinic Keratosis | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT02226757 | Paclitaxel-trastuzumab in EGFR-mutated NSCLC Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02159820 | Intraperitoneal tgDCC-E1 and Intravenous Paclitaxel in Women With Platinum-Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04004871 | Induction Chemotherapy With Nab-paclitaxel, Cisplatin and Fluorouracil for Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00042861 | UCN-01, Fluorouracil, and Leucovorin in Treating Patients With Metastatic or Unresectable Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT05229497 | FOLFIRI + Cetuximab + Avelumab RAS Wild-type CRC | cancer | Fluorouracil (NPC75844) | |
| NCT00303771 | Combination Chemotherapy as First-Line Therapy in Treating Older Patients With Metastatic Colorectal Cancer That Cannot Be Removed By Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00050167 | Evaluation of Differing Taxanes/Taxane Combinations on the Outcome of Patients With Operable Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00294762 | Erlotinib (Tarceva) as a Single Agent or Intercalated With Combination Chemotherapy in Patients With EGFR Positive NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02993731 | A Study of Napabucasin Plus Nab-Paclitaxel With Gemcitabine in Adult Patients With Metastatic Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02106884 | Quality of Life in Locally Advanced or Metastatic Pancreatic Cancer Treated With Gemcitabine and Nab-paclitaxel | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00005059 | Carboplatin and Paclitaxel in Treating Older Patients With Metastatic or Recurrent Unresectable Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01983969 | Aza-SAHA-GBM With AutoSCT for Refractory Lymphoma | lymphoma;cancer | Glutamine (NPC197087) | |
| NCT03342352 | Nivolumab Plus Epacadostat in Combination With Chemotherapy Versus the EXTREME Regimen in Squamous Cell Carcinoma of the Head and Neck (CheckMate 9NA/ECHO-310) | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT01523977 | Everolimus With Multiagent Re-Induction Chemotherapy in Pediatric Patients With ALL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00468169 | Cetuximab (ERBITUX®) Added to Two Concurrent Chemoradiotherapy Platforms in Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT03797443 | High Dose Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) in Patients Who Have Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00004125 | Combination Chemotherapy in Treating Women With Stage II or Stage IIIA Breast Cancer That Has Spread to the Lymph Nodes | breast cancer | Paclitaxel (NPC208553) | |
| NCT00296621 | Effect of Oral Glutamine on Muscle Mass and Function in Duchenne Muscular Dystrophy | Duchenne muscular dystrophy | Glutamine (NPC197087) | |
| NCT05111860 | Neoadjuvant Bevacizumab + Chemotherapy Combined With Short-course Radiotherapy | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01324180 | Vincristine, Dexamethasone, Doxorubicin, and PEG-asparaginase (VPLD) and Metformin for Relapsed Childhood Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01263145 | MK2206 and Paclitaxel in Treating Patients With Locally Advanced or Metastatic Solid Tumors or Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04024462 | A Two-Arm Study to Evaluate the Pharmacokinetics, Efficacy, and Safety of Subcutaneous Administration of the Fixed-Dose Combination of Pertuzumab and Trastuzumab in Combination With Chemotherapy in Chinese Participants With HER2-Positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03829722 | Radiotherapy, Carboplatin/Paclitaxel and Nivolumab for High Risk HPV-related Head and Neck Cancer | oropharynx squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05088057 | Neoadjuvant Camrelizumab Plus Chemotherapy in Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01554059 | Neoadjuvant Chemoradiation With Bevacizumab for Chinese Rectal Cancer Patients | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01100944 | A Phase 1/2 Study of PXD101 (Belinostat) in Combination With Cisplatin, Doxorubicin and Cyclophosphamide in the First Line Treatment of Advanced or Recurrent Thymic, Malignancies | Thymic Carcinoma;Thymoma | Doxorubicin (NPC261012) | |
| NCT00193310 | Preoperative or Postoperative Therapy of Patients With Stages IB, II, IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01198548 | High-Dose Cholecalciferol in Treating Patients Receiving Combination Chemotherapy and Bevacizumab as First-Line Therapy For Metastatic Colorectal Cancer | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT00356811 | Combined Treatment of Cetuximab and Paclitaxel in Basal Like Breast Carcinoma | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00427570 | Fluorouracil, Semustine, and Vincristine Compared With BCG in Treating Patients With Dukes' B or Dukes' C Colon Cancer That Has Been Removed By Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00272987 | Comparison of Safety and Efficacy of TOCOSOL(R) Paclitaxel Versus Taxol(R) for Treatment of Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00367471 | Lapatinib Combined With Paclitaxel For Patients With First-Line ErbB2-Amplified Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02733380 | Chidamide Combined With VDDT Regimen in the Relapse and Refractory Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00054054 | S0216, Combination Chemotherapy and RT in Treating Patients With Stage III or Stage IV Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00368992 | S0536: Cetuximab, Paclitaxel, Carboplatin, and Bevacizumab in Treating Patients With Advanced Non-Small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05173987 | Study of Pembrolizumab (MK-3475) Versus Chemotherapy in Mismatch Repair Deficient (dMMR) Advanced or Recurrent Endometrial Carcinoma (MK-3475-C93/KEYNOTE-C93/GOG-3064/ENGOT-en15) | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT02367794 | A Study of Atezolizumab in Combination With Carboplatin + Paclitaxel or Carboplatin + Nab-Paclitaxel Compared With Carboplatin + Nab-Paclitaxel in Participants With Stage IV Squamous Non-Small Cell Lung Cancer (NSCLC) [IMpower131] | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03677141 | A Phase Ib/II Study Investigating the Safety, Tolerability, Pharmacokinetics, and Efficacy of Mosunetuzumab (BTCT4465A) in Combination With CHOP or CHP-Polatuzumab Vedotin in Participants With B-Cell Non-Hodgkin Lymphoma | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT00014599 | Paclitaxel in Treating Patients With Locally Advanced, Metastatic, or Recurrent Cancer of the Vulva | vulva cancer | Paclitaxel (NPC208553) | |
| NCT01843829 | A Feasibility Study of Chemo-radiotherapy to Treat Operable Oesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00052910 | Chemotherapy and Radiation Therapy After Surgery in Treating Patients With Stomach or Esophageal Cancer | esophageal cancer;gastric cancer | Fluorouracil (NPC75844) | |
| NCT01704690 | Combination Treatment of S-1 With Paclitaxel in Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT03493854 | A Study to Evaluate the Pharmacokinetics, Efficacy, and Safety of Subcutaneous Administration of the Fixed-Dose Combination of Pertuzumab and Trastuzumab in Combination With Chemotherapy in Participants With HER2-Positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00004164 | Combination Chemotherapy in Treating Patients With Stage IIB, Stage III, or Stage IV Cancer of the Nasopharynx | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT05290090 | ZR2 Followed by Immunochemotherapy in Elderly Patients With Newly-diagnosed DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02041481 | MEK Inhibitor MEK162 in Combination With Leucovorin Calcium, Fluorouracil, and Oxaliplatin in Treating Patients With Advanced Metastatic Colorectal Cancer | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT04188860 | Immunotherapy for Recurrent Cervical Cancer Refractory to Platinum-based Chemotherapy | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT04004234 | A Phase I/II Study of the Pan-immunotherapy in Patients With Local Advanced/Metastatic BTC | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT00960284 | Gemcitabine or Combination Chemotherapy Followed by Chemoradiation for Stage IB, II, or III Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03322969 | Receiving Modified Chemotherapy Followed With Radical Resection After Neoadjuvant Chemotherapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01566240 | Induction Chemotherapy Plus Chemoradiation as First Line Treatment for Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02051751 | Paclitaxel and BKM120 Before Surgery in Treating Patients With Stage II or III Estrogen Receptor-Positive and HER2-Negative Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00287937 | Vorinostat, Paclitaxel, and Carboplatin in Treating Patients With Advanced or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03340376 | Doxorubicin Alone Versus Atezolizumab Alone Versus Doxorubicin and Atezolizumab in Recurrent Cervical Cancer | cervical cancer | Doxorubicin (NPC261012) | |
| NCT00028860 | Combination Chemotherapy Following Surgery in Treating Patients With Urinary Tract Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00780039 | A Study to Evaluate the Safety and Efficacy of Caelyx in Combination With Carboplatin in Patients With Ovarian Cancer Recurrent Within Six to Twelve Months After Initial Carboplatin and Paclitaxel Chemotherapy (P03625) | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT02501902 | Dose-Escalation Study Of Palbociclib + Nab-Paclitaxel In mPDAC | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00083109 | Fluorouracil and Low-Dose Suramin as Chemosensitization in Treating Patients With Metastatic Renal Cell (Kidney) Cancer | renal cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00072007 | Cladribine and Rituximab as Remission Induction Therapy Followed By Rituximab and Stem Cell Mobilization in Treating Patients With CLL | leukemia | Doxorubicin (NPC261012) | |
| NCT04611724 | Neo-adjuvant Short Course Chemo-radiation Therapy in Locally Advanced Rectal Cancer Patients | cancer | Fluorouracil (NPC75844) | |
| NCT00401817 | Bevacizumab + CHOP-Rituximab in Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00003049 | Surgery Followed by Radiation Therapy and Chemotherapy in Treating Patients With Cancer of the Pancreas | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT04072263 | Apatinib and Etoposide Capsule Versus Weekly Paclitaxel in Patients With Platinum Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02607202 | A Study of Nab-paclitaxel-based Doublet as First Line Therapy in Patients With Cancer of Unknown Primary (CUP) | neoplasm | Paclitaxel (NPC208553) | |
| NCT02826837 | LEAC-102 for Advanced Colorectal Cancer | colorectal cancer | Fluorouracil (NPC75844) | |
| NCT00004173 | Oxaliplatin and Paclitaxel in Treating Patients With Metastatic or Unresectable Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT02483247 | A Safety, Tolerability, and Pharmacokinetics Study of MLN0128 as a Single Agent and in Combination With Paclitaxel in Adults With Advanced Nonhematologic Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT01413022 | FOLFIRINOX Plus PF-04136309 in Patients With Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma | pancreatic neoplasm | Fluorouracil (NPC75844) | |
| NCT04624984 | PD-1 Inhibitor or PD-1 Inhibitor Plus GVD for Relapsed/Refractory CHL | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT02682693 | Denosumab as an add-on Neoadjuvant Treatment (GeparX) | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00003018 | S9700 Combination Chemotherapy in Treating Patients With Stage II or Stage III Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00722137 | Study of the Combination of Rituximab, Cyclophosphamide, Doxorubicin, VELCADE, and Prednisone or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01889420 | Phase I Trial of Everolimus, Pomalidomide and Dexamethasone in Patients With Relapsed/Refractory Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT05203913 | Cisplatin, Nab-paclitaxel, Nivolumab With Radiotherapy After Resection of Non-Metastatic Muscle Invasive Bladder Cancer | urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT00660842 | Study Comparing Weekly Versus Every 3 Week Chemotherapy in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03497702 | Neo-adjuvant Chemotherapy With Letrozole in Patients With Estrogen Receptor Positive/HER-2 Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01015222 | Dasatinib, Bevacizumab, Paclitaxel in Patients With Advanced Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT04000295 | An Open-label, Phase I/II Study of the Pan-immunotherapy in Patients With Relapsed/Refractory Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04418895 | Neoadjuvant Therapy for Localized Rectal Adenocarcinoma | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00112918 | Combination Chemotherapy With or Without Bevacizumab in Treating Patients Who Have Undergone Surgery for High Risk Stage II or Stage III Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02414568 | Bendamustine Study in Classical Hodgkin Lymphoma Patients Over 60 Treated by Prednisone, Vinblastine and Doxorubicin | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT04539808 | NeoOPTIMIZE: Early Switching of mFOLFIRINOX or Gemcitabine/Nab-Paclitaxel Before Surgery for the Treatment of Resectable, Borderline Resectable, or Locally-Advanced Unresectable Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03152370 | Preoperative Radiotherapy and E7046 in Rectum Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT05497778 | A Phase 1b Study of Gemcitabine and Nab-paclitaxel in Combination With IM156 in Patients With Advanced Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03437070 | Trabectedin, Doxorubicin and Olaratumab in Patients With Metastatic or Recurrent Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT02948075 | Pembrolizumab, Paclitaxel, and Carboplatin in Patients With Advanced Stage Epithelial Ovarian Cancer (EOC). | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00299182 | Study of AMG 531 to Evaluate the Safety & Efficacy in Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04150575 | A Clinical Study to Evaluate Efficacy and Safety of HLX10 Combined With Albumin-Bound Paclitaxel in Patients With Advanced Cervical Cancer Who Have Progressive Disease or Intolerable Toxicity After First-Line Standard Chemotherapy | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01024712 | Paclitaxel, Carboplatin, and Gefitinib in Treating Patients With Advanced Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00373217 | Vaccine Therapy, Paclitaxel, and Carboplatin in Treating Patients Who Are Undergoing Surgery for Stage III or Stage IV Ovarian Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01120171 | Myocet Plus Endoxan for Older Patients With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01248403 | A Randomized, Double Blind Study Evaluating Paclitaxel With and Without RAD001 in Patients With Gastric Carcinoma After Prior Chemotherapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00151060 | Estramustine, Etoposide and Paclitaxel Treatment for Hormonally Responsive Adenocarcinoma of the Prostate | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03812783 | HAIC of FOLFOX Plus Sorafenib vs HAIC of FOLFIRINOX Plus Sorafenib for Advcanced HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00791947 | A Nordic Phase II Study of PTCL Based on Dose-intensive Induction and High-dose Consolidation With ASCT | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04426955 | Study of Camrelizumab (SHR-1210) in Combination With Concurrent Chemoradiotherapy in Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03943901 | Split-Dose R-CHOP for Older Adults With DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00088556 | Carboplatin, Paclitaxel and TLK286 (Telcyta) as First-Line Therapy in Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05024097 | A Phase II Study to Test the Efficacy of AB928 (Dual Adenosine Receptor Antagonist) and AB122 (a PD1 Checkpoint Inhibitor) in Combination With Short Course Radiotherapy and Consolidation Chemotherapy for Rectal Cancer. | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04289792 | Split-course SBRT for Borderline Resectable and Locally Advanced Pancreatic Cancer | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00003064 | Combination Chemotherapy and Peripheral Stem Cell Transplantation in Treating Patients With Recurrent or Persistent Epithelial Ovarian Cancer, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01767155 | Zoptarelin Doxorubicin (AEZS 108) as Second Line Therapy for Endometrial Cancer | endometrial cancer | Doxorubicin (NPC261012) | |
| NCT01730833 | Pertuzumab, Trastuzumab, and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With HER2-Positive Advanced Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00524303 | EndoTAG-1 in Triple Receptor Negative Breast Cancer Patients | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00186888 | Study of Treatment for Patients With Cancer of the Eye -Retinoblastoma | retinoblastoma | Doxorubicin (NPC261012) | |
| NCT04056949 | Efficacy and Safety of IBI308 and Paclitaxel/Albumin Paclitaxel for SCLC Patients Who Failed Etoposide Chemotherapy | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00258323 | Radiotherapy,Chemotherapy,Before and After Surgery in Advanced Esophageal or Gastroesophageal Junction Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT01244789 | Chemotherapy or Observation in Stage I-II Intermediate or High Risk Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02420314 | Pharmacological Ascorbate for Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00005870 | Nitrocamptothecin Compared With Other Chemotherapy in Treating Patients With Recurrent or Refractory Cancer of the Pancreas | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00567554 | Bevacizumab, Everolimus (RAD001), and Lapatinib as Neoadjuvant Chemotherapy Regimes for Primary Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00580073 | Phase II Trial of Neoadjuvant FOLFOX4 and Cetuximab for Localized Adenocarcinoma of Rectum | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00002523 | Radiation Therapy, Surgery, and Chemotherapy in Treating Patients With Rectal Cancer That Can Be Surgically Removed | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00378313 | A Study of Gemcitabine, Epirubicin, and Paclitaxel Combination Chemotherapy Given Before Surgery to Patients With Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05351346 | Genotype-guided Treatment in DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03595059 | A Study With ABBV-155 Alone and in Combination With Taxane Therapy in Adults With Relapsed and/or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00778102 | A Study of Avastin (Bevacizumab) in Combination With mFOLFOX-6 or FOLFOXIRI in Patients With Metastatic Colorectal Cancer. | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00248248 | DOXIL for Consolidation Therapy in Ovarian Cancer. | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT00839111 | Sorafenib and FOLFIRI Regimen in 2nd Colorectal Cancer (CRC) After Failure of Oxaliplatin Treatment | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00268398 | Combination Chemotherapy in Treating Patients With Colorectal Cancer and Resectable Metastases | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00657917 | Topical Treatment of Recalcitrant Ulcerative Old World Leishmaniasis With WR 279,396 | cutaneous Leishmaniasis | Gentamicin (NPC471628) | |
| NCT00334802 | Combination Chemotherapy of Gemcitabine and Paclitaxel for Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00603941 | A Phase 1/2 Study of CS7017, an Oral PPARγ Agonist, in Combination With Paclitaxel | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT01938833 | Romidepsin and Abraxane in Treating Patients With Metastatic Inflammatory Breast Cancer | inflammatory breast carcinoma;male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02228772 | Phase I Study of MLN 9708 in Addition to Chemotherapy for the Treatment of Acute Lymphoblastic Leukemia in Older Adults | B-cell acute lymphoblastic leukemia;lymphoblastic lymphoma;T-cell acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01570582 | TRINOVA-3: A Study of AMG 386 or AMG 386 Placebo in Combination With Paclitaxel and Carboplatin to Treat Ovarian Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03503786 | Carboplatin, Paclitaxel With or Without Avelumab in Advanced or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01579578 | Assess the Efficacy of AZD8931 in Combination With Paclitaxel Versus Paclitaxel Alone in Patients With Gastric Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT00003862 | Chemotherapy and Radiation Therapy in Treating Patients With Stomach Cancer | gastric cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT04990037 | A Study of the Safety and Tolerance of CAN04 in Combination With FOLFIRINOX in Subjects With Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01572324 | Study of Hepatic Arterial Infusion to Treat Biliary Tract Carcinomas | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT02648711 | Alternative Dosing for CRLX101(NLG207) Alone, With Avastin and With mFOLFOX6 in Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT00915018 | Study Evaluating Neratinib Plus Paclitaxel VS Trastuzumab Plus Paclitaxel In ErbB-2 Positive Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00193206 | Neo-adjuvant Gemcitabine, Epirubicin, ABI-007 (GEA) in Locally Advanced or Inflammatory Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02515448 | A Pharmacokinetic-pharmacodynamic Dose Comparison Study of 8 mg/kg of Inhaled or Parenteral Gentamicin in 12 Mechanically Ventilated Critically Ill Patients Treated for Ventilator-associated Pneumonia | ventilator-associated pneumonia | Gentamicin (NPC471628) | |
| NCT00360360 | Paclitaxel/Carboplatin Plus Bevacizumab/Erlotinib in the First Line Treatment of Carcinoma of Unknown Primary Site | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003001 | Combination Chemotherapy in Treating Patients With Recurrent or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02050009 | GANNET53: Ganetespib in Metastatic, p53-mutant, Platinum-resistant Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT04190745 | Toripalimab Combined With Apatinib Mesylate for the Treatment of Gastric Adenocarcinoma in a Prospective Randomized Multicenter Phase II Clinical Study | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00168883 | Study for Patients With Non Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03435250 | Study of AG-270 in Participants With Advanced Solid Tumors or Lymphoma With MTAP Loss | lymphoma | Paclitaxel (NPC208553) | |
| NCT02273713 | The Addition of Nab-paclitaxel (Abraxane) to First Line Treatment of Metastasized Oesophagogastric Carcinoma (ACTION) | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00005584 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00571740 | Cetuximab and Bevacizumab as First-Line Therapy Followed By Combination Chemotherapy and Bevacizumab With or Without Cetuximab as Second-Line Therapy in Treating Patients With Stage IV Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00787527 | SAHA + CHOP in Untreated T-cell Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02930902 | Pembrolizumab and Paricalcitol With or Without Chemotherapy in Patients With Pancreatic Cancer That Can Be Removed by Surgery | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01893801 | Nab-Pac+Cis+Gem in Pts w Previously Untreated Metastatic PDA | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02739529 | Study to Evaluate the Efficacy and Safety of Genexol-PM Once a Week for Gynecologic Cancer | female reproductive system neoplasm | Paclitaxel (NPC208553) | |
| NCT00390611 | Paclitaxel and Carboplatin With Or Without Sorafenib In The First-Line Treatment Of Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00728845 | Hydroxychloroquine, Carboplatin, Paclitaxel, and Bevacizumab in Recurrent Advanced Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03224221 | Apatinib Combined With Chemotherapy for Esophageal Squamous Cell Cancer After the Failure of Standard Treatment | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT02163291 | Study on Neoadjuvant Chemotherapy for Advanced Gastric Cancer | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT04749108 | Study Evaluating the Tailored Management of Locally-advanced Rectal Carcinoma | rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04021992 | GVD±R Regimen for ASCT-eligible Patients With Refractory/Relapsed DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00795899 | Taxol Epirubicin Cyclophosphamide Herceptin Neoadjuvant (TECHNO) | breast cancer | Paclitaxel (NPC208553) | |
| NCT04130854 | INNATE: Immunotherapy During Neoadjuvant Therapy for Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03646123 | Clinical Trial of Brentuximab Vedotin in Classical Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00254592 | Neoadjuvant Treatment of Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00533936 | Trastuzumab or Observation After Combination Chemotherapy and Trastuzumab in Treating Patients Undergoing Surgery for Stage II or Stage III Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01925612 | Study of Brentuximab Vedotin Combined With RCHOP or RCHP in Front-line Treatment of Patients With Diffuse Large B-cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03175939 | Elimination of Adjuvant Chemotherapy for Selected Stage II and III Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT01864109 | Irinotecan and Temozolomide in Combination With Existing High Dose Alkylator Based Chemotherapy for Treatment of Patients With Newly Diagnosed Ewing Sarcoma | Ewing sarcoma | Doxorubicin (NPC261012) | |
| NCT05238064 | Parsaclisib in Combination With CHOP in Participants With Previously Untreated PTCL | mature T-cell and NK-cell non-Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT01536223 | Efficacy Study of Neoadjuvant Chemotherapy With Chemoradiation Therapy for Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT02834975 | Ruxolitinib Phosphate, Paclitaxel, and Carboplatin in Treating Patients With Stage III-IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT04808323 | MRI-Guided Adaptive Radiation Therapy for Organ Preservation in Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00217425 | Bevacizumab and Combination Chemotherapy in Treating Patients With Peripheral T-Cell Lymphoma or Natural Killer Cell Neoplasms | lymphoma | Doxorubicin (NPC261012) | |
| NCT00180908 | Comparison of High-Dose Methotrexate (HDM) Plus Doxorubicin to HDM Plus Etoposide-Ifosfamide in Osteosarcoma Children | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT03240016 | Abraxane With Anti-PD1/PDL1 in Patients With Advanced Urothelial Cancer | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT00002772 | S9623, Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Women With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00448305 | Neoadjuvant Study With Chemotherapy, Lapatinib And Trastuzumab In Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01390584 | Chemotherapy Based on PET Scan in Treating Patients With Stage I or Stage II Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00514020 | Fluorouracil, Oxaliplatin, and Leucovorin in Treating Patients With Metastatic Stomach Cancer or Gastroesophageal Junction Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT05078827 | Therapeutic Equivalence of Fluorouracil Cream, 5% Compared With Fluorouracil 5% Topical Cream of MylanPharmaceuticals Inc., U.S.A in the Treatment of Actinic Keratosis | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT04139304 | A Study of Daratumumab and Dose-Adjusted EPOCH in Plasmablastic Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03294577 | Plinabulin vs. Pegfilgrastim in Prevention of TAC Induced Neutropenia | neutropenia | Doxorubicin (NPC261012) | |
| NCT00108953 | A Research Study to Treat Patients With Advanced Hepatocellular Carcinoma | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00003401 | Peripheral Stem Cell Transplantation Plus Combination Chemotherapy in Treating Patients With Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT01158144 | Concurrent Endostar, Paclitaxel/Carboplatin and Radiotherapy for Locally Advanced Non-small Cell Lung (RT0902) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00319865 | PAD Combination Therapy Followed by Thal/Dex for Relapsed or Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01820325 | Safety and Efficacy of Buparlisib (BKM120) in Patients With Untreated Squamous Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01669239 | Study of Neoadjuvant Myocet®, Paclitaxel, Pertuzumab, and Trastuzumab in HER2-positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00002318 | A Comparison of DOX-SL Versus Adriamycin Plus Bleomycin Plus Vincristine in the Treatment of Severe AIDS-Related Kaposi's Sarcoma | Kaposi's sarcoma | Doxorubicin (NPC261012) | |
| NCT00486954 | Lapatinib in Combination With Weekly Paclitaxel in Patients With ErbB2 Amplified Advanced Gastric Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT04492033 | A Study of CTX-009 (ABL001) in Combination With Irinotecan or Paclitaxel in Advanced or Metastatic Solid Tumor Patients | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT00070278 | Neoadjuvant Epirubicin, Cyclophosphamide, and Paclitaxel With or Without Gemcitabine in Treating Women Who Are Undergoing Surgery for Early Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00462423 | Abraxane and Avastin as Therapy for Patients With Malignant Melanoma, a Phase II Study | melanoma | Paclitaxel (NPC208553) | |
| NCT05467670 | Safety and Efficacy of Anti-CD47, ALX148 in Combination With Liposomal Doxorubicin and Pembrolizumab in Recurrent Platinum-resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT04968106 | Neoadjuvant Chemotherapy and Retifanlimab in Patients With Selected Sarcomas (TORNADO) | sarcoma | Doxorubicin (NPC261012) | |
| NCT00425425 | Cetuximab, Oxaliplatin, Fluorouracil, and Radiation Therapy in Treating Patients With Stage II or Stage III Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT04698941 | Combined Simvastatin and Albumin Paclitaxel in Treating ES-SCLC Patients Relapsed From 1st Chemotherapy | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04594798 | A Study of Polatuzumab Vedotin, Rituximab and Dose Attenuated CHP in Older Patients With DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00002579 | Tamoxifen Following Combination Chemotherapy in Treating Women With Operable Invasive Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00912639 | A Clinical Trial of Paclitaxel Loaded Polymeric Micelle in Patients With Taxane-Pretreated Recurrent Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00005847 | Chemotherapy With or Without Biological Therapy in Treating Patients With Metastatic Prostate Cancer That Has Not Responded to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT05022030 | First-line mCapOX+Cetuximab vs. mFOLFOX6+Cetuximab for Metastatic Left-sided CRC With Wild-type RAS/BRAF Genes | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01526135 | Trial Comparing Adjuvant Chemotherapy With Gemcitabine Versus mFolfirinox to Treat Resected Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00004265 | Paclitaxel in Treating Patients With Recurrent or Refractory Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01852292 | Study of Efficacy and Safety of Buparlisib (BKM120) Plus Paclitaxel Versus Placebo Plus Paclitaxel in Recurrent or Metastatic Head and Neck Cancer Previously Pre-treated With a Platinum Therapy | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00136435 | A Study in Adults With Untreated Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00558636 | A Trial Comparing Safety and Efficacy of Carboplatin and Paclitaxel Plus or Minus Sorafenib (BAY43-9006) in Chemonaive Patients With Stage IIIB-IV Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01235897 | MK-2206, Paclitaxel and Trastuzumab in Treating Patients With HER2-overexpressing Solid Tumor Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT03970252 | Nivolumab in Combination With Chemotherapy Pre-Surgery in Treating Patients With Borderline Resectable Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03937830 | Combined Treatment of Durvalumab, Bevacizumab, Tremelimumab and Transarterial Chemoembolization (TACE) in Subjects With Hepatocellular Carcinoma or Biliary Tract Carcinoma | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT03150693 | Inotuzumab Ozogamicin and Frontline Chemotherapy in Treating Young Adults With Newly Diagnosed B Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00003376 | Two-Drug Combination Chemotherapy Compared With Four-Drug Combination Chemotherapy in Treating Patients With Advanced Cancer of the Urothelium | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01205711 | Irinotecan Hydrochloride, Fluorouracil, and Leucovorin Calcium With or Without Zibotentan in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00451074 | Six Month Study of Gentamicin in Duchenne Muscular Dystrophy With Stop Codons | Duchenne muscular dystrophy | Gentamicin (NPC471628) | |
| NCT00369551 | Bevacizumab, Paclitaxel, Carboplatin, and Radiation Therapy to the Chest in Treating Patients With Locally Advanced Non-Small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01702714 | A Study of RO5083945 in Combination With Cisplatin and Gemcitabine or Carboplatin and Paclitaxel in Patients With Advanced or Recurrent Non-Small Cell Lung Cancer of Squamous Histology Who Have Not Received Prior Chemotherapy for The Metastatic Disease | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004921 | High-Dose Chemotherapy Compared With Standard Chemotherapy in Treating Patients With Stage III or Stage IV Ovarian Epithelial Cancer That Has Been Removed During Surgery | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02995772 | Neoadjuvant Hormonal Therapy Compared to Neoadjuvant Chemotherapy in Stage IIIB/C and IV Breast Cancer Patients | breast cancer | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT03722498 | Phase Ⅱ Study of HAIC of FOLFOX vs. Sorafenib in HCC Refractory to TACE | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00954174 | Paclitaxel and Carboplatin or Ifosfamide in Treating Patients With Newly Diagnosed, Persistent or Recurrent Uterine, Ovarian, Fallopian Tube, or Peritoneal Cavity Cancer | Fallopian Tube Carcinoma;Uterine Carcinosarcoma | Paclitaxel (NPC208553) | |
| NCT00723125 | Carboplatin, Abraxane, Avastin as Neoadjuvant Therapy for Her2-Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00006111 | Surgery Plus Combination Chemotherapy and Radiation Therapy in Treating Patients With Cancer of the Urinary Tract | urethra cancer;urinary bladder cancer | Fluorouracil (NPC75844) | |
| NCT04307576 | A Treatment Study Protocol for Participants 0-45 Years With Acute Lymphoblastic Leukaemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT02881086 | Optimization of Therapy in Adult Patients With Newly Diagnosed Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma by Individualised, Targeted and Intensified Treatment | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00005775 | Glutamine Supplementation to Prevent Death or Infection in Extremely Premature Infants | fetal growth restriction;premature birth;Sepsis | Glutamine (NPC197087) | |
| NCT01435018 | Three Chemo Regimens as an Adjunct to ART for Treatment of Advanced AIDS-KS | HIV-1 infection | Paclitaxel (NPC208553) | |
| NCT00691912 | Therapy of Metastatic Breast Cancer With Paclitaxel and Liposomal Doxorubicin | breast cancer | Paclitaxel (NPC208553) | |
| NCT00635193 | Efficacy and Safety Study of M200(Volociximab in Combination With Liposomal Doxorubicin) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02838225 | DA Versus DAC as Postoperative Adjuvant Treatment for Early-stage Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00903656 | Lapatinib Plus Caelyx in Patients With Advanced Metastatic Breast Cancer Following Failure of Trastuzumab Therapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT01177683 | Bendamustine in Combination With Bortezomib and Pegylated Liposomal Doxorubicin for Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00709761 | Neo ALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) Study | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT05213312 | Study of Neoadjuvant Nivolumab or Placebo Plus Chemotherapy Followed by Surgery and Adjuvant Treatment in Subjects With Resectable ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02319304 | Pelvic Radiotherapy With Concurrent Neoadjuvant FOLFOX for Patients With Newly Diagnosed Rectal Adenocarcinoma | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04604132 | Derazantinib Alone or in Combination With Paclitaxel, Ramucirumab or Atezolizumab in Gastric Adenocarcinoma | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00455533 | Study to Assess Effectiveness of Giving Combination of Standard Chemotherapy Drugs Versus Combination of Standard Chemotherapy and New Drug Ixabepilone When Given Before Surgical Removal of Early Stage Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03894540 | Dose Escalation and Dose Expansion Study of IPN60090 in Patients With Advanced Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT04561817 | To Evaluate the Safety and Efficacy of Ipatasertib (GDC-0068) in Combination With Paclitaxel in Platinum-resistant Recurrent Epithelial Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT05367206 | Neoadjuvant Chemotherapy Followed by Chemoradiation Versus Chemoradiation for Stage IIIC Cervical Cancer Patients: A Randomized Phase III Trial | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02717091 | Neoadjuvant FOLFIRINOX or Nab-paclitaxel With Gemcitabine for Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02867566 | A Study Comparing the Efficacy and Safety Between I-CHOP and R-CHOP in Untreated CD20-Positive Diffuse Large B-cell Lymphoma Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00634179 | A Phase I/II Trial of VR-CHOP in Lymphoma Patients | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT00003133 | Combination Chemotherapy Following Surgery in Treating Patients With Advanced Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT03639246 | A Study of the Efficacy and Safety of Bevacizumab in Chinese Women With Newly Diagnosed, Previously Untreated Stage III or Stage IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03940196 | Chiauranib in Combination With Chemotherapy in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00427310 | Liver Infusions of Fluorouracil in Treating Patients With Dukes' A, Dukes' B, or Dukes' C Colon Cancer Undergoing Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03705429 | Study of Boserolimab (MK-5890) as Monotherapy and in Combination With Pembrolizumab (MK-3475) in Adults With Advanced Solid Tumors (MK-5890-001) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01376453 | Pre-operative 5-Fluorouracil (5-FU) and Sorafenib With External Radiation in Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00003812 | Chemotherapy Plus Radiation Therapy in Treating Patients With Limited-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00537823 | Pre- and Post-operative FOLFOX Based Therapy for Patients With Colorectal Cancer With Liver Involvement | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02112552 | Paclitaxel and Intraperitoneal Carboplatin Followed by Radiation Therapy in Treating Patients With Stage IIIC-IV Uterine Cancer | uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT01702844 | Single Arm on the Tolerability of Weekly Nab-paclitaxel | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03548961 | Organ Preservation in Early Rectal Cancer Patients | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00291850 | Phase II Trial of Dose-dense Paclitaxel and Cisplatin as Neo-adjuvant Chemotherapy for Operable Stage II and IIA NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02157870 | Neoadjuvant Chemotherapy in Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00318136 | A Study of Bevacizumab Plus Carboplatin and Paclitaxel in Subjects With Advanced, Previously Untreated, Squamous Non-Small Cell Lung Cancer (BRIDGE) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01297452 | BKM120 + Carboplatin + Paclitaxel for Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00083551 | UARK 98-026 TT II: Multiple Myeloma Evaluating Anti-Angiogenesis With Thalidomide and Post-Transplant Consolidation Chemotherapy | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00193141 | Chemotherapy With or Without Surgical Resection in Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02246127 | Efficacy and Safety of Everolimus and (STZ-5FU) Given One Upfront the Other Upon Progression in Advanced Pancreatic Neuroendocrine Tumor (pNET) | neuroendocrine neoplasm | Fluorouracil (NPC75844) | |
| NCT00954642 | A Study of MNRP1685A in Combination With Bevacizumab With or Without Paclitaxel in Patients With Locally Advanced or Metastatic Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT00002894 | Platinum-based Chemotherapy With or Without Paclitaxel in Treating Patients With Relapsed Ovarian Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02083679 | Sym004 in Subjects With Stage IV Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00679029 | Combination Chemotherapy and Bevacizumab in Treating Women With HER2/Neu-Negative Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00607048 | Dose Finding Study Of CP-870,893, An Immune System Stimulating Antibody, In Combination With Paclitaxel And Carboplatin For Patients With Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02047201 | Assessing Tumor Response and IMRT Treat Plan After IC Based on FDG-PET/CT for Locally Advanced HNSCC | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00626561 | Bevacizumab and Paclitaxel for Neuroendocrine Tumors of the Cervix | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00659646 | The Safety and Efficacy of an Antibiotic Sponge in Diabetic Patients With Moderately Infected Foot Ulcers | diabetic foot | Gentamicin (NPC471628) | |
| NCT05381909 | Study Investigating BGB-24714 as Monotherapy and in Combination With Chemotherapy in Participants With Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04225364 | Efficacy of Neoadjuvant PD-1 Blockade Plus Chemotherapy for Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00136175 | Paclitaxel, Carboplatin and Gemcitabine in the Treatment of Patients With Advanced Transitional Cell Cancer of the Bladder | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00586209 | L-Glutamine Therapy for Sickle Cell Anemia | Thalassemia;sickle cell anemia | Glutamine (NPC197087) | |
| NCT01285609 | Phase 3 Trial in Squamous Non Small Cell Lung Cancer Subjects Comparing Ipilimumab Versus Placebo in Addition to Paclitaxel and Carboplatin | lung cancer | Paclitaxel (NPC208553) | |
| NCT00001387 | Phase I and Pharmacokinetic Trial of Paclitaxel (Taxol) Given as a 3-Hour Infusion in Pediatric Patients With Refractory Malignancy | neoplasm | Paclitaxel (NPC208553) | |
| NCT01332968 | A Study of Obinutuzumab (RO5072759) Plus Chemotherapy in Comparison With Rituximab Plus Chemotherapy Followed by Obinutuzumab or Rituximab Maintenance in Patients With Untreated Advanced Indolent Non-Hodgkin's Lymphoma (GALLIUM) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00003589 | Combination Chemotherapy in Treating Patients With Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04085276 | Toripalimab in Combination With Nab-Paclitaxel For Patients With Metastatic or Recurrent Triple-Negative Breast Cancer (TNBC) With or Without Systemic Treatment | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT02383212 | Phase I Study of Oral BAY 1217389 in Combination With Intravenous Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT00493870 | TAC Versus TC for Adjuvant Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00580333 | Preoperative Cisplatin and Bevacizumab in ER-, PR-, HER2 Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05322499 | Phase II Clinical Study of Camrelizumab Combined With Chemotherapy or Anlotinib in Advanced Esophageal Squamous Cell Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02047513 | Neoadjuvant Plus Adjuvant or Only Adjuvant Nab- Paclitaxel Plus Gemcitabine for Resectable Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01628211 | Second Look Laparoscopy in Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04257448 | Safety and Tolerance of Epigenetic and Immunomodulating Drugs Combined With Chemotherapeutics in Patients Suffering From Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02588105 | Study to Assess the Safety and Preliminary Efficacy of AZD0156 at Increasing Doses Alone or in Combination With Other Anti-cancer Treatment in Patients With Advanced Cancer | neoplasm | Fluorouracil (NPC75844) | |
| NCT02672475 | Galunisertib and Paclitaxel in Treating Patients With Metastatic Androgen Receptor Negative (AR-) Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT05099666 | Lurbinectedin + Doxorubicin In Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT02545504 | Andecaliximab With mFOLFOX6 as First Line Treatment for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01011075 | Study of Gleevec and Weekly Paclitaxel in Patients Aged 70 or Older With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04032964 | Dose Finding Study of L19TNF and Doxorubicin in Patients With STS | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00866528 | Study of Pazopanib and Paclitaxel in Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00384826 | Therapeutic Strategy in Advanced Bronchioloalveolar Carcinoma | bronchoalveolar adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01591733 | FOLFIRINOX + RT for Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00417209 | Larotaxel Compared To Continuous Administration of 5-FU in Advanced Pancreatic Cancer Patients Previously Treated With A Gemcitabine-Containing Regimen | pancreatic neoplasm | Fluorouracil (NPC75844) | |
| NCT01218516 | A Safety and Efficacy Study of Farletuzumab in Participants With Adenocarcinoma of the Lung | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03714555 | Disulfiram-Copper Gluconate in Met Pancreas Cancer w Rising CA19-9 on Abraxane-Gemzar, FOLFIRINOX or Gemcitabine | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00483301 | A Phase II Trial OF Carboplatin, ABI-007 (Abraxane) And Sorafenib (BAY 43-9006) in Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT01442649 | Efficacy of Chemotherapy, Associated to Either Cetuximab or Bevacizumab, in KRAS Wild-type Metastatic Colorectal Cancer Patients With Progressive Disease After Receiving First-line Treatment With Bevacizumab | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00002937 | Paclitaxel With or Without PSC 833 in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02340117 | Study of Combined SGT-53 Plus Gemcitabine/Nab-Paclitaxel for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00666991 | Pharmacokinetic, Safety and Efficacy Study of Nanoparticle Paclitaxel in Patients With Peritoneal Cancers | peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT02855788 | Metronomic Chemotherapy in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00003350 | Paclitaxel Compared With Doxorubicin in Treating Patients With Advanced AIDS-Related Kaposi's Sarcoma | Kaposi's sarcoma | Paclitaxel (NPC208553) | |
| NCT00722592 | Platinum Resistant Ovarian Cancer Evaluation of Doxil and Vintafolide (MK-8109, EC145) Combination Therapy (8109-009, EC-FV-04) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03957590 | Study of Tislelizumab (BGB-A317) Versus Placebo in Combination With Chemoradiotherapy in Participant With ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01784120 | A Phase II Trial of Doxorubicin and Genexol-PM in Patients With Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01591135 | A Phase III Study of Comparing Paclitaxel Plus 5-Fluorouracil Versus Cisplatin Plus 5-Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02449252 | Efficacy of Consolidative Involved-site Radiotherapy for Patients With Limited-stage Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT00191789 | Neoadjuvant Sequential Administration of Two Gemcitabine Combinations in Operable Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02012608 | Glutamine for the Prevention of Radiation Toxicity in Subjects Conserving Therapy | breast cancer | Glutamine (NPC197087) | |
| NCT00572169 | UARK 2006-66, Total Therapy 3B: An Extension of UARK 2003-33 Total Therapy | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00929591 | SWOG-8814 Tamoxifen With or Without Combination Chemotherapy in Postmenopausal Women Who Have Undergone Surgery for Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01730586 | Abraxane in CIMP-High Colorectal and Small Bowel Adenocarcinomas | colorectal carcinoma | Paclitaxel (NPC208553) | |
| NCT04035473 | A Study to Determine the Bioequivalence of Oraxol in Cancer Patients Treated With Intravenous Paclitaxel | neoplasm | Paclitaxel (NPC208553) | |
| NCT02890602 | Erythropoietin for Management of Anemia Caused by Chemotherapy | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00646659 | Cetuximab, Combination Chemotherapy, and Radiation Therapy in Treating Patients With Newly Diagnosed Stage III or Stage IV Head and Neck Cancer That Cannot Be Removed By Surgery | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT05044871 | Oregovomab in Combination With Bevacizumab Plus Chemo in BRCA Wild Type Platinum Sensitive Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03491540 | Mechanical Bowel Preparation and Oral Antibiotics Before Rectal Cancer Surgery | rectum cancer | Gentamicin (NPC471628) | |
| NCT02622074 | Phase II Study With Trastuzumab + Paclitaxel in Locally Advanced HER2+ Tumors or Epirubicin + Taxotere in HER2- Tumors | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02520154 | Dose-Escalation Study of Intraperitoneal (IP) Cisplatin, IV/IP Paclitaxel, IV Bevacizumab, and Oral Olaparib for Newly Diagnosed Ovarian, Primary Peritoneal, and Fallopian Tube Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00024401 | DHA-Paclitaxel in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Paclitaxel (NPC208553) | |
| NCT02483104 | Open-label Phase 1b Study of ARQ 092 in Combination With Anastrozole | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05185869 | SHR6390 Plus Nab-paclitaxel and Gemcitabine in Advanced/Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00831181 | Neo-adjuvant Chemoradiation With Oxaliplatin/5-FU in Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03807999 | Nab-Paclitaxel Plus Gemcitabine Versus Gemcitabine For The First Line Treatment of Pancreas Cancer | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00038545 | A Phase II Study of Paclitaxel and Topotecan With Filgrastim-SD/01 Support For Relapsed and Refractory Aggressive Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT00003377 | Radiation Therapy, Paclitaxel, and Cisplatin in Treating Patients With Cancer of the Cervix | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01873326 | Paclitaxel, Ifosfamide and Cisplatin (TIP) Versus Bleomycin, Etoposide and Cisplatin (BEP) for Patients With Previously Untreated Intermediate- and Poor-risk Germ Cell Tumors | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT02744898 | A Phase 2 Feasibility Study of Abraxane and Carboplatin in Epithelial Neoplasms of the Uterus | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00002814 | Combination Chemotherapy for Patients With Brain Cancer | Central Nervous System Neoplasm | Paclitaxel (NPC208553) | |
| NCT01704716 | High Risk Neuroblastoma Study 1.8 of SIOP-Europe (SIOPEN) | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT02023710 | Bevacizumab Combined With Carboplatin Plus Paclitaxel Chemotherapy to Treat Metastatic Mucosal Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT02492503 | Effectiveness of Chemotherapy in Metastatic or Recurrent Carcinoma Cervix | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT01383148 | Phase IIB/III Of TG4010 Immunotherapy In Patients With Stage IV Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05190445 | Cinrebafusp Alfa in Combination With Ramucirumab and Paclitaxel in HER2-High Gastric or GEJ Adenocarcinoma and in Combination With Tucatinib in HER2-Low Gastric or GEJ Andenocarinoma | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00505492 | Malignant Mixed Mesodermal Tumor (MMMT) - Early Stage With Postoperative XRT/Chemotherapy | uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT04563975 | Efficacy and Safety of Toripalimab Combined With Docetaxel or Nab-paclitaxel in Patients With Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04714190 | A Study of RC48-ADC in Local Advanced or Metastatic Gastric Cancer With the HER2-Overexpression | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT02128100 | Effects of Folfirinox and Stereotactic Body Radiation Therapy for Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00006479 | Surgery With or Without Combination Chemotherapy in Treating Patients With Liver Metastases From Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00004005 | Irinotecan Followed By Fluorouracil and Leucovorin in Treating Patients With Stage III or Stage IV Colorectal Carcinoma (Cancer), Other Refractory Carcinoma, or Metastatic Adenoma (Cancer) of Unknown Primary Origin | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00915005 | Trial of Image-Guided Adaptive Conformal Photon vs Proton Therapy, With Concurrent Chemotherapy, for Locally Advanced Non-Small Cell Lung Carcinoma: Treatment Related Pneumonitis and Locoregional Recurrence | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00669877 | Rituximab and Hyper-CVAD (Cyclophosphamide, Vincristine, Adriamycin, and Dexamethasone) for Burkitt's and Burkitt's -Like Leukemia/Lymphoma | Burkitts lymphoma | Doxorubicin (NPC261012) | |
| NCT00274872 | Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer That Cannot Be Removed By Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01567618 | Dose-dense Biweekly Docetaxel Combined With 5-fluorouracil as First-line Treatment in Advanced Gastric Cancer | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT04568200 | Study of Anti-PD-L1 in Combination With Chemo(Radio)Therapy for Resectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02352337 | First-line Metastatic Pancreatic Cancer : FOLFIRINOX +/- LV5FU2 in Maintenance Versus Firgem | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02623972 | A Phase 2 Study of Eribulin Followed by AC as Preoperative Therapy for HER2-negative Inflammatory Breast Cancer | inflammatory breast carcinoma | Doxorubicin (NPC261012) | |
| NCT00407888 | Doxorubicin Hydrochloride, Cyclophosphamide, and Filgrastim Followed By Paclitaxel Albumin-Stabilized Nanoparticle Formulation With or Without Trastuzumab in Treating Patients With Breast Cancer Previously Treated With Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT01215344 | First Autologous Transplant on Minimal Residual Disease Markers in Previously Untreated Myeloma Undergoing Initial Treatment With Velcade | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT02563054 | A Study of Capecitabine (Xeloda) in Participants With Advanced or Metastatic Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02317874 | Testing the Addition of the Anti-Cancer Drug Talazoparib to the Combination of Carboplatin and Paclitaxel for the Treatment of Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00498953 | Combination Chemotherapy and Radiation Therapy With or Without Lapatinib in Treating Patients With Locally Advanced Cancer of the Larynx or Hypopharynx | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00255762 | Carboplatin, Paclitaxel, and Bevacizumab in Treating Patients With Stage IV Melanoma That Cannot Be Removed By Surgery | melanoma | Paclitaxel (NPC208553) | |
| NCT00496028 | Phase I Study in Patients With Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT05171530 | Lenvatinib With Taxane Drugs Treatment for Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03574779 | A Study to Evaluate the Efficacy and Safety of Novel Treatment Combinations in Participants With Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT02041533 | An Open-Label, Randomized, Phase 3 Trial of Nivolumab Versus Investigator's Choice Chemotherapy as First-Line Therapy for Stage IV or Recurrent PD-L1+ Non-Small Cell Lung Cancer (CheckMate 026) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00259402 | Oxaliplatin in Esophagus Cancer (Advanced) 1st Line | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT01881230 | Evaluate Risk/Benefit of Nab Paclitaxel in Combination With Gemcitabine and Carboplatin Compared to Gemcitabine and Carboplatin in Triple Negative Metastatic Breast Cancer (or Metastatic Triple Negative Breast Cancer) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT04229459 | Trial of Nivolumab and Cetuximab After Chemoradiation in Esophageal Squamous Cell Carcinoma Patients. | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT04814485 | Efficacy and Safety of SHR-1020 Combined With Albumin-bound Paclitaxel in the Second-line Treatment of Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05179733 | The Efficacy and Safety of ZR2 Versus R-miniCHOP in the Treatment of Unfit or Frail de Novo Diffuse Large B-cell Lymphoma Patients Aged Older Than or Equal to 70 Years | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04824092 | Tafasitamab + Lenalidomide + R-CHOP Versus R-CHOP in Newly Diagnosed High-intermediate and High Risk DLBCL Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02322281 | TIGER-3: Open Label, Multicenter Study of Rociletinib (CO-1686) Mono Therapy Versus Single-agent Cytotoxic Chemotherapy in Patients With Mutant EGFR NSCLC Who Have Failed at Least One Previous EGFR-Directed TKI and Platinum-doublet Chemotherapy | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01374425 | Study of Bevacizumab + mFOLFOX6 Versus Bevacizumab + FOLFIRI With Biomarker Stratification in Participants With Previously Untreated Metastatic Colorectal Cancer (mCRC) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02289456 | Phase II Safety and Tolerability Trial With Nab-Paclitaxel Plus Carboplatin Followed by Nab-Paclitaxel for First Line Treatment of NSCLC Subjects With ECOG PS 2 | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00422682 | A Study Evaluating BSI-201 in Combination With Chemotherapeutic Regimens in Subjects With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00323011 | FOLFIRI + Bevacizumab With or Without Dalteparin in First Line Treatment of Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00601627 | Panitumumab, Chemotherapy, and External-Beam Radiation Therapy in Treating Patients With Locally Advanced Pancreatic Cancer That Cannot be Removed by Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03323463 | Major De-escalation to 30 Gy for Select Human Papillomavirus Associated Oropharyngeal Carcinoma | oropharynx squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT05062889 | Exploiting Circulating Tumour DNA to Intensify the Postoperative Treatment Resected Colon Cancer Patients | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00129727 | Carboplatin Taxol Avastin in Ovarian Cancer (OVCA) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01264328 | Study of the Combination of Panitumumab With Paclitaxel as First-line Treatment of Subjects With Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00436566 | Doxorubicin and Cyclophosphamide Followed By Trastuzumab, Paclitaxel, and Lapatinib in Treating Patients With Early-Stage HER2-Positive Breast Cancer That Has Been Removed By Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT01491217 | A Study of Oraxol® in Gastric Cancer Patients | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00494780 | Ofatumumab (Humax-CD20) With CHOP (Cyclophosphamide,Doxorubicin, Vincristine, Predisolone) in Follicular Lymphoma (FL) Patients | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT04790903 | A Study Evaluating the Safety, Efficacy and Pharmacokinetics of Venetoclax in Combination With Polatuzumab Vedotin Plus Rituximab (R) and Cyclophosphamide, Doxorubicin, Prednisone (CHP) in Participants With Untreated BCL-2 Immunohistochemistry (IHC)-Positive Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02326025 | A Study of Olaratumab and Doxorubicin in Participants With Advanced Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT04224337 | Phase II Study of Durvalumab ,Doxorubicin, and Ifosfamide in Pulmonary Sarcomatoid Carcinoma | non-small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT03358472 | Pembrolizumab Plus Epacadostat, Pembrolizumab Monotherapy, and the EXTREME Regimen in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (KEYNOTE-669/ECHO-304) | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT03908333 | High Dose Ascorbic Acid and Nanoparticle Paclitaxel Protein Bound and Cisplatin and Gemcitabine (AA NABPLAGEM) in Patients Who Have Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00004160 | Chemotherapy Plus Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Surgically Removed | lung cancer | Paclitaxel (NPC208553) | |
| NCT02048540 | Neoadjuvant Bev Plus DOF vs DOF in LAGC and Its Association With Circulating Tumor Cell | gastric carcinoma | Fluorouracil (NPC75844) | |
| NCT00016952 | Irinotecan or Fluorouracil Plus Leucovorin in Treating Patients With Previously Treated Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03852979 | Neo-Adjuvant Chemotherapy and Conservative Surgery in Cervical Cancer to Preserve Fertility | cervical cancer | Paclitaxel (NPC208553) | |
| NCT05312372 | S095033 in Combination With Paclitaxel as 2nd- or 3rd-line Treatment in Participants With Advanced or Metastatic ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003206 | Carboplatin and Paclitaxel in Treating Patients With Locally Recurrent or Metastatic Nasopharyngeal Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT03306121 | TPF+CCRT vs.CCRT+PF in High Risk Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00365417 | Therapy With Bevacizumab (BEV), Doxorubicin, and Cyclophosphamide Followed by BEV, Docetaxel, and Capecitabine Before Surgery Followed by BEV Alone After Surgery for Women With Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00004179 | Combination Chemotherapy With or Without Rituximab in Treating Patients With Relapsed Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00599131 | Cetuximab and Radiation Therapy in Laryngeal Cancer Patients Who Have Responded to One Cycle of Chemotherapy (SPORE) | laryngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT02448329 | Study of AZD1775 in Combination With Paclitaxel, in Advanced Gastric Adenocarcinoma Patients Harboring TP53 Mutation as a Second-line Chemotherapy | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04859582 | Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy in Participants Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (MK-3475-859/KEYNOTE-859)-China Extension | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT00096668 | Evaluation of Safety and Efficacy of TOCOSOL(R) Paclitaxel as Initial Treatment for Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03189446 | Vaginal Cuff Brachytherapy Followed by Chemotherapy in Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00844649 | Phase III Study of ABI-007(Albumin-bound Paclitaxel) Plus Gemcitabine Versus Gemcitabine in Metastatic Adenocarcinoma of the Pancreas | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05346146 | A Prospective, Single-arm, Exploratory Clinical Study of Sintilimab Injection in Combination With Paclitaxel (Albumin-bound) and Gemcitabine in Translational Therapy in Patients With Unresectable Locally Advanced Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00963729 | Chemotherapy or Letrozole Before Surgery in Treating Postmenopausal Women With Breast Cancer That Can Be Removed By Surgery | breast cancer | Fluorouracil (NPC75844) | |
| NCT00077233 | FOLFIRI or FOLFOX With or Without Cetuximab in Patients With Metastatic Adenocarcinoma of the Colon or Rectum | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00289263 | Maintenance Chemotherapy in Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01250782 | Effectiveness of Dipeptide N (2)-L-Alanyl-L-Glutamine in Trauma ICU Patients: Pilot, Prospective, Randomized and Double Blind Study. | injury | Glutamine (NPC197087) | |
| NCT00434226 | A Study of Sunitinib in Combination With Bevacizumab, Carboplatin, and Paclitaxel in Patients With Advanced Non-Small Cell Lung Cancer (SABRE-L) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00546156 | Preoperative Dose-dense Doxorubicin and Cyclophosphamide Followed by Paclitaxel With Bevacizumab in Operable Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01905150 | Ph 2 Trial of Vitamin C & G-FLIP (Low Doses Gemcitabine, 5FU, Leucovorin, Irinotecan, Oxaliplatin) for Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02319018 | Alisertib and Combination Chemotherapy in Treating Patients With Gastrointestinal Tumors | digestive system cancer | Fluorouracil (NPC75844) | |
| NCT03023358 | Compared the Efficacy and Safety of CDOP Combined With Chidamide and CDOP in de Novo Peripheral T Cell Lymphoma Patients | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01356680 | HD17 for Intermediate Stage Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00005867 | Combination Chemotherapy in Treating Patients With Aggressive Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02430311 | The Pharmacokinetics and Safety of Olaparib Alone and With Paclitaxel in Chinese Patients With Advanced Solid Tumour. | neoplasm | Paclitaxel (NPC208553) | |
| NCT00729612 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients With Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00850577 | Ph II of a Novel Anti-angiogenic Agent in Combination With Chemotherapy for the Treatment of Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00346229 | Temperature-Sensitive Liposomal Doxorubicin and Hyperthermia in Treating Women With Locally Recurrent Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01847677 | Neoadjuvant Therapy in Advanced Ovarian Cancer With Avastin | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT03407144 | Safety and Efficacy of Pembrolizumab (MK-3475) in Children and Young Adults With Classical Hodgkin Lymphoma (MK-3475-667/KEYNOTE-667) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00002627 | Addition of Paclitaxel to High-Dose Combination Chemotherapy in Treating Patients With High-Risk Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02379585 | Fasting on Newly Diagnosed Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03563170 | QUILT-3.072: NANT Hepatocellular Carcinoma (HCC) Vaccine | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02976909 | Efficacy and Safety of TPF Induction Chemotherapy for Borderline-resectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02103062 | Phase 2 Study With Abraxane (Nab®Paclitaxel) in Metastatic Colorectal Cancer | colorectal neoplasm | Paclitaxel (NPC208553) | |
| NCT00002557 | Combination Chemotherapy in Patients With Advanced or Recurrent Mycosis Fungoides | lymphoma | Doxorubicin (NPC261012) | |
| NCT03082209 | First in Human Study of IBI308 in Chinese Subjects With Advanced Solid Tumors | cancer | Fluorouracil (NPC75844) | |
| NCT00640081 | Combination Chemotherapy and Cetuximab as First-Line Therapy in Treating Patients With Advanced and/or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02138812 | A Phase 1, Dose Escalation Study to Assess the Safety and Tolerability of ASP9853 With Either Docetaxel or Paclitaxel in Patients With Advanced Non-hematologic Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT05054439 | A Clinical Study of SI-B001 in Combination With Paclitaxel in the Treatment of Recurrent and Metastatic HNSCC | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04999969 | Safety, Pharmacokinetics and Clinical Activity of AZD0171 in Combination With Durvalumab and Chemotherapy in Locally Advanced or Metastatic Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003642 | Radiation Therapy and Chemotherapy Following Surgery in Treating Patients With Stage II or Stage III Bladder Cancer | urinary bladder cancer | Fluorouracil (NPC75844) | |
| NCT04827953 | Study to Evaluate the Safety and Efficacy of Treatment With NLM-001 and Standard Chemotherapy Plus Zalifrelimab in Patients With Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04170530 | Neoadjuvant mFOLFOXIRI Chemotherapy Alone for Extramural Vascular Invasion Positive Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01755845 | Trial of Postoperative Chemoradiotherapy With or Without Consolidation Chemotherapy for Cervical Cancer Patients | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01516580 | Intergroup Randomized Trial for Children or Adolescents With B-Cell Non Hodgkin Lymphoma or B-Acute Leukemia: Rituximab Evaluation in High Risk Patients | leukemia;non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01010945 | Erlotinib, Gemcitabine and Nab-Paclitaxel in Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003251 | Amifostine Plus Chemotherapy and Radiation Therapy in Treating Patients With Advanced, Unresectable Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00479583 | Combination Trial of BMS-690514 in Combination With FOLFIRI and FOLFOX | cancer | Fluorouracil (NPC75844) | |
| NCT03421353 | AZD9150 Plus Durvalumab Alone or in Combination With Chemotherapy in Patients With Advanced, Solid Tumours and in Patients With Non-Small-Cell Lung Cancer | neoplasm | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00795613 | Positron Emission Tomography (PET)-Adapted Chemotherapy In Advanced Hodgkin Lymphoma (HL) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01269229 | A Trial of Neoadjuvant FOLFOX6 With Short Course Radiotherapy in Patients With Unresectable Rectal Cancer and Liver Metastasis | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00093665 | Fluorouracil, Cisplatin, and Radiation Therapy in Treating Patients With Stage II, Stage III, or Stage IV Nasopharyngeal Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT03869034 | HAIC Combined With PD-1 Inhibitor in Potentially Resectable Locally Advanced HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00001442 | A Pilot Study of Paclitaxel With Radiation Therapy for Locally Advanced Head and Neck Cancer | squamous cell carcinoma;upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT02012062 | Concurrent Nimotuzumab Versus Cisplatin With Radiotherapy for Locoregionally Advanced NPC | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00165178 | Treatment of Acute Lymphoblastic Leukemia in Children | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT02483247 | A Study of BBI503 in Combination With Selected Anti-Cancer Therapeutics in Adult Patients With Advanced Cancer | cancer | Doxorubicin (NPC261012) | |
| NCT02583477 | Phase Ib/II Study of MEDI4736 Evaluated in Different Combinations in Metastatic Pancreatic Ductal Carcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00003680 | Standard Chemotherapy Compared With High-Dose Chemotherapy Plus Peripheral Stem Cell Transplant in Treating Women With Advanced or Inflammatory Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00473720 | Phase I Study of the Combination of Satraplatin and Abraxane in Advanced Cancers | cancer | Paclitaxel (NPC208553) | |
| NCT02172846 | Hypofractionated Proton Beam Radiation Therapy, Paclitaxel, and Carboplatin in Treating Patients With Stage II-III Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02247869 | Dose-dense ABVD First Line Therapy in Early Stage Unfavorable Hodgkin's Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02455141 | A Study Assessing the Safety and Efficacy of Adding Ipatasertib to Paclitaxel Treatment in Participants With Breast Cancer That Has Spread Beyond the Initial Site, and the Cancer Does Not Have Certain Hormonal Receptors | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00877006 | Study of Bendamustine Hydrochloride and Rituximab (BR) Compared With R-CVP or R-CHOP in the First-Line Treatment of Patients With Advanced Indolent Non-Hodgkin's Lymphoma (NHL) or Mantle Cell Lymphoma (MCL) - Referred to as the BRIGHT Study | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04195828 | Camrelizumab Combined With Apatinib Mesylate Tablets, Nab-paclitaxel and S-1 in the Treatment of Locally Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02280070 | RP II Study of SOX vs mFOLFOX6 in Patients With Resectable Rectal Cancer (KSCC1301). | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00053820 | Interferon Alfa With or Without Interleukin-2 and Fluorouracil in Treating Patients With Advanced Metastatic Kidney Cancer | kidney cancer | Fluorouracil (NPC75844) | |
| NCT03336216 | A Study of Cabiralizumab Given With Nivolumab With and Without Chemotherapy in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03901118 | Efficacy and Safety of Paclitaxel for Injection (Albumin-bound) for First-line Chemotherapy of Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02911350 | Safety Study of Combination of Hormone Therapy, Paclitaxel and Radiation Therapy to Treat Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT02641847 | TA(E)C-GP Versus A(E)C-T for the High Risk TNBC Patients and Validation of the mRNA-lncRNA Signature | breast cancer | Doxorubicin (NPC261012) | |
| NCT00072319 | Neoadjuvant or Adjuvant Epirubicin, Cyclophosphamide, and Paclitaxel in Treating Women With Stage I, Stage II, or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01757288 | Phase I/II NabPaclitaxel, Paclitaxel&Carboplatin w RTX Followed by Consolidation in Patients w Favorable Prognosis Inoperable Stage IIIA/B NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01040871 | Study of the Combination of VELCADE, Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Non-Germinal Center B-Cell Subtype of Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00719472 | A Study of Rituximab Alternative Dosing Rate in Patients With Previously Untreated Diffuse Large B-cell or Follicular Non-Hodgkin's Lymphoma (RATE) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00394251 | Study of Dose-dense Adriamycin Plus Cytoxan (AC) Followed by Either ABI-007 (Abraxane) or Taxol With Bevacizumab as Adjuvant Therapy for Patients With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003128 | Ifosfamide With or Without Paclitaxel in Treating Patients With Advanced, Refractory, or Recurrent Cancer of the Uterus | sarcoma | Paclitaxel (NPC208553) | |
| NCT01197664 | Paricalcitol, Fluorouracil, and Radiation Therapy in Treating Patients With Rectal Cancer That Can Be Removed in Surgery | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00003413 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Stage III or Stage IV Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01746173 | CHOEP + High Dose Therapy + Auto SCT for T-Cell Lymphoma | T-cell non-Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT04883281 | A Prospective Study of Daily Adaptive Radiotherapy to Better Organ-at-Risk Doses in Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00004059 | Fluorouracil Plus UCN-01 in Treating Patients With Advanced or Refractory Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT04551950 | Bintrafusp Alfa Combination Therapy in Participants With Cervical Cancer (INTR@PID 046) | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00954512 | Study of Robatumumab (SCH 717454, MK-7454) in Combination With Different Treatment Regimens in Participants With Advanced Solid Tumors (P04722, MK-7454-004) | neoplasm | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03467178 | Pembrolizumab With Chemotherapy in Front Line Advanced Ovarian, Primary Peritoneal and Fallopian Tube Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003539 | Paclitaxel Plus Monoclonal Antibody Therapy in Treating Women With Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00102622 | Intraperitoneal tgDCC-E1 and Intravenous Paclitaxel in Women With Platinum-Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03081143 | Ramucirumab Plus FOLFIRI Versus Ramucirumab Plus Paclitaxel in Patients With Advanced or Metastatic Gastric Cancer, Who Failed One Prior Line of Palliative Chemotherapy | gastric carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03871036 | Improve Checkpoint-blockade Response in Advanced Urothelial Cancer | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT00033683 | Combination Chemotherapy in Treating Women With Resected Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT05222204 | DDP ip Combined With AG in PDAC With Peritoneal Metastasis | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00398086 | Gemcitabine Plus Albumin-bound Paclitaxel In Patients With Advanced Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00872625 | Radiation Therapy and Docetaxel Followed by Standard Therapy in Treating Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT04081389 | Chemokine Modulation Therapy and Standard Chemotherapy Before Surgery for the Treatment of Early Stage Triple Negative Breast Cancer | breast carcinoma in situ | Doxorubicin (NPC261012) | |
| NCT00960960 | A Study of PI3-Kinase Inhibitor GDC-0941 in Combination With Paclitaxel, With and Without Bevacizumab or Trastuzumab, and With Letrozole, in Participants With Locally Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00808899 | Neuroblastoma Protocol 2008: Therapy for Children With Advanced Stage High Risk Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT04025593 | Biomarker Guided Treatment in DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00003893 | Adjuvant Chemotherapy Plus Radiation Therapy in Treating Women With Early-Stage Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02598687 | Testing TH-302, in Combination With Preoperative Chemoradiotherapy, in Esophageal Cancer. | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03907475 | Durvalumab in Combination With Chemotherapy in Treating Patients With Advanced Solid Tumors, (DURVA+ Study) | neoplasm | Paclitaxel (NPC208553) | |
| NCT00509964 | Second-Line Irinotecan vs. ILF for AGC | gastric carcinoma | Fluorouracil (NPC75844) | |
| NCT03756818 | TAK-659 and Paclitaxel in Treating Patients With Advanced Solid Tumors | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT04177108 | A Study Of Ipatasertib in Combination With Atezolizumab and Paclitaxel as a Treatment for Participants With Locally Advanced or Metastatic Triple-Negative Breast Cancer. | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03393884 | A Study of Apatinib Treatment in for Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01187199 | Phase I Trial of Bevacizumab and Temsirolimus in Combination With 1) Carboplatin, 2) Paclitaxel, 3) Sorafenib for the Treatment of Advanced Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT00004010 | Combination Chemotherapy and Radiation Therapy in Treating Children With Previously Untreated Stage II, Stage III, or Stage IV Hodgkin's Disease | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003592 | Methotrexate Compared With Paclitaxel in Treating Patients With Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01820754 | Evaluation of Circulating T Cells and Tumor Infiltrating Lymphocytes (TILs) During / After Pre-Surgery Chemotherapy in Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00070564 | S0221 Adjuvant Doxorubicin, Cyclophosphamide, and Paclitaxel in Treating Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04660799 | A Study on Pharmacokinetics (PK), Efficacy and Safety of Subcutaneous (SC) Versus Intravenous (IV) Rituximab, in Combination With CHOP (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone) in Previously Untreated Participants With CD20 Positive Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01558492 | Carboplatin and Paclitaxel in Patients With Metastatic, Castrate-Resistant Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT01566435 | Induction Chemotherapy With ACF Followed by Chemoradiation Therapy for Adv. Head & Neck Cancer | upper aerodigestive tract neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02987699 | Phase II Study of HAIC Using Cisplatin,Leucovorin and 5-Fluorouracil | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT01234038 | Safety and Tolerability Study of ISIS EIF4E Rx in Combination With Carboplatin and Paclitaxel | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03368859 | A Study to Evaluate Efficacy in Subjects With Esophageal Cancer Treated With Nivolumab and Ipilimumab or Nivolumab Combined With Fluorouracil Plus Cisplatin Versus Fluorouracil Plus Cisplatin | cancer | Fluorouracil (NPC75844) | |
| NCT01196234 | Paclitaxel/Carboplatin (PC) Followed by Gefitinib Versus PC in Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02098343 | p53 Suppressor Activation in Recurrent High Grade Serous Ovarian Cancer, a Phase Ib/II Study of Systemic Carboplatin Combination Chemotherapy With or Without APR-246 | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03108300 | Use of Propranolol Hydrochloride in the Treatment of Metastatic STS | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00042874 | Combination Chemotherapy in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT04926753 | Induction Chemotherapy Combined With Toripalimab in Locoregionally-Advanced Laryngo-Hypopharyngeal Squamous Cell Cancer | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00559741 | Cetuximab and Combination Chemotherapy in Treating Patients With Advanced or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00025090 | Radiation Therapy Plus Fluorouracil With or Without Additional Chemotherapy in Treating Patients With Primary Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT00513383 | Panitumumab Chemoradiotherapy Chemotherapy for Squamous Cancer of the Head and Neck | head and neck squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01523457 | Study of Modified FOLFIRINOX in Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00378716 | Combination Chemotherapy in Treating Patients With Resected Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00003558 | Chemotherapy in Treating Patients With Cancer of Unknown Primary Origin | carcinoma | Fluorouracil (NPC75844) | |
| NCT02377752 | A Study of Olaratumab in Japanese Participants With Advanced Cancer | neoplasm | Doxorubicin (NPC261012) | |
| NCT00143403 | Comparing Irinotecan and 5 FU/FA To 5-FU/FA After Resection Of Liver Metastases For Colorectal Cancer | colorectal neoplasm;hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00984464 | Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin in Patients With Metastatic Melanoma | metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT04581265 | Nab-PTX, Ifosfamide and Cisplatin in the Treatment of Pediatric Extracranial Germ Cell Tumor. | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT03169777 | QUILT-3.050: NANT Colorectal Cancer (CRC) Vaccine: Combination Immunotherapy in Subjects With Recurrent or Metastatic CRC | colorectal carcinoma | Paclitaxel (NPC208553) | |
| NCT00004112 | Combination Chemotherapy With or Without Rituximab in Treating Patients With Newly Diagnosed Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00788931 | A Trial l of Panobinostat Given in Combination With Trastuzumab and Paclitaxel in Adult Female Patients With HER2 Positive Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00586846 | Phase II Study of Chemotherapy and Pamidronate for the Treatment of Newly Diagnosed Osteosarcoma | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT01676649 | Ipilimumab With Carboplatin and Paclitaxel in Patients With Unresectable Stage III and Stage IV Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT04882462 | Sintilimab and Nimotuzumab Combined With Chemotherapy for the Treatment of R/M HNSCC. | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01200758 | A Study of Rituximab (MabThera) Subcutaneous (SC) Versus Rituximab (MabThera) Intravenous in Participannts With Follicular Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04084158 | A Study of Toripalimab Combined With Concurrent Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma. | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00089024 | Combination Chemotherapy, and Radiation Therapy in Treating Patients With Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00748553 | A Phase I/II Clinical Trial of Vidaza With Abraxane in Patients With Advanced/Metastatic Solid Tumors and Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00054028 | Suramin and Paclitaxel in Treating Women With Stage IIIB-IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01594099 | Concurrent Chemoradiotherapy for Cervical Cancer in Elderly Women | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00004150 | High-Dose Fluorouracil With or Without Leucovorin Compared With Standard Fluorouracil Plus Leucovorin Following Surgery in Treating Patients With Stage III Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00111761 | Evaluating Panitumumab (ABX-EGF) in Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00003594 | Combination Chemotherapy in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04348032 | Apatinib Combined With PLD vs PLD for Platinum-resistant Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00890656 | Study of Augmented Hyper-CVAD in Acute Lymphoblastic Leukemia Salvage | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03975270 | Sintilimab in Combination With Nab-paclitaxel in Patients With HNSCC (Head and Neck Squamous Cell Carcinoma ) | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003927 | Combination Chemotherapy, Amifostine, and Peripheral Stem Cell Transplantation in Treating Patients With Stage II, Stage III, or Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01220128 | Evaluation of an Anti-cancer Immunotherapy Combined With Standard Neoadjuvant Treatment in Patients With WT1-positive Primary Invasive Breast Cancer | breast neoplasm | Fluorouracil (NPC75844) | |
| NCT02218164 | Capecitabine or 5-FU With Pegylated Interferon Alpha-2b in Unresectable/Metastatic Cutaneous Squamous Cell Carcinoma | skin carcinoma;squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00231582 | High-dose Chemotherapy With Autologous Stem Cell Transplantation in Poor Prognosis Germ-cell Tumors: TAXIF II | testicular neoplasm | Paclitaxel (NPC208553) | |
| NCT00002917 | Paclitaxel in Treating Patients With Early-Stage Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00215501 | Capecitabine or 5-Fluorouracil in Combination With Irinotecan and Cisplatin in Patients With Solid Tumor Malignancies | neoplasm | Fluorouracil (NPC75844) | |
| NCT01963325 | S-1 in Combination With Abraxane in Treating Cholangiocarcinoma | cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT00245154 | Paclitaxel + Carboplatin With/Out Cediranib Maleate in Stage III or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00209222 | Efficacy of R-CHOP vs R-CHOP/R-DHAP in Untreated MCL | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04649476 | Neoadjuvant PD-1 Blockade in Resectable Oral Squamous Cell Carcinoma | oral squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00038168 | Intravenous Estramustine With Taxol in Hormone Refractory Prostate Adenocarcinoma | prostate cancer | Paclitaxel (NPC208553) | |
| NCT02481635 | A Study of Gemcitabine/Nab-paclitaxel and Radiation Therapy Followed by Surgery in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00669773 | Validate Gene Expression and Proteomic Signatures Predictive of Treatment for Response for Breast Cancer Patient | breast cancer | Doxorubicin (NPC261012) | |
| NCT03534713 | Induction Chemotherapy Followed by Standard Therapy in Cervical Cancer With Aortic Lymph Node Spread | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01940497 | A Study of the Safety of Subcutaneously Administered Trastuzumab (Herceptin) in Participants With Early and Locally Advanced Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00202787 | Safety and Efficacy Study of Combination Therapy With Cetuximab and FOLFOX4 in Patients With Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03126812 | Olaparib +/- Cediranib or Chemotherapy in Patients With Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02382263 | Nab-paclitaxel in Combination With Gemcitabine in Fragile Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00042835 | Erlotinib and Radiation Therapy Plus Combination Chemotherapy in Treating Patients With Inoperable Stage III Non-Small Cell Lung Cancer | lung adenocarcinoma;squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00807612 | QUILT-2.017: Phase 1b/2 Study of AMG 479 in Combination With Paclitaxel and Carboplatin for 1st Line Treatment of Advanced Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003899 | Chemotherapy Plus Bone Marrow or Peripheral Stem Cell Transplantation in Treating Patients With Recurrent or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02456714 | Second Line Chemotherapy FOLFIRINOX in Irresectable Cholangiocarcinoma | cholangiocarcinoma | Fluorouracil (NPC75844) | |
| NCT04084496 | mFOLFIRINOX as Adjuvent Chemotherapy in Treating Chinese Pancreatic Cancer Patients | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01779050 | Effect of Trastuzumab on Disease Free Survival in Early Stage HER2-Negative Breast Cancer Patients With ERBB2 Expressing Disseminated Tumor Cells | breast neoplasm | Fluorouracil (NPC75844) | |
| NCT00281658 | ErbB2 Over-expressing Metastatic Breast Cancer Study Using Paclitaxel, Trastuzumab, and Lapatinib | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04683939 | Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Efficacy Trial of BNT141 in Patients With Unresectable or Metastatic CLDN18.2-positive Gastric, Pancreatic, Ovarian and Biliary Tract Tumors | cholangiocarcinoma;gastric cancer;pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00710736 | A Study of ARRY-334543 and Capecitabine in Patients With Advanced Cancer | cancer | Fluorouracil (NPC75844) | |
| NCT00499603 | Paclitaxel Followed by FEC Versus Paclitaxel and RAD001 Followed by FEC In Women With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00324415 | Combined Modality Therapy for Patients With With HIV and Stage I, Stage II, or Stage III Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT02372409 | Using MRI-Guided Laser Heat Ablation to Induce Disruption of the Peritumoral Blood Brain Barrier to Enhance Delivery and Efficacy of Treatment of Pediatric Brain Tumors | anaplastic astrocytoma;glioblastoma multiforme;oligoastrocytoma;oligodendroglioma;optic nerve glioblastoma;pilocytic astrocytoma | Doxorubicin (NPC261012) | |
| NCT02593175 | Women's MoonShot: Neoadjuvant Treatment With PaCT for Patients With Locally Advanced TNBC | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03517449 | Lenvatinib in Combination With Pembrolizumab Versus Treatment of Physician's Choice in Participants With Advanced Endometrial Cancer (MK-3475-775/E7080-G000-309 Per Merck Standard Convention [KEYNOTE-775]) | endometrial neoplasm | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT00026364 | ZD 1839 Plus Combination Chemotherapy in Treating Patients With Locally Advanced, Locally Recurrent, or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01525329 | Combination Therapy With 5-Fluorouracil and Photodynamic Therapy in Post-transplant Premalignant Skin Disease | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT04523818 | Short-Course Chemoradiotherapy Followed by Chemotherapy for the Treatment of Resectable Gastric Adenocarcinoma | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00459121 | Vandetanib, Carboplatin, and Paclitaxel in Treating Patients With Stage I, Stage II, or Stage III Non-Small Cell Lung Cancer That Can Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT04729608 | Alpelisib Plus Olaparib in Platinum-resistant/Refractory, High-grade Serous Ovarian Cancer, With no Germline BRCA Mutation Detected | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03805022 | Benefit of Intensified Peri-operative Chemotherapy Within High-risk CINSARC Patients With Resectable Soft-tissue Sarcomas | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02362165 | CyBorD vs. PAD in the Treatment of Newly Diagnosed Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00979212 | Chemotherapy and Radiation Therapy With or Without Panitumumab in Treating Patients With Stage IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01455532 | A Dose Escalation Study of Iniparib as a Single Agent and in Combination in Solid Tumors | neoplasm | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT00133302 | Study of Standard CHOP Versus Biweekly CHOP in Aggressive Non-Hodgkin's Lymphoma (JCOG9809) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00038558 | Prophylactic Use of Filgrastim SD/01 in Patients With Hodgkin's Disease Receiving ABVD Chemotherapy | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03048123 | HAIC Versus TACE for Large and Unresectable Hepatocellular Carcinoma Staged BCLC A/B | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT03601897 | A Phase 1b/2 Study of Rebastinib (DCC-2036) in Combination With Paclitaxel in Patients With Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00941655 | Prospective Randomized Trial Comparing Gastrectomy, Metastasectomy Plus Systemic Therapy Versus Systemic Therapy Alone: GYMSSA Trial | gastric cancer | Fluorouracil (NPC75844) | |
| NCT03286244 | Second-line CM082 Combined With Paclitaxel For Patients With Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00014222 | Combination Chemotherapy With or Without Colony-stimulating Factors in Treating Women With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01473303 | Combination Chemotherapy With or Without Ganitumab in Treating Patients With Previously Untreated Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01248949 | A Study to Evaluate the Safety and Antitumor Activity in Subjects With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04431258 | ABTL0812 in Combination With FOLFIRINOX for First-line Treatment of Metastatic Pancreatic Study | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00634205 | Phase II Study of Valproate and Doxorubicin in Malignant Mesothelioma | mesothelioma | Doxorubicin (NPC261012) | |
| NCT01711970 | SGI-110 in Combination With Carboplatin in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02375672 | Study of Pembrolizumab in Combination With Chemotherapy for Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02588443 | Study of Neo-adjuvant RO7009789 Alone or Neo-adjuvant RO7009789 Plus Nab-Paclitaxel and Gemcitabine Followed by Adjuvant RO7009789 Plus Nab-Paclitaxel and Gemcitabine for Patients With Newly Diagnosed Resectable Pancreatic Carcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00290498 | Study of Rituximab-HCVAD Alternating With Rituximab-Methotrexate-Cytarabine Versus Standard Rituximab-CHOP Every 21 Days for Patients With Newly Diagnosed High Risk Aggressive B-Cell Non-Hodgkin's Lymphomas in Patients 60 Years Old or Younger | lymphoma | Doxorubicin (NPC261012) | |
| NCT01351350 | Dose Escalation Study of MLN0128 in Combination With Paclitaxel, With/Without Trastuzumab, in Subjects With Advanced Solid Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT03899636 | A Pivotal Study of Safety and Effectiveness of NanoKnife IRE for Stage 3 Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02301143 | Phase 2 Nab® -Paclitaxel (Abraxane®) Plus Gemcitabine in Subjects With Locally Advanced Pancreatic Cancer (LAPC) | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT04094688 | Vitamin D3 With Chemotherapy and Bevacizumab in Treating Patients With Advanced or Metastatic Colorectal Cancer | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT02319889 | Feasibility Study of SBRT Plus Chemotherapy for Non-Small Cell Lung Carcinoma | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00819221 | AZD2281 in Combination With Liposomal Doxorubicin in Advanced Solid Tumours | neoplasm | Doxorubicin (NPC261012) | |
| NCT00093795 | Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Positive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01880385 | Efficacy and Safety of Bevacizumab in the Neodjuvant Treatment of Inflammatory Breast Cancer | inflammatory breast carcinoma | Fluorouracil (NPC75844) | |
| NCT02283489 | Recombinant Human Endostatin Adenovirus Combined With Chemotherapy for Advanced Head and Neck Malignant Tumors | upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT00004202 | Combination Chemotherapy, Radiation Therapy, and RSR13 in Treating Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00691379 | Weekly Paclitaxel/Carboplatin/Bevacizumab as First Line Therapy for Triple Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00189137 | Evaluation of Side Effects and Relative Activity of Two Chemotherapy Regimens in the Treatment Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT00369070 | A Phase 2 Trial of AMG 706 or Bevacizumab in Combination With Chemo for Advanced NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03050814 | Standard of Care Alone or in Combination With Ad-CEA Vaccine and Avelumab in People With Previously Untreated Metastatic Colorectal Cancer QUILT-2.004 | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00055133 | A Study Using Intravenous Paxceed™ to Treat Patients With Rheumatoid Arthritis | rheumatoid arthritis | Paclitaxel (NPC208553) | |
| NCT02281682 | IM Versus 5-FU Versus IMI Versus MAL-PDT in Treatment of Actinic Keratosis | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT00653952 | CAELYX Versus Paclitaxel HCl in Patients With Epithelial Ovarian Carcinoma Following Failure of First-Line, Platinum-Based Chemotherapy | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT02131480 | Study of Doxorubicin and Trabectedin in First Line Treatment on Patients With Metastatic Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00323583 | Weekly Dosing of an Integrative Chemotherapy Combination to Treat Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03043729 | mFOLFOX6 vs. mFOLFOX6 + Aflibercept as Neoadjuvant Treatment in MRI-defined T3-rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00122460 | Cetuximab (Erbitux) in Combination With Cisplatin or Carboplatin and 5-Fluorouracil in the First Line Treatment of Subjects With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck (EXTREME) | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT02593708 | Phase1 of Neratinib+Trastuzumab, Pertuzumab, Paclitaxel in Patients With Advanced Solid Tumors/HER2+ | neoplasm | Paclitaxel (NPC208553) | |
| NCT00062426 | Oxaliplatin and Bevacizumab (Avastin™) With Either Fluorouracil and Leucovorin or Capecitabine in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03002103 | A Trial Evaluating the Efficacy and Safety of EndoTAG®-1 in Combination With Paclitaxel and Gemcitabine Compared With Paclitaxel and Gemcitabine as First-line Therapy in Patients With Visceral Metastatic Triple-negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00003422 | Radiation Therapy Before Surgery Compared With Chemotherapy Plus Radiation After Surgery in Treating Patients With Rectal Cancer That Can Be Surgically Removed | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00016874 | 3-AP Plus Cisplatin and Paclitaxel in Treating Patients With Advanced or Metastatic Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00058201 | Two Chemotherapy Regimens Compared With Observation in Treating Patients With Completely Resected Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00080990 | Alvocidib, Oxaliplatin, Fluorouracil, and Leucovorin Calcium in Treating Patients With Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT00003260 | Combination Chemotherapy in Treating Patients With Recurrent Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03464734 | Pembrolizumab and Nab Paclitaxel in Patients With Metastatic Urothelial Carcinoma | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT00193440 | Rituximab and Chemotherapy Followed by Ibritumomab Tiuxetan as Treatment for Low Grade Follicular Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00045747 | UCN-01 and Fluorouracil in Treating Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02595554 | Neoadjuvant Chemotherapy and Radical Surgery in Stage IIB Cervical Cancer | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT00004137 | S9914: Combination Chemotherapy Plus Filgrastim in Untreated Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02240212 | A Phase I Trial of the Combination of AZD2014 and Weekly Paclitaxel. | cancer | Paclitaxel (NPC208553) | |
| NCT05017012 | A Study to Evaluate the Bioavailability of Pembrolizumab (MK-3475) Via Subcutaneous (SC) Injection of MK-3475A (Pembrolizumab Formulated With MK-5180) In Advanced Solid Tumors (MK-3475A-C18) | neoplasm | Paclitaxel (NPC208553) | |
| NCT05235542 | A Study to Investigate Safety and Tolerability of TransCon IL-2 β/γ Alone or in Combination With Pembrolizumab and/or Chemotherapy or TransCon TLR7/8 Agonist in Adult Participants With Locally Advanced or Metastatic Solid Tumor Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00768469 | Study Evaluating Safety And Tolerability, Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT04722133 | Trastuzumab-pkrb Combined With Modified FOLFOX-6 in Biliary Tract Cancer Patients Progressed on First Line Therapy | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT03699449 | Efficacy and Safety Study of AVB-S6-500 in Patients With Platinum-Resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03181100 | Atezolizumab With Chemotherapy in Treating Patients With Anaplastic or Poorly Differentiated Thyroid Cancer | thyroid carcinoma | Paclitaxel (NPC208553) | |
| NCT04764227 | Phase II Study of Postoperative Concurrent Chemoradiotherapy for Esophageal Squamous Cell Carcinoma (ESO- Shanghai 17) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03699319 | CPI-613 in Combination With Modified FOLFIRINOX in Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00147225 | AMG 531 in Patients With Advanced Malignancy Receiving Treatment With Carboplatin | cancer | Doxorubicin (NPC261012) | |
| NCT00993655 | Two Different Schedules of Carboplatin, Paclitaxel, Gemcitabine, and Surgery in Treating Patients With Newly Diagnosed Stage IIIC or Stage IV Primary Epithelial Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer | fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01647828 | A Phase 1b/2 Study of OMP-59R5 (Tarextumab) in Combination With Nab-Paclitaxel and Gemcitabine in Subjects With Previously Untreated Stage IV Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003242 | Bryostatin 1 Plus Paclitaxel and Cisplatin in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00062179 | Paclitaxel and Carboplatin With or Without Celecoxib Before Surgery in Treating Patients With Stage IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03731442 | Salvage Chemoradiation Therapy for Recurrence After Radical Surgery or Palliative Surgery in Esophageal Cancer Patients | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00251355 | Gemcitabine, 5-Fluorouracil, and Radiation Therapy in the Treatment of Non-Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01279291 | Study of Anti-HB-EGF Antibody KHK2866 in Subjects With Advanced Solid Tumors and Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT00983541 | Chemoradiation +Gemcitabine +Continuous 5-FU (Fluorouracil) Followed by High Dose Brachytherapy/Stereotactic Radiation Boost in Locally Advanced Intra/Extrahepatic Cholangiocarcinoma | cancer | Fluorouracil (NPC75844) | |
| NCT01705691 | Comparison of Neoadjuvant Chemotherapy With Weekly Paclitaxel or Eribulin Followed by A/C in Women With Locally Advanced HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03515200 | Treatment With Combination Chemotherapy for Relapsed or Refractory Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03600623 | Folfirinox or Gemcitabine-Nab Paclitaxel Followed by Stereotactic Body Radiotherapy for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT03161574 | FOLFOXIRI Chemotherapy Alone as Neoadjuvant Treatment for Circumferential Radial Margin (CRM) Positive Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01179269 | Pazopanib and Paclitaxel for Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01889069 | A Study to Evaluate Safety, Efficacy and Pharmacokinetics of Rituximab (MabThera/Rituxan) in Participants With Diffuse Large B Cell Lymphoma (DLBCL) or Follicular Lymphoma (FL) | lymphoma | Doxorubicin (NPC261012) | |
| NCT04742036 | Capivasertib China PK Study | neoplasm | Paclitaxel (NPC208553) | |
| NCT03036488 | Safety and Efficacy Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy as Neoadjuvant Treatment for Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-173/KEYNOTE-173) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01839487 | PEGPH20 Plus Nab-Paclitaxel Plus Gemcitabine Compared With Nab-Paclitaxel Plus Gemcitabine in Participants With Stage IV Untreated Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01608464 | Pre-op Chemo-RT w/CDDP/5FU vs Chemo w/ Docetaxel/Irinotecan in PET Non Responder Resectable Esophagus Cancer | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT00008138 | S0009 Combination Chemo and Surgery in Stage III or Stage IV Ovarian Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00903942 | Abraxane and RT for Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02774187 | Sorafenib Alone Versus Sorafenib Combined With HAIC for Advanced HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT01992705 | Borderline Pancreas Study: FOLFIRINOX +SBRT | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03165994 | APX005M With Concurrent Chemoradiation for Resectable Esophageal and Gastroesophageal Junction Cancers | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01222052 | 6xFU/Epirubicin/Cyclophosphamide (FEC) Compared to 3xFEC-3xDocetaxel in High-risk Node-negative Breast Cancer Patients | breast cancer | Fluorouracil (NPC75844) | |
| NCT03349281 | Pevonedistat With VXLD Chemotherapy for Adolescent/Young Adults With Relapsed/Refractory ALL or Lymphoblastic NHL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03579771 | Gemcitabine, Cisplatin, and Nab-Paclitaxel Before Surgery in Patients With High-Risk Liver Bile Duct Cancer | intrahepatic cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT01333085 | Everolimus, Carboplatin, and Paclitaxel in Locally Advanced Head and Neck Cancer That Cannot Be Removed by Surgery | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02969473 | Definitive Concurrent Chemoradiotherapy With Docetaxel Plus Cisplatin Versus 5-fluorouracil Plus Cisplatin in Patients With Esophageal Squamous Cell Carcinoma | neoplasm of esophagus | Fluorouracil (NPC75844) | |
| NCT00800202 | A Study of Avastin (Bevacizumab) in Patients With Non-Squamous Non-Small Cell Lung Cancer With Asymptomatic Untreated Brain Metastasis | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02916511 | Study of Extensive Clinical Target Volumes in Postoperative Radiotherapy Concurrent With Chemotherapy for Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03292822 | Effects of (Licochalcone A) and Paclitaxel on Human Oral Squamous Cell Carcinoma Cell Line | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00313872 | Phase III Trial of DP Followed by FOLFIRI or the Reverse Sequence in Unresectable Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT04572100 | Pilot Study of Chemotherapy for HPV-Associated Oropharyngeal Cancer | oropharynx squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03603184 | Atezolizumab Trial in Endometrial Cancer - AtTEnd | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00276588 | Gemcitabine and Carboplatin Followed by Paclitaxel in Treating Patients With Stage III or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00702975 | Study of Combination Therapy of Carboplatin -Gemcitabine Plus Bevacizumab Beyond Progression in Patients With Locally Advanced and/or Metastatic Non-small Cell Lung Cancer (NSCLC) Who Have Not Received Prior Systemic Therapy | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01608009 | [18F]Fluciclatide-PET, Pazopanib and Paclitaxel in Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00004863 | Paclitaxel and GEM 231 in Treating Patients With Recurrent or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01777152 | ECHELON-2: A Comparison of Brentuximab Vedotin and CHP With Standard-of-care CHOP in the Treatment of Patients With CD30-positive Mature T-cell Lymphomas | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00933816 | Study of Sorafenib In Combination With Low-dose 5-fluorouracil/Cisplatin (FP) Intraarterial Infusion Chemotherapy | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02979392 | Phase I Study of TENPA in Advanced Solid Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00004257 | Oxaliplatin and Fluorouracil Plus Radiation Therapy in Treating Patients With Primary Esophageal or Stomach Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT05493332 | HAIC Combined With Donafenib Tosilate and Toripalimab for Unresectable HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00465673 | Liposomal Doxorubicin (Lipo-Dox) in Patients With Brain Metastasis From Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04149015 | Neoadjuvant POF in the Treatment of Locally Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02012192 | A Study to Evaluate the Effect of the Combination of Pertuzumab With Carboplatin-Based Standard Chemotherapy in Patients With Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05192798 | Albumin-Bound Paclitaxel Combined With Antiangiogenic Agents in First-line Treatment of Relapsed or Metastatic TNBC | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00403130 | Phase 2 Study of Gemzar, Taxol & Avastin Combination as 1st Line Treatment for Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01220154 | Neoadjuvant Chemotherapy IV Carboplatin With Weekly Paclitaxel Bevacizumab for Primary Ovarian | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00296608 | Radiation Therapy With or Without Chemotherapy Before Surgery in Treating Patients With Stage II or Stage III Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00061646 | Safety and Effectiveness of ABT-510 Plus Combination Chemotherapy in Subjects With Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00825201 | A Phase I/II Study of Paclitaxel Plus Carboplatin Plus Vorinostat in Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03056833 | Ribociclib (Ribociclib (LEE-011)) With Platinum-based Chemotherapy in Recurrent Platinum Sensitive Ovarian Cancer | peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT04887961 | Reprab Study: PLD + Trabectedin Rechallenge | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01783197 | Study of Selumetinib in Patients With Previously Treated or Untreated Advanced/Metastatic NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02382406 | Carboplatin/Nab-Paclitaxel and Pembrolizumab in NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04016142 | Carboplatin-Paclitaxel Adjuvant Chemotherapy in the Treatment of Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02730416 | Combination Chemotherapy With Nintedanib / Placebo in Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02181738 | Study of Nivolumab in Patients With Classical Hodgkin's Lymphoma (Registrational) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00433420 | Combination Chemotherapy With or Without Fluorouracil and/or Pegfilgrastim in Treating Women With Node-Positive Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02107937 | Phase II Study DCVAC/OvCa Added to First Line Carboplatin and Paclitaxel Newly Diagnosed Epithelial Ovarian Carcinoma | malignant epithelial tumor of ovary | Paclitaxel (NPC208553) | |
| NCT02792491 | Phase II Prospective Trial of Addition of Rituximab to Reduced Dose CHOP Chemotherapy in DLBC L Patients Aged 65 Years and Over | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00392704 | Treatment of Head & Neck Cancer With Chemotherapy and Radiation | head and neck malignant neoplasia | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT04460352 | Chemoradiotherapy Followed by Planned Surgery or by Surveillance and Surgery Only When Needed for Oesophageal Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00003585 | Biological Therapy Plus Chemotherapy in Treating Patients With Metastatic or Recurrent Kidney Cancer | kidney cancer | Fluorouracil (NPC75844) | |
| NCT00782041 | Patients Who Have Failed First Line Treatment for Locally Advanced and/or Metastatic Cervical Cancer | uterine neoplasm | Fluorouracil (NPC75844) | |
| NCT00006257 | SU5416 and Paclitaxel in Treating Patients With Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT02574663 | TGR-1202 Alone and in Combination With Either Nab-paclitaxel + Gemcitabine or With FOLFOX in Patients With Select Relapsed or Refractory Solid Tumors | gastric cancer;pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00882973 | Trial to Determine the Maximum Tolerated Dose of Genexol-PM Plus Gemcitabine and Evaluate Efficacy and Safety of Genexol-PM Regimens in Subjects With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03536039 | RCHOP Chemoimmunotherapy Preceded BY BBB Permeabilization by t-NGR Necrosis Factor | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02246049 | A Multicenter, Clinical Study of FOLFOXIRI With Bevacizumab As First-line Therapy in Patients With mCRC | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00017303 | Combination Chemotherapy Plus IM-862 in Treating Patients With Resected Stage III Ovarian Cancer or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00005791 | Combination Chemotherapy in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT00036868 | Combination Chemotherapy With Trastuzumab in Treating Women With Metastatic Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT03309150 | ATRi Transition Rollover Study | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003449 | Combination Chemotherapy in Treating Patients With Platinum-Resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00066716 | Celecoxib, Paclitaxel, and Carboplatin in Treating Patients With Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01515787 | PROSPECT: Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01133990 | FOLFIRI Alone Versus FOLFIRI Plus Bevacizumab Versus FOLFIRI Plus E7820 as Second-Line Therapy in Patients With Locally Advanced or Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00193531 | Paclitaxel, Carboplatin, and Oral Etoposide Followed by Weekly Paclitaxel in High Grade Neuroendocrine Carcinoma | neuroendocrine carcinoma | Paclitaxel (NPC208553) | |
| NCT00434031 | CETRA: Neoadjuvant Caelyx and Trastuzumab in Her-2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00786110 | Sorafenib Plus Paclitaxel in Adreno-Cortical-Cancer Patients | adrenal cortex carcinoma | Paclitaxel (NPC208553) | |
| NCT04844385 | Toripalimab Plus Neoadjuvant Chemotherapy Combined With Chemoradiotherapy for Locally Advanced Unresectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03776812 | Study of Relacorilant in Combination With Nab-Paclitaxel for Patients With Recurrent Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | Fallopian Tube Carcinoma | Paclitaxel (NPC208553) | |
| NCT03017326 | Paediatric Hepatic International Tumour Trial | Hepatoblastoma | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT02551159 | Phase III Open Label Study of MEDI 4736 With/Without Tremelimumab Versus Standard of Care (SOC) in Recurrent/Metastatic Head and Neck Cancer | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00002939 | Irinotecan and Paclitaxel in Treating Patients With Metastatic or Recurrent Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00028990 | Paclitaxel With or Without Bevacizumab in Treating Patients With Locally Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04338399 | The BURAN Study of Buparlisib in Patients With Recurrent or Metastatic HNSCC | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00577109 | A Study of Avastin (Bevacizumab) in Combination With Chemotherapy in Patients With Previously Untreated Metastatic Colorectal Cancer. | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00859495 | Trimodal Lung-Sparing Treatment of Pleural Mesothelioma | mesothelioma | Doxorubicin (NPC261012) | |
| NCT00357149 | Neoadjuvant Docetaxel Plus Cisplatin and 5-fluorouracil (TPF) Followed by Concomitant Chemoradiotherapy and Chemoradiotherapy Alone in Locally Advanced Squamous Cell Carcinoma of the Head and Neck Patients (SCCHNS) | upper aerodigestive tract neoplasm | Fluorouracil (NPC75844) | |
| NCT00077246 | ABI-007 in Treating Patients With Chemotherapy-Naïve Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01229930 | Carboplatin and Paclitaxel With or Without Cediranib Maleate in Treating Patients With Metastatic or Recurrent Cervical Cancer That Cannot Be Removed by Surgery | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00789581 | A Randomized Trial of Ixempra Versus Taxol in Adjuvant Therapy of Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02513563 | AZD1775 Plus Carboplatin-Paclitaxel in Squamous Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04581343 | A Phase 1B Study of Canakinumab, Spartalizumab, Nab-paclitaxel, and Gemcitabine in Metastatic PC Patients | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00379262 | Therapeutic Gain by Induction-concurrent Chemoradiotherapy and/or Accelerated Fractionation for Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00270790 | EVALUATION OF AMIFOSTINE FOR MUCOSAL AND HEMOPOETIC PROTECTION AND CARBOPLATIN, TAXOL, RADIOTHERAPY IN THE MANAGEMENT OF PATIENTS WITH HEAD AND NECK CANCER.(GCC 0202) | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01142414 | Chemotherapy and Radiation Therapy With or Without Panitumumab in Treating Patients Who Have Undergone Surgery for Advanced Hypopharyngeal Cancer, Oropharyngeal Cancer, Laryngeal Cancer, or Oral Cavity Cancer at High Risk of Recurrence | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00003158 | S9712: Radiation Therapy and Combination Chemotherapy in Treating Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00517621 | Use of FACT-GOG/NTX Questionnaire in Peripheral Neurotoxicity & Validation of a French Version of This Questionnaire | fallopian tube cancer;ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00756470 | Phase II Neoadjuvant in Inflammatory Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00671658 | Modified Hyper-CVAD (Cyclophosphamide, Vincristine, Adriamycin, and Dexamethasone) Program for Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT04943653 | Intraperitoneal Paclitaxel With XELOX in Gastric Cancer With Peritoneal Metastasis | stomach neoplasm | Paclitaxel (NPC208553) | |
| NCT00331097 | ELDA: Elderly Breast Cancer - Docetaxel in Adjuvant Treatment | breast cancer | Fluorouracil (NPC75844) | |
| NCT00209209 | Induction Chemotherapy (R-CHOP Vs. R-FC) Followed by Interferon Maintenance Versus Rituximab Maintenance in MCL | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05177796 | Panitumumab and Pembrolizumab in Combination With Neoadjuvant Chemotherapy for the Treatment of Stage III-IV Triple Negative Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT01858025 | Concurrent Chemoradiation + 5-FU + Mitomycin-C in Anal Carcinoma | carcinoma | Fluorouracil (NPC75844) | |
| NCT03542669 | Study of 6b11-OCIK Injection Treatment in Patients With Recurrent Drug-resistant Ovarian Cancer | ovarian carcinoma | Doxorubicin (NPC261012) | |
| NCT00300885 | A Randomized Controlled Trial Comparing Safety and Efficacy of Carboplatin and Paclitaxel Plus or Minus Sorafenib (BAY 43-9006) in Chemonaive Patients With Stage IIIB-IV Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00944047 | Evaluate Trastuzumab Plus Standard Chemotherapy Given Before Surgery in Breast Cancer Patients With Low HER 2 Expression | breast cancer | Doxorubicin (NPC261012) | |
| NCT05316376 | MITO 35B: Olaparib Beyond Progression Compared to Platinum Chemotherapy After Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer Patients. | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003013 | Chemotherapy Plus Surgery in Treating Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT03169738 | QUILT-3.044: NANT Non-small Cell Lung Cancer (NSCLC) Vaccine: Combination Immunotherapy in Subjects With NSCLC Who Have Progressed After Treatment With PD-1/PD-L1 Inhibitors | non-small cell lung carcinoma | Fluorouracil (NPC75844) | |
| NCT00915369 | A Clinical Trial to Study the Effects of Nanoparticle Based Paclitaxel Drug, Which Does Not Contain the Solvent Cremophor, in Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02402842 | Clinical and Biological Interest of Taxanes in Advanced Squamous Cell Anal Carcinoma | anal carcinoma | Fluorouracil (NPC75844) | |
| NCT00499603 | Paclitaxel Followed by FEC Versus Paclitaxel and RAD001 Followed by FEC In Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00668616 | Adjuvant Treatment of Breast Cancer With 1-3 Aflicted Lymph Nodes | breast cancer | Paclitaxel (NPC208553) | |
| NCT01270438 | Combination Chemotherapy and Bevacizumab With or Without RO4929097 in Treating Patients With Metastatic Colorectal Cancer | colorectal adenocarcinoma;malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT01302613 | Altered Chemotherapy Sequencing During Neoadjuvant Therapy for Patients With Stage II or III Rectal Adenocarcinoma | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00003392 | High-Dose Chemotherapy and Peripheral Stem Cell Transplantation in Treating Patients With Recurrent or Refractory Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04101812 | Pegylated Liposomal Doxorubicin, PD-1 in Treating Muscle Invasive Bladder Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT05294900 | Trial of Neoadjuvant Therapy With Paclitaxel and Carboplatin in Operable Locally Advanced Head and Neck Cancer Patients | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00194792 | Hormone Therapy and Combination Chemotherapy Before and After Surgery in Treating Patients With Stage I-IIIA Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00998192 | A Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin in Patients With Squamous Cell Carcinoma of the Lung | squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00053911 | Combination Chemotherapy Compared With Observation After Surgery in Treating Women With Relapsed Nonmetastatic Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00703170 | Phase I Study of DOXIL and Temsirolimus in Resistant Solid Malignancies | cancer | Doxorubicin (NPC261012) | |
| NCT01281176 | High-Dose or Low-Dose Vorinostat in Combination With Carboplatin or Paclitaxel in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00102609 | A Safety Study Utilizing Yondelis and Doxorubicin in Patients With a Type of Cancer Called Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT03977220 | Nab-paclitaxel Combined With S-1 Treating Diffuse Type of Stage Ⅲ Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00054873 | Tezacitabine With or Without 5-Fluorouracil (5-FU) for Advanced Esophageal Cancer or Gastric Cancer | esophageal carcinoma;stomach neoplasm;adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT04469556 | Pancreatic Adenocarcinoma Signature Stratification for Treatment | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT04068103 | Circulating Tumor DNA Testing in Predicting Treatment for Patients With Stage IIA Colon Cancer After Surgery | colon adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03643276 | Treatment Protocol for Children and Adolescents With Acute Lymphoblastic Leukemia - AIEOP-BFM ALL 2017 | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00555672 | Study Of Sunitinib In Combination With Cisplatin And 5-Fluorouracil In Patients With Advanced Gastric Cancer | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT00004093 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00027833 | Vaccine Therapy and Chemotherapy With or Without Tetanus Toxoid Compared With Chemotherapy Alone in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01966003 | Efficacy and Safety Study of ABP 215 Compared With Bevacizumab in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04089150 | MFOLFIRINOX And Stereotactic Radiotherapy (SBRT) for Pancreatic Cancer With High Risk and Locally Advanced Disease | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00770809 | Paclitaxel and Trastuzumab With or Without Lapatinib in Treating Patients With Stage II or Stage III Breast Cancer That Can Be Removed by Surgery | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01358747 | Study of a Treatment Driven by Early PET Response to a Treatment Not Monitored by Early PET in Patients With AA Stage 3-4 or 2B HL | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03740165 | Study of Chemotherapy With Pembrolizumab (MK-3475) Followed by Maintenance With Olaparib (MK-7339) for the First-Line Treatment of Women With BRCA Non-mutated Advanced Epithelial Ovarian Cancer (EOC) (MK-7339-001/KEYLYNK-001/ENGOT-ov43/GOG-3036) | peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT02767219 | The Use of Bevacizumab as a Modulator of Wound Healing Following Trabeculectomy Surgery | glaucoma | Fluorouracil (NPC75844) | |
| NCT00017160 | Combination Chemotherapy, Radiation Therapy, and Surgery in Treating Patients With Primary or Recurrent Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01220128 | Lapatinib or Trastuzumab Given Prior to Surgery With Chemotherapy in Patients With Early Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00038402 | Evaluation of the Addition of Herceptin to Standard Chemotherapy in the Neoadjuvant Setting for Operable Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00002928 | Paclitaxel in Treating Patients With Recurrent or Progressive Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00722293 | A Study to Determine the Activity of Caelyx With Trastuzumab and Docetaxel in the Treatment of Metastatic Breast Cancer (Study P03679) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT04241276 | Phase IIb Randomised Trial of ATRA in a Novel Drug Combination for Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00777673 | Preoperative Chemotherapy in Triple Negative Invasive Breast Cancer That Can be Removed by Surgery. | breast cancer | Doxorubicin (NPC261012) | |
| NCT03101748 | Neratinib and Paclitaxel With or Without Pertuzumab and Trastuzumab Before Combination Chemotherapy in Treating Patients With Metastatic or Locally Advanced Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT02299999 | SAFIR02_Breast - Efficacy of Genome Analysis as a Therapeutic Decision Tool for Patients With Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00064077 | Comparison of Four Combination Chemotherapy Regimens Using Cisplatin in Treating Patients With Stage IVB, Recurrent, or Persistent Cancer of the Cervix | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00077129 | Paclitaxel and Carboplatin in Treating Patients With Locally Advanced or Metastatic Renal Cell Cancer | kidney cancer | Paclitaxel (NPC208553) | |
| NCT04864782 | QL1604 Plus Chemotherapy Versus Chemotherapy in Subjects With Stage ⅣB, Recurrent, or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01333709 | Multicenter Phase 2 Trial: a Tailored Strategy for Locally Advanced Rectal Carcinoma | rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02539537 | A Randomized Phase III Trial Comparing Folfirinox to Gemcitabine in Locally Advanced Pancreatic Carcinoma | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00204646 | Neoadjuvant Adriamycin and Ifosfamide Plus High-Dose ICE in Patients With Soft Tissue Sarcoma (STS) | sarcoma | Doxorubicin (NPC261012) | |
| NCT00440726 | Bortezomib With Chemotherapy for Relapsed Pediatric Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00823186 | Dose-finding Study of Weekly Paclitaxel and Cisplatin in FIGO IB2 and Bulky IIA Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00266799 | The Efficacy and Safety of Pegylated Liposomal Doxorubicin Compared With Capecitabine as First Line Chemotherapy for Metastatic Breast Cancer (P04445/MK-2746-071) | breast cancer | Doxorubicin (NPC261012) | |
| NCT04199026 | Implantable Microdevice for the Delivery of Drugs and Their Effect on Tumors in Patients With Metastatic or Recurrent Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT04835870 | Zanubrutinib Plus R-CHOP for Patients With Newly Diagnosed Untreated Non-GCB DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00024414 | DHA-Paclitaxel in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00003835 | Combination Chemotherapy in Treating Patients With Stage III Colon Cancer | colorectal adenocarcinoma;malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00933803 | Hydroxychloroquine, Carboplatin, Paclitaxel, and Bevacizumab in Recurrent Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03825861 | Neoadjuvant FOLFIRINOX in the Treatment of Locally Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00117572 | Docetaxel Based Chemotherapy Plus or Minus Induction Chemotherapy to Decrease Events in Head and Neck Cancer (DeCIDE) | laryngeal neoplasm;oral cavity cancer;paranasal sinus neoplasm;pharynx cancer | Fluorouracil (NPC75844) | |
| NCT03335241 | Study of Fludarabine With Pegylated Liposomal Doxorubicin Versus Pegylated Liposomal Doxorubicin Alone In Patients With Platinum Resistant/Refractory Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00455247 | Efficacy of an Oral Formula in Prevention of Anti-cancer Therapy Side Effects | cancer | Glutamine (NPC197087) | |
| NCT01168791 | Study of Palifosfamide-tris in Combination With Doxorubicin in Patients With Front-line Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00561119 | Maintenance Versus Observation After 6 Cycles of Gemcitabine Plus Paclitaxel in Pts With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05189184 | Study of Camrelizumab in Combination With Apatinib Mesylate Plus Albumin-bound Paclitaxel and Cisplatin as the First Line Treatment of Recurrent or Metastatic, UnresectableHead and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02453282 | Phase III Open Label First Line Therapy Study of MEDI 4736 (Durvalumab) With or Without Tremelimumab Versus SOC in Non Small-Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01148628 | Dose-finding Study of CAELYXTM and RAD001 in Patients With Advanced Solid Tumors | neoplasm | Doxorubicin (NPC261012) | |
| NCT00006366 | Radiation Therapy Plus Chemotherapy Followed by Surgery in Treating Patients With Locally Advanced Cancer of the Rectum | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00269828 | A Survival Study for Women With Advanced Lung Cancer Who Have Not Previously Received Chemotherapy. | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02412722 | Study of REGN2810 (Anti-PD-1) in Patients With Advanced Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00768131 | A Study to Determine Whether EGFR Status by FISH Can Predict Results in Non Small Cell Lung Cancer (NSCLC) Patients Treated With Cetuximab, Carboplatin and Paclitaxel | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00331422 | Carboplatin, Paclitaxel, and Surgery in Treating Patients With Advanced Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cavity Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00174434 | Study Of SU011248 In Combination With Paclitaxel In Patients With Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00198432 | Chemoradiotherapy of NSCLC Stage IIIB | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00869232 | UARK 2008-02 A Trial for High-risk Myeloma Evaluating Accelerating and Sustaining Complete Remission | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT04278092 | Paclitaxel Plus Cetuximab After First-line Checkpoint Inhibitor Failure | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01275677 | Chemotherapy With or Without Trastuzumab After Surgery in Treating Women With Invasive Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT05067530 | Study Assessing the Efficacy and Safety of Alpelisib + Nab-paclitaxel in Subjects With Advanced TNBC Who Carry Either a PIK3CA Mutation or Have PTEN Loss | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03425656 | Comparing Efficacy and Safety of AryoGen Pharmed Biosimilar Trastuzumab (AryoTrust) Versus Herceptin® in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00350025 | Study of Cetuximab With and Without Weekly Paclitaxel for Patients With Previously Treated Advanced Urothelial Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00101894 | Safety of AMG 706 Plus Panitumumab Plus Chemotherapy in the Treatment of Subjects With Metastatic Colorectal Cancer | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT02038647 | Phase 2 Study of Alisertib (MLN8237) in Combination With Paclitaxel Versus Placebo in Combination With Paclitaxel as Second Line Therapy for Small Cell Lung Cancer (SCLC) | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02448537 | A Phase II Multi-Strata Study of PM01183 as a Single Agent or in Combination With Conventional Chemotherapy in Metastatic and/or Unresectable Sarcomas | sarcoma | Doxorubicin (NPC261012) | |
| NCT00002565 | Combination Chemotherapy in Treating Patients With Intermediate-Grade or Immunoblastic Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04176952 | PRIMUS002: Looking at 2 Neo-adjuvant Treatment Regimens for Resectable and Borderline Resectable Pancreatic Cancer | neoplasm | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT01998906 | A Study of Herceptin (Trastuzumab) in Combination Chemotherapy in Women With Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02050009 | Nab-Paclitaxel and Bevacizumab in Treating Patients With Unresectable Stage IV Melanoma or Gynecological Cancers | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03301350 | Neoadjuvant Carbo/Paclitaxel Followed by Doxorubicin/Cyclophosphamide in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01268059 | A Study of Carboplatin and Paclitaxel With or Without MEDI-575 in Untreated, Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004057 | Paclitaxel Plus L-778,123 in Treating Patients With Recurrent or Refractory Solid Tumors or Lymphomas | lymphoma | Paclitaxel (NPC208553) | |
| NCT05214339 | A Trial of Hepatic Arterial Infusion Combined With Bevacizumab and Sintilimab for Unresectable A-staged Hepatocellular Carcinoma in BCLC Classification (D-TRIPLET) | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00930605 | The Effectiveness of Alemtuzumab Given in Combination With CHOP and ESHAP in Patients Newly Diagnosed With Peripheral T-Cell Lymphoma (PTCL) | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00995293 | Taxotere (Docetaxel) New Indication: Squamous Cell Carcinoma of the Head and Neck (SCCHN) Treatment Registration Trial | upper aerodigestive tract neoplasm | Fluorouracil (NPC75844) | |
| NCT04888403 | Exploring the Safety and Effectiveness of Toripalimab Combined With Neoadjuvant Radiotherapy and Chemotherapy in the Locally Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00600340 | 2-arm Trial of Paclitaxel Plus Bevacizumab vs. Capecitabine Plus Bevacizumab | breast cancer | Paclitaxel (NPC208553) | |
| NCT00736320 | HD16 for Early Stage Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00003576 | Surgery and Radiation Therapy Compared With Chemotherapy and Radiation Therapy in Treating Patients With Stage III or Stage IV Head and Neck Cancer That Can Be Removed During Surgery | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00003799 | Chemotherapy, Radiation Therapy, and Surgery in Treating Patients With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04877275 | ATG-010(Selinexor) in Combination With Chemotherapy in RRMM | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00871013 | Trial for Patients Not Qualifying for TT4 and TT5 Protocols Because of Prior Therapy | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01702129 | Anti-EGFR Immunoliposomes in Solid Tumors | neoplasm | Doxorubicin (NPC261012) | |
| NCT00118209 | Rituximab and Combination Chemotherapy in Treating Patients With Diffuse Large B-Cell Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00018109 | A Multicenter Randomized Placebo-Controlled Double-Blind Study to Assess Efficacy and Safety of Glutamine and Creatine Monohydrate in Duchenne Muscular Dystrophy (DMD) | Duchenne muscular dystrophy | Glutamine (NPC197087) | |
| NCT00145769 | A Randomised Trial of Preoperative Radiotherapy for Stage T3 Adenocarcinoma of Rectum | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00107341 | Bortezomib, Paclitaxel, and Carboplatin in Treating Patients With Unresectable, Metastatic Cancer of the Esophagus or Gastroesophageal Junction | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02405910 | Ph2 Nab-paclitaxel With Gemcitabine to Determine Efficacy in Advanced Non-squamous NSCLC. | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00883116 | A Study of Ixabepilone as Second-line Therapy for Locally Advanced, Recurrent, or Metastatic Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT01763645 | A Safety and Efficacy Study of BCD-021 With Paclitaxel and Carboplatin Compared to Avastin With Paclitaxel and Carboplatin in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01515306 | A Study of Ramucirumab (IMC-1121B) and Paclitaxel in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02405585 | Immunotherapy and SBRT Study in Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01522989 | PD-0332991, 5-FU, and Oxaliplatin for Advanced Solid Tumor Malignancies | neoplasm | Fluorouracil (NPC75844) | |
| NCT01831505 | Feasibility of Assessing Lymphoma Response to Precise Local Injection of Candidate Chemotherapy Agents | lymphoma | Doxorubicin (NPC261012) | |
| NCT02303977 | Phase II Study of Abraxane and Gemicitabine in Patients With Advanced Adenocarcinoma Non-Small Cell Lung Cancer Progressing After First-Line Platinum-Based Chemotherapy | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00087178 | Comparison of Two Combination Chemotherapy Regimens in Treating Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT00990860 | Study in Asia of the Combination of TACE With Sorafenib in HCC Patients | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00773695 | A Study of Bevacizumab (Avastin) in Combination With Neoadjuvant Treatment Regimens in Participants With Primary Human Epidermal Growth Factor Receptor 2 (HER2) Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03330106 | A Study to Evaluate the Effects of Pevonedistat on the Corrected QT (QTc) Interval in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00041132 | S0213 Chemotherapy Plus Rituximab in Treating Patients With Mantle Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00967616 | Study of CS-7017 in Combination With FOLFIRI in Subjects With Metastatic Colorectal Cancer Who Failed First-Line Therapy | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01340131 | CKD-828 (40/5mg) Pharmacokinetic Study | hypertension | Paclitaxel (NPC208553) | |
| NCT00026208 | Combination Chemotherapy Plus Low-Dose Radiation Therapy in Treating Patients With Stage I or Stage IIA Hodgkin's Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00001384 | A Pilot Trial of AC (Adriamycin, Cyclophosphamide) Chemotherapy With G-CSF (Granulocyte Colony-Stimulating Factor) Followed by Infusional Taxol (Paclitaxel) as Adjuvant Treatment for High Risk Stage II and Stage III Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003281 | Topotecan and Paclitaxel in Treating Patients With Recurrent or Refractory Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003873 | Fluorouracil With or Without Eniluracil in Treating Patients With Advanced Colorectal Cancer | colorectal adenocarcinoma;malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT00920153 | Three Different Therapy Regimens in Treating Patients With Previously Untreated Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02395705 | Neoadjuvant Chemotherapy Versus Surgery Alone for Esophageal Squamous Cell Carcinoma | neoplasm of esophagus | Paclitaxel (NPC208553) | |
| NCT03059615 | A Phase 2a, Open-Label, Two Stage Study of Nerofe or Nerofe With Doxorubicin in Subjects With AML or MDS | acute myeloid leukemia;myelodysplastic syndrome | Doxorubicin (NPC261012) | |
| NCT05047991 | Study of Irinotecan Liposome Injection-containing Regimens Versus Nab-paclitaxel Plus Gemcitabine in Patients With Previously Untreated, Metastatic Pancreatic Adenocarcinoma | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT02625610 | Avelumab in First-Line Maintenance Gastric Cancer (JAVELIN Gastric 100) | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00811447 | Taxotere New Indication - Gastric Cancer Treatment Registration Trial | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT01705158 | Myocet ® - Carboplatine in Ovarian Cancer in Relapse, Sensitive to the Platinum | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03783442 | A Study of Tislelizumab (BGB-A317) in Combination With Chemotherapy as First Line Treatment in Participants With Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00424827 | A Trial of Chemo & Radiation Therapy for Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00677118 | Adjuvant Chemotherapy in Patients With Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00609765 | Avastin, Fluorouracil, Doxorubicin and Streptozocin in Locally Advanced and Metastatic Pancreatic Endocrine Tumors | pancreatic carcinoma | Doxorubicin (NPC261012) | |
| NCT02340949 | Induction FOLFOX With or Without Aflibercept Followed by Chemoradiation in High Risk Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01616303 | A Controlled Study of the Effectiveness of Oregovomab (Antibody) Plus Chemotherapy in Advanced Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00002525 | Perioperative Chemotherapy in Treating Patients With Colon Cancer That Can Be Surgically Removed | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02723331 | Perioperative Therapy for Resectable and Borderline-Resectable Pancreatic Adenocarcinoma With Molecular Correlates | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01238133 | Gamma-Secretase/Notch Signalling Pathway Inhibitor RO4929097, Paclitaxel, and Carboplatin Before Surgery in Treating Patients With Stage II or Stage III Triple-Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00002913 | Paclitaxel, Cisplatin, and Topotecan With or Without Filgrastim in Treating Patients With Newly Diagnosed Stage III or Stage IV Epithelial Ovarian Cancer | ovarian serous cystadenocarcinoma;endometrioid carcinoma | Paclitaxel (NPC208553) | |
| NCT02926183 | Study of NAC of GA Therapy for Patients With BRPC | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03834948 | AO-176 in Multiple Solid Tumor Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT00107094 | Study of Adriamycin Plus Cyclophosphamide Followed by Abraxane as Adjuvant Therapy for Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00887536 | A Clinical Trial Comparing the Combination of TC Plus Bevacizumab to TC Alone and to TAC for Women With Node-Positive or High-Risk Node-Negative, HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05332002 | A Safety and Efficacy Study of Treatment Combinations With and Without Chemotherapy in Adult Participants With Advanced Upper Gastrointestinal Tract Malignancies | cancer | Fluorouracil (NPC75844) | |
| NCT02210559 | A Study of Gemcitabine Plus Nab-paclitaxel With or Without FG-3019 in Participants With Locally Advanced, Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01621243 | M402 in Combination With Nab-Paclitaxel and Gemcitabine in Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00645177 | Phase 2 Study of ABT-869 in Combination With Paclitaxel Versus Paclitaxel Alone to Treat Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03872791 | A Study of KN046 in Subjects With Locally Advanced or Metastatic Triple-negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03810872 | An Explorative Study of Afatinib in the Treatment of Advanced Cancer Carrying an EGFR, a HER2 or a HER3 Mutation | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00119262 | Bevacizumab and Combination Chemotherapy in Patients With Lymph Node Positive Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00174655 | BIG 02/98 Docetaxel - Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT04322318 | A Study of Combination Chemotherapy for Patients With Newly Diagnosed DAWT and Relapsed FHWT | kidney Wilms tumor | Doxorubicin (NPC261012) | |
| NCT05472259 | A Study Evaluating the Efficacy of 5-FU + NALIRI and 5-FU + NALIRINOX for PDAC (NALPAC) | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03887442 | Paclitaxel vs Paclitaxel + Cetuximab in Recurrent - Metastatic Head & Neck Carcinoma After Failure of a 1º Chemotherapy | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT05254171 | Study of Nab-Paclitaxel and Gemcitabine With or Without SBP-101 in Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00452803 | Pre-operative Chemotherapy Versus Concurrent Chemoradiotherapy in N2 Positive IIIA Non Small Cell Lung Cancer (NSCLC) | lung cancer | Paclitaxel (NPC208553) | |
| NCT02885753 | Systemic Oxaliplatin or Intra-arterial Chemotherapy Combined With LV5FU2 +/- Irinotecan and an Target Therapy in First Line Treatment of Metastatic Colorectal Cancer Restricted to the Liver | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT04802980 | A Study of HB002.1T Plus Chemotherapy in Subjects With Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT02630823 | MK-3475 Immunotherapy in Endometrial Carcinoma | endometrial carcinoma | Paclitaxel (NPC208553) | |
| NCT02017600 | A Phase II Trial of Induction Chemotherapy With ND-420, Cisplatin and Fluorouracil Followed by Surgery in the Treatment of Patients With Localized Squamous Cell Carcinoma of the Esophagus | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00003092 | Paclitaxel in Treating Older Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00790244 | A Scandinavian Sarcoma Group Protocol for Patients With High-risk Soft Tissue Sarcoma of the Extremities and Trunk Wall | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02440425 | A Study of CRLX101(NLG207) in Combination With Weekly Paclitaxel in Patients With Recurrent or Persistent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003065 | Topotecan and Paclitaxel in Treating Patients With Recurrent or Metastatic Cancer of the Cervix | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00748163 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Sunitinib as First-Line Therapy in Treating Patients With Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00550784 | Combination Chemotherapy and Autologous Peripheral Stem Cell Transplant in Treating Patients With Stage III, Stage IV, or Recurrent Ovarian Epithelial Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03435289 | A Study of CPI-613 With Gemcitabine and Nab-paclitaxel for Patients With Advanced or Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00654836 | Carboplatin, Paclitaxel, and Bevacizumab in Treating Patients With Locally Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02025023 | Local 5-Fluorouracil Injection for the Treatment of Chalazia: A Prospective, Comparative Study | meibomian cyst | Fluorouracil (NPC75844) | |
| NCT00662129 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation, Gemcitabine, and Bevacizumab in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01014351 | Study of Everolimus With Paclitaxel and Carboplatin in Patients With Metastatic Melanoma | metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT00551733 | Carboplatin With Either Paclitaxel Poliglumex or Paclitaxel in Treating Women With Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003120 | S9701 Paclitaxel in Treating Patients With Advanced Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Remission | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02440958 | Modified FOLFIRINOX for Gemcitabine Refractory Pancreatic Cancer: A Phase II Multicenter Trial | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01716949 | Neoadjuvant Chemoradiation With 5-FU(or Capecitabine) and Oxaliplatin Combined With Hyperthermia in Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03250832 | Study of TSR-033 With an Anti-programmed Cell Death-1 Receptor (PD-1) in Participants With Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT00857246 | Pre-operation Chemo and Antibody Therapy Followed by Surgical Resection and Adjuvant Chemoradiation for Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00889343 | Study to Evaluate the Effects of Sorafenib if Combined With Chemotherapy (FOLFOX6 or FOLFIRI) in the Second-Line Treatment of Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT01705483 | A Study of MK-8109 (Vintafolide) Given Alone or With Chemotherapy in Participants With Advanced Cancers (MK-8109-001) | cancer | Paclitaxel (NPC208553) | |
| NCT01100372 | Liposome-Encapsulated Doxorubicin Citrate With or Without Gemcitabine Hydrochloride in Treating Patients With Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cavity Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Doxorubicin (NPC261012) | |
| NCT00796991 | Drug-Drug Interaction - 3 Arm - Carboplatin/Paclitaxel, Dacarbazine | melanoma | Paclitaxel (NPC208553) | |
| NCT01081041 | A Study in Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00094497 | Trial in Locally Advanced and Metastatic Adrenocortical Carcinoma Treatment (FIRM-ACT) | carcinoma | Doxorubicin (NPC261012) | |
| NCT02449265 | Efficacy of Consolidative Involved-site Radiotherapy for Patients With Limited-stage Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00008060 | Combination Chemotherapy in Treating Patients With Unresectable Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00189384 | Efficacy Study of Community-Based Treatment of Serious Bacterial Infections in Young Infants | Sepsis;bacterial disease | Gentamicin (NPC471628) | |
| NCT03169764 | QUILT-3.047: NANT Head and Neck Squamous Cell Carcinoma (HNSCC) Vaccine: Combination Immunotherapy in Subjects With HNSCC Who Have Progressed on or After Chemotherapy and PD-1/PD-L1 Therapy | head and neck squamous cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02753881 | Pharmacokinetics of Doxorubicin in cTACE of Liver Cancer | liver cancer | Doxorubicin (NPC261012) | |
| NCT03678883 | 9-ING-41 in Patients With Advanced Cancers | kidney cancer;lung neoplasm;metastasis | Paclitaxel (NPC208553) | |
| NCT00314678 | Cisplatin Induction With Paclitaxel Consolidation for Stage III-IV Epithelial Ovarian and Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04159155 | A Study of Various Treatments in Serous or p53 Abnormal Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01204801 | Randomized Study of Combination Chemotherapy With or Without Focused Microwave Thermotherapy Before Surgery in Treating Women With Large Breast Cancer Tumors | breast cancer | Doxorubicin (NPC261012) | |
| NCT00193037 | Doxorubicin HCI Liposome Injection Versus Weekly Docetaxel in Patients First Relapse Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003216 | Fluorouracil, Gemcitabine, and Radiation Therapy in Treating Patients With Cancer of the Pancreas | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00026273 | Fluorouracil and Leucovorin With or Without Irinotecan in Treating Patients Following Surgery for Stage III Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03023046 | Etoposide, Prednisone, Vincristine Sulfate, Cyclophosphamide, and Doxorubicin in Treating Patients With Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT04038489 | COX Inhibition and Biomarkers During Neoadjuvant Chemoendocrine Therapy for ER+, HER2- Stage I-III Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05189730 | Selected Chemotherapy Combined Immunotherapy Treated High Risk Patient After NCRT in Resected Locally Advanced ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03815461 | Nab-paclitaxel Combined With S-1 as Induction Therapy for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01493505 | EP-100 Plus Paclitaxel Versus Paclitaxel Alone in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00838370 | Pharmacogenomic and Pharmacokinetic Safety and Cost-saving Analysis in Patients Treated With Fluoropyrimidines | neoplasm | Fluorouracil (NPC75844) | |
| NCT04650984 | A Study Comparing the Efficacy of L19TNF+Doxorubicin vs Doxorubicin Alone as First-line Therapy in Patients With Advanced or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00093145 | Study of Albumin-bound Paclitaxel (Abraxane) in Combination With Carboplatin and Herceptin in Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00231075 | Evaluation of the Safety and Efficacy of Paraplatin in Combination With Taxol in Patients of 70 Years and Older With Ovary Cancer F.I.G.O. Stages III-IV | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02783599 | A Study of Olaratumab (LY3012207) in Participants With Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00136539 | Neoadjuvant Therapy With Herceptin and Taxol for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03607656 | The Effect of Traditional Chinese Treatment Combined Adjuvant Chemotherapy in IIIb and IIIc Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT01627379 | Cisplatin and 5-FU +/- Panitumumab for Patients With Nonresectable,Advanced or Metastatic Esophageal Squamous Cell Cancer | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT04526470 | Alpelisib and Paclitaxel in PIK3CA-altered Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03197584 | QUILT-3.051: NANT Ovarian Cancer Vaccine: Combination Immunotherapy in Subjects With Epithelial Ovarian Cancer Who Have Progressed on or After Standard-of-care (SoC) Therapy | ovarian cancer | Fluorouracil (NPC75844) | |
| NCT00070629 | CPG 7909 Injection in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00340184 | A Safety and Efficacy of CCRT With Paclitaxel/Carboplatin as Adjuvant Therapy to Post-operative Cervical Cancer Patients | cervical cancer | Paclitaxel (NPC208553) | |
| NCT05189197 | A Study to Evaluate Efficacy and Safety of Zanubrutinib With R-CHOP in Newly Diagnosed Non-GCB DLBCL Patients With Double Expression | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00861705 | Paclitaxel With or Without Carboplatin and/or Bevacizumab Followed by Doxorubicin and Cyclophosphamide in Treating Patients With Breast Cancer That Can Be Removed by Surgery | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04303429 | Adjuvant Chemotherapy in High Risk Stage II Colon Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT02426281 | Nab-pacliatxel Plus Gemcitabine in Korean Patients With Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04324307 | Study of Immunotherapy Combined With Chemotherapy in Locally Advanced and Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00352118 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT03409198 | Phase IIb Study Evaluating Immunogenic Chemotherapy Combined With Ipilimumab and Nivolumab in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02445404 | Compare Efficacy of CHOP Versus Fractionated ICED in Transplant-eligible Patients With Previously Untreated PTCL | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04221828 | Trial of NanoPac Focal Therapy for Prostate Cancer | prostate adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04862585 | Safely Stopping Pre-medications in Patients With Breast Cancer Who Are Receiving Paclitaxel | breast carcinoma in situ | Paclitaxel (NPC208553) | |
| NCT00686114 | Concurrent Chemoradiotherapy Containing Paclitaxel&Cisplatin With/Without Tarceva in Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02487277 | PEGPH20, Gemicitabine and Nab-Paclitaxel for Pancreatic Ductal Adenocarcinoma | adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01486602 | Specialized Radiation Therapy and Chemotherapy in Treating Patients With Stage III Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00395161 | The CRISIS Prevention Study | Sepsis | Glutamine (NPC197087) | |
| NCT00005581 | Combination Chemotherapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01338558 | A Study of Avastin (Bevacizumab) in Combination With mFOLFOX6 in Treatment-Naïve Patients With Metastatic Colorectal Cancer With or Without K-RAS Mutations, and Comparison to Cetuximab | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01353638 | Safety and Efficacy of the Addition of Alanyl-Glutamine-Dipeptide to Dialysis Solution in Peritoneal Dialysis | chronic kidney disease | Glutamine (NPC197087) | |
| NCT00126256 | Trial Comparing Two Strategies of Chemotherapy for Metastatic Colorectal Cancer | colorectal carcinoma;metastasis | Fluorouracil (NPC75844) | |
| NCT00980954 | Chemotherapy and Pelvic Radiation Therapy With or Without Additional Chemotherapy in Treating Patients With High-Risk Early-Stage Cervical Cancer After Radical Hysterectomy | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01047007 | A Dose Escalation Study of MK1775 in Combination With 5-FU or 5-FU/CDDP in Patients With Advanced Solid Tumor (1775-005) | neoplasm | Fluorouracil (NPC75844) | |
| NCT01312350 | Neoadjuvant Chemotherapy for Locally Advanced Squamous Cell Cancer of the Head and Neck (SCCHN) | hypopharyngeal carcinoma | Fluorouracil (NPC75844) | |
| NCT01590719 | A Study of Onartuzumab (MetMAb) in Combination With mFOLFOX6 in Patients With Metastatic Human Epidermal Growth Factor Receptor 2 (HER2) Negative Gastroesophageal Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT03326947 | Phage II Trial of Stathmin as Predictive Biomarker for TPF Induction Chemotherapy in OSCC | upper aerodigestive tract neoplasm | Fluorouracil (NPC75844) | |
| NCT00334321 | Pelvic IMRT With Tomotherapy in Post-Hysterectomy Endometrial Cancer Patients | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT03426904 | Neoadjuvant FOLFOX Chemotherapy for Patients With Locally Advanced Colon Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00189553 | Caelyx Plus Carboplatin Versus Paclitaxel Plus Carboplatin in Patients With Epithelial Ovarian Cancer in Late Relapse | fallopian tube cancer;ovarian cancer | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT00366106 | Alternative Schedule of Velcade/Dexamethasone Plus Doxil for Patients With Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01527422 | Cyclophosphamide, Doxorubicin, Vincristine, Prednisone, Rituximab Pateinets With Aggresive NHL | lymphoma | Doxorubicin (NPC261012) | |
| NCT00005048 | Estramustine and Paclitaxel in Treating Patients With Prostate Cancer That Has Not Responded to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03723967 | FRAIL-IMMUNE (GORTEC 2018-03) - Combination of Durvalumab With Carboplatin/Paclitaxel | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00697905 | Combination of Gemcitabine and Carboplatin in Metastatic or Recurrent Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT05319730 | A Study to Evaluate Pembrolizumab (MK-3475) in Participants With Advanced Esophageal Cancer Previously Exposed to Programmed Cell Death 1 Protein (PD-1)/ Programmed Cell Death Ligand 1 (PD-L1) Treatment | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03780634 | HAIC Plus PD-1 Antibody vs HAIC Plus Sorafenib for Advanced HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00021073 | Combination Chemotherapy in Treating Patients With Advanced Cancer | neoplasm | Fluorouracil (NPC75844) | |
| NCT02506777 | Neoadjuvant FDC With Melatonin or Metformin for Locally Advanced Breast Cancer. | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003399 | Peripheral Stem Cell Transplantation Plus Combination Chemotherapy in Treating Patients With Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT00020917 | Cetuximab Plus Combination Chemotherapy in Treating Patients With Stage IV Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04859465 | Albumin-bound Paclitaxel Combined With Liposomal Doxorubicin in the Treatment of Advanced or Unresectable Angiosarcoma | angiosarcoma | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT00933426 | Lenalidomide and Paclitaxel in Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT02392507 | A Study of Nab-Paclitaxel and Carboplatin Plus Necitumumab (LY3012211) in Participants With Stage IV Squamous NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03603756 | SHR-1210 in Combination With Apatinib and Chemotherapy in Patients With Advanced Esophageal Squamous Cell Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01857934 | Therapy for Children With Advanced Stage Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT03042780 | FOLFIRINOX in Metastatic High Grade Gastroenteropancreatic Neuroendocrine Carcinomas | pancreatic endocrine carcinoma;pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01854255 | Intraperitoneal Aerosol Chemotherapy in Gastric Cancer | gastric cancer | Doxorubicin (NPC261012) | |
| NCT04729387 | Alpelisib Plus Olaparib in Platinum-resistant/Refractory, High-grade Serous Ovarian Cancer, With no Germline BRCA Mutation Detected | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00721513 | Docetaxel, Cisplatin, and Fluorouracil Followed By Cetuximab and Radiation Therapy in Treating Patients With Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT01693276 | Gemcitabine/Abraxane Chemotherapy and Dose Escalated Radiotherapy for Locally Advanced, Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00374036 | Metastatic Gastric Cancer FFCD 03-07 | gastric cancer;metastasis | Fluorouracil (NPC75844) | |
| NCT00937560 | ZD4054 (Zibotentan) or Placebo Plus Chemotherapy in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003042 | Chemotherapy and Stem Cell Transplantation in Treating Patients With Stage IIIB Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03387098 | QUILT-3.070:Pancreatic Cancer Vaccine: Subjects With Pancreatic Cancer Who Have Progressed on or After Standard-of-care Therapy | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT04510064 | PD-1 Antibody Combined With Modified FLOT Regimen in the Treatment of Unresectable Locally Advanced or Limited Metastatic Gastric Cancer | gastric adenocarcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00546156 | Preoperative Dose-dense Doxorubicin and Cyclophosphamide Followed by Paclitaxel With Bevacizumab in Operable Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05498896 | Investigate the Contribution of Ipatasertib to Neoadjuvant Chemotherapy Plus Atezolizumab in TNBC | breast cancer | Doxorubicin (NPC261012) | |
| NCT02124707 | Weekly Carboplatin, Paclitaxel and Cetuximab Treatment for Patients With Recurrent or Metastatic SCCHN | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01560949 | Preoperative Folfirinox, Radiation Therapy for Resectable Adenocarcinoma of the Pancreas | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00550537 | Proteomic Profiling in Predicting Response in Patients Receiving Erlotinib for Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00129389 | FAC Versus FAC Plus Weekly Paclitaxel as Adjuvant Treatment of Node Negative High Risk Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT00309543 | Randomized Trial on Adjuvant Chemotherapy in Colon Carcinoma Dukes B | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00971867 | Rollover Study of Weekly Paclitaxel (BMS-181339) in Patients With Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT03496662 | BMS-813160 With Nivolumab and Gemcitabine and Nab-paclitaxel in Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma (PDAC) | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03393884 | Neo-adjuvant Pembrolizumab in Primary Stage IV Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00081042 | ABI-007 in Treating Patients With Inoperable Locally Recurrent or Metastatic Melanoma | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT04643405 | APG-1387 Plus Chemotherapy in Advanced Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00019812 | Monoclonal Antibody Plus Chemotherapy in Treating Patients With Metastatic Breast Cancer That Overexpresses HER2 | breast cancer | Paclitaxel (NPC208553) | |
| NCT02359162 | Efficacy and Safety Study of P-Gemox vs.EPOCH as First-line Chemotherapy to Treat NK/T-cell Lymphoma With Early Stage | lymphoma | Doxorubicin (NPC261012) | |
| NCT02730481 | A Study to Evaluate the Safety, Tolerability, MTD, PK, and Activity of Oraxol in Subjects w Adv. Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT05322720 | HR Positive, HER2 Negative Advanced Breast Cancer With Progression After Endocrine Therapy | neoplasm | Paclitaxel (NPC208553) | |
| NCT05189483 | Albumin-bound Paclitaxel Plus Camrelizumab for Advanced Soft Tissue Sarcoma. | soft tissue sarcoma | Paclitaxel (NPC208553) | |
| NCT01478126 | Effects of Glutamine in Ischemic Heart Disease Patients Following Cardiopulmonary Bypass | coronary artery disease | Glutamine (NPC197087) | |
| NCT03317457 | Durvalumab and Tremelimumab Compared to Doxorubicin in Patients With Advanced or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02096588 | Detection and Prevention of Anthracycline-Related Cardiac Toxicity With Concurrent Simvastatin | breast cancer | Doxorubicin (NPC261012) | |
| NCT00625573 | Phase II Trial of Abraxane and Capecitabine in Metastatic Colon Cancer (COL 01) | colorectal carcinoma | Paclitaxel (NPC208553) | |
| NCT05201430 | Neoadjuvant FOLFOXIRI Versus CapeOX Chemotherapy for Local Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00369681 | Phase 2 Study of Rituximab-ABVD in Classical Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT05112536 | Trilaciclib, a CDK4/6 Inhibitor, in Patients With Early-Stage Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00433420 | Combination Chemotherapy With or Without Fluorouracil and/or Pegfilgrastim in Treating Women With Node-Positive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00055601 | Combination Chemotherapy and Radiation Therapy With/Without Surgery In Patients With Stage II/III Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01889303 | Phase II Study of DC Versus 5-FU/CF as Chemotherapy and Concurrent Chemoradiotherapy for Locally Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00828841 | Phase 2b Study of Cetuximab With Platinum-Based Chemo as First Line Treatment of Recurrent or Advanced NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02451956 | Study of AZD5363 in Combination With Paclitaxel, in Advanced Gastric Adenocarcinoma Patients Harboring PIK3CA Mutation and/or PIK3CA Amplification as a Second-line Chemotherapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03776812 | A Phase 1b Study of Sevacizumab in Combination With Chemotherapy in Chinese Patients With Platinum-Resistant Recurrent Ovarian Cancer. | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01081951 | Intraperitoneal vs Intravenous Chemotherapy Following Neoadjuvant Chemotherapy in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01809379 | Intraperitoneal Aerosol High-pressure Chemotherapy for Women With Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT05218889 | Surufatinib Plus Camrelizumab and AS in First Line Treatment of Advanced Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00005581 | Combination Chemotherapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04516616 | Pd-1 Antibody Combined Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01070862 | Multiple Myeloma Treated With Thalidomide Before Autotransplant or With Conventional Chemotherapy and as Consolidation/Maintenance Treatment in Young and Elderly Patients : 3 Randomized Studies. | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01087424 | Mini-CHOP and Rituximab in Patients Aged Over 80 Years | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04832776 | FOLFOXIRI Plus Cetuximab Versus FOLFOXIRI Plus Bevacizumab in Conversion Therapy of Right-sided Colon Cancer Liver Metastases | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT02121990 | Dose Escalation Study of OMP-54F28 in Combination With Paclitaxel and Carboplatin in Patients With Recurrent Platinum-Sensitive Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03803254 | HAIC Plus Lenvatinib and PD-1 Antibody Versus HAIC Plus Lenvatinib for Advanced HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02307227 | A Study to Evaluate the Potential Benefit of the Addition of BYL719 to Paclitaxel in the Treatment of Breast Cancer and Head-and-neck Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT05371093 | Study of Axicabtagene Ciloleucel Versus Standard of Care Therapy in Participants With Relapsed/Refractory Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02858258 | ASCT After a Rituximab/Ibrutinib/Ara-c Containing iNduction in Generalized Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00473889 | A Clinical Trial of Vorinostat (MK0683, SAHA) in Combination With FDA Approved Cancer Drugs in Patients With Advanced Non-Small Cell Lung Cancer (NSCLC)(0683-056) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03064126 | RANGER™ Paclitaxel Coated Balloon vs Standard Balloon Angioplasty | peripheral arterial disease | Paclitaxel (NPC208553) | |
| NCT01646762 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Relapsed or Refractory Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT03392909 | Intravenous Gentamicin Therapy for Recessive Dystrophic Epidermolysis Bullosa (RDEB) | recessive dystrophic epidermolysis bullosa | Gentamicin (NPC471628) | |
| NCT02606305 | Study of Mirvetuximab Soravtansine in Combination With Bevacizumab, Carboplatin, Pegylated Liposomal Doxorubicin, Pembrolizumab, or Bevacizumab + Carboplatin in Participants With Folate Receptor Alpha (FRα) Positive Advanced Epithelial Ovarian Cancer, Primary Peritoneal, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02732938 | Ph1b/2 Study of PF-04136309 in Combination With Gem/Nab-P in First-line Metastatic Pancreatic Patients | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03678883 | 9-ING-41 in Patients With Advanced Cancers | malignant glioma;sarcoma | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT03825328 | A Trial of NS/GEMOX Chemotherapy in Patients With Untreated Pancreatic Cancer ( HZ-NS/GEMOX-PC ) | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02264990 | Study Comparing Veliparib Plus Carboplatin and Paclitaxel Versus Investigator's Choice of Standard Chemotherapy in Adults Receiving First Cytotoxic Chemotherapy for Metastatic or Advanced Non-Squamous Non-Small Cell Lung Cancer (NSCLC) and Who Are Current or Former Smokers | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00058474 | Radiation Therapy and Either Capecitabine or Fluorouracil With or Without Oxaliplatin Before Surgery in Treating Patients With Resectable Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00204568 | Trofosfamide Versus Adriamycin in Elderly Patients With Soft Tissue Sarcoma (STS) | sarcoma | Doxorubicin (NPC261012) | |
| NCT00135499 | R-ACVBP Versus R-CHOP in Patients Aged 60-65 With Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00460317 | MONET1-MOtesanib NSCLC Efficacy and Tolerability Study | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01541332 | Pomalidomide, Dexamethasone and Pegylated Liposomal Doxorubicin for Relapsed/Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT02272790 | Adavosertib Plus Chemotherapy in Platinum-Resistant Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | Fallopian Tube Carcinoma;ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00002689 | Radiation Therapy Plus Chemotherapy in Treating Patients With Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01107665 | Pazopanib and Paclitaxel as First-Line Treatment for Subjects With Unresectable Stage III and Stage IV Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT02415699 | DC-CIK Immunotherapy Plus Chemotherapy vs Chemotherapy Alone in the Adjuvant Treatment of Stage III Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00075270 | Paclitaxel With / Without GW572016 (Lapatinib) As First Line Therapy For Women With Advanced Or Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00442260 | Doxorubicin HCL Liposome Injection (DOXIL) in Combination With Abraxane in Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00088114 | STA-4783 and Paclitaxel for Treatment of Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00033696 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Limited-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003254 | SWOG-S9635 Fluorouracil Plus Ethynyluracil in Advanced Colorectal Cancer Not Responded to Fluorouracil | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02228512 | Study of Pomalidomide Combined With Modified DA-EPOCH and Rituximab in KSHV-Associated Lymphomas | Castleman disease;diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04282109 | Study to Assess the Efficacy and Safety of Nivolumab in Combination With Paclitaxel in Subjects With Head and Neck Cancer Unable for Cisplatin-based Chemotherapy (NIVOTAX) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01233843 | Chemoradiotherapy HNSCC, Randomized Study, Docetaxel,Cisplatin, 5FU,Erbitux. | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT03553537 | Efficacy and Safety of Decitabine Plus CHOP vs CHOP in Patients With Untreated Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02890511 | A Study DHP107, a Novel Oral Paclitaxel Formulation, in Patients With Advanced Solid Tumours or Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00194779 | Combination Chemotherapy and Filgrastim Before Surgery in Treating Patients With HER2-Positive Breast Cancer That Can Be Removed By Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT04002947 | Acalabrutinib With DA-EPOCH-R or R-CHOP for People With Untreated Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04661007 | To Assess the Safety and Tolerability of Tafasitamab Alone or in Combination With Other Drugs in Japanese Participants With Non-Hodgkins Lymphoma (NHL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00850512 | Study to Evaluate the Efficacy and Safety of Subsequent Treatment With the Zevalin (Ibritumomab Tiuxetan) in Elderly (More Than 60 Years) Patients With Diffuse Large B Cell Lymphoma After 4 Cycles of CHOP21-Rituximab (CHOP21-R) Therapy | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05100628 | A Study of NOX66 Plus Doxorubicin in Anthracycline-naïve, Adult Patients With Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01739218 | A Phase I/II Trial of VB-111 and Paclitaxel for Recurrent Platinum-Resistant Müllerian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00101101 | Universal Granulocyte Macrophage-colony Stimulating Factor (GM-CSF)-Producing and GM.CD40L for Autologous Tumor Vaccine in Mantle Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02527772 | Liposomal Doxorubicin Plus Gemcitabine Versus Oxaliplatin Plus Fluorouracil/Leucovorin for Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT04231448 | Phase III Study of Tucidinostat in Combination With R-CHOP in Patients With Newly Diagnosed Double-Expressor DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00006377 | Doxorubicin, Paclitaxel, and Carboplatin in Treating Patients With Primary Stage III, Stage IV, or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01637532 | Feasibility of the Combination of Chemotherapy (Carbo/Caelyx or Carbo/Doxorubicin) With Tocilizumab (mAb IL-6R) and Peg-Intron in Patients With Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02244502 | Safety, Feasibility and Effect of TTFields (200 kHz) Concomitant With Weekly Paclitaxel in Recurrent Ovarian Carcinoma (INNOVATE) | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT04005339 | NAPOLI-2: Fluorouracil, Leucovorin, and Nanoliposomal Irinotecan in Biliary Cancer | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT00158379 | Taxol Carboplatin and Erythropoetin | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00864318 | Dose Intensification Study in Refractory Germ Cell Tumors With Relapse and Bad Prognosis | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT00495170 | Concurrent Proton and Chemotherapy in Locally Advanced Stage IIIA/B Non-Small Cell Lung Cancer (NSCLC) | lung cancer | Paclitaxel (NPC208553) | |
| NCT00152477 | A Study of Paclitaxel/Carboplatin With or Without CDP791 in Patients With Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02975141 | Afatinib and Gemcitabine/Nab-paclitaxel in Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00199797 | Phase I Trial of huA33 Plus Chemotherapy in Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00803556 | Clinical Trial of the Combination of Intravenous Alvespimycin (KOS-1022), Trastuzumab With or Without Paclitaxel in Patients With Advanced Solid Tumor Malignancies or Her2 Positive Metastatic Breast Cancer Who Have Previously Failed Trastuzumab Therapy | breast cancer | Paclitaxel (NPC208553) | |
| NCT01332604 | GDC-0980 in Combination With a Fluoropyrimidine, Oxaliplatin, and Bevacizumab in Patients With Advanced Solid Tumors | cancer | Fluorouracil (NPC75844) | |
| NCT01413750 | Carboplatin and Paclitaxel With or Without Vorinostat in Treating Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05174169 | Colon Adjuvant Chemotherapy Based on Evaluation of Residual Disease | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT01827111 | Phase II Study of Abraxane Plus Ipilimumab in Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00659178 | Combination Study Of SB-485232 (Interleukin 18) And Doxil For Advanced Stage Epithelial Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT04679064 | Trial on NIraparib-TSR-042 (Dostarlimab) vs Physician's Choice CHEmotherapy in Recurrent, Ovarian, Fallopian Tube or Primary Peritoneal Cancer Patients Not Candidate for Platinum Retreatment | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT04223024 | Concurrent Chemoradiotherapy With Nimotuzumab for High Risk Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT01821729 | Proton w/FOLFIRINOX-Losartan for Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00639522 | Dose Escalation Study of Liposomal Paclitaxel With/Without Capecitabine in Patients With Advanced Gastric Carcinoma | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT00807859 | Safety Study of AMG 386 to Treat HER2-positive Locally Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02754726 | Combination Therapy for Patients With Untreated Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03804866 | NGR-hTNF in Combination With an Anthracycline in Platinum-resistant Ovarian Cancer (NGR018) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02769832 | Nab-Paclitaxel With Gemcitabine for Relapsed Small Cell Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05052099 | Phase Ib/II Single-arm Study of mFOLFOX6, Bevacizumab and Atezolizumab in Advanced Biliary Tract Cancer | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT00603538 | Study of CP-751,871 in Combination With Carboplatin and Paclitaxel in Advanced Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04644250 | Combination of Toripalimab and Neoadjuvant Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00009776 | Monoclonal Antibody Therapy, Paclitaxel, and Cyclosporine in Treating Patients With Recurrent or Refractory Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT02685657 | Neoadjuvant Chemotherapy Docetaxel With or Without SELUMETINIB in Patients With Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00499083 | Paclitaxel, Cyclophosphamide, and Doxorubicin Followed by Autologous Dendritic Cells and Surgery With or Without Radiation Therapy and/or Hormone Therapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00426556 | Efficacy and Safety of Everolimus in Combination Therapy, in Patients With HER2-overexpressing Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03432598 | Anti-PD-1 in Combination With Chemotherapy as First-Line Treatment to Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003624 | Amifostine in Treating Women With Ovarian, Peritoneal, Cervical, Fallopian Tube, Uterine, or Endometrial Cancer | cervical cancer;endometrial cancer;fallopian tube cancer;ovarian cancer;peritoneum cancer;sarcoma | Paclitaxel (NPC208553) | |
| NCT00802529 | Transtympanic Gentamicin vs. Steroids in Refractory Meniere's Disease | Meniere disease | Gentamicin (NPC471628) | |
| NCT00126191 | Intensive Chemotherapy and Rituximab in the Treatment of Burkitt Lymphoma | Burkitts lymphoma | Doxorubicin (NPC261012) | |
| NCT03855384 | Study of TQB2450 in Patients With Recurrent or Metastatic Squamous Cell Cancer of the Head and Neck(R/M SCCHN) | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT01650376 | Phase Ib Study of Olaparib Plus Weekly Carboplatin and Paclitaxel in Relapsed Ovarian Cancer | uterine cancer | Paclitaxel (NPC208553) | |
| NCT04354961 | Almonertinib Versus Paclitaxel Plus Carboplatin as First-line Treatment in Patients With EGFR Mutation Positive Locally Advanced or Metastatic Pulmonary Adenosquamous Carcinoma (ARISE) | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01711697 | An Alternative Radiation Fractionation Strategy for Locally Advanced Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00774826 | Multicentric Study, Three Randomized Arms (R-CVP vs R-CHOP vs R-FM),for Patients With Stage II-IV Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT00274456 | Phase II Trial Comparing ABI-007 (Abraxane®, Nab®-Paclitaxel) to Taxotere in First Line Therapy of Patients With Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00002896 | Radiation Therapy Plus Chemotherapy Before Surgery With or Without Chemotherapy After Surgery in Treating Patients With Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03669783 | Clinical Trial for Patients With a Stage IV Childhood Renal Tumor, Comparing Upfront Vincristine, Actinomycin-D and Doxorubicin (Standard Arm) With Upfront Vincristine, Carboplatin and Etoposide (Experimental Arm) | childhood kidney neoplasm | Doxorubicin (NPC261012) | |
| NCT03056001 | Safety, Tolerability, and Efficacy of Doxorubicin and Pembrolizumab for Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00006229 | Paclitaxel + Carboplatin With/ut BMS-275291 in Advanced or Metastatic Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01271166 | Glivec® Plus m-FOLFOX Avastin® in Advanced Colorectal Cancer | colorectal cancer | Fluorouracil (NPC75844) | |
| NCT00083915 | DTPACE Followed by Tandem Transplant With Melphalan (MEL) 200 Versus MEL/Dexamethasone/Thalidomide (DT) Platinol/Adriamycin/Etoposide (PACE) Hybrid and DTPACE Consolidation | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01165216 | Japanese Study of Ipilimumab Administered in Combination With Paclitaxel/Carboplatin in Patients With Nonsmall-cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04745832 | Phase 3 Study of Zandelisib (ME-401) in Combination With Rituximab in Patients With iNHL - (COASTAL) | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT00041119 | Four Versus Six Cycles of Cyclophosphamide/Doxorubicin or Paclitaxel in Adjuvant Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00008047 | Combination Chemotherapy Combined With Radiation Therapy in Treating Patients Who Have Stage II or Stage III Cancer of the Esophagus | esophageal cancer | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00511849 | Study Of SU011248 In Combination With Paclitaxel/Carboplatin In Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00344552 | Phase II Study of Weekly Paclitaxel (BMS-181339)in Patients With Advanced or Recurrent Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02611700 | Nimotuzumab in Combined With Paclitaxel and Cisplatin for Treatment of Metastatic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01347645 | Irinotecan Plus E7820 Versus FOLFIRI in Second-Line Therapy in Patients With Locally Advanced or Metastatic Colon or Rectal Cancer | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT04778839 | Study of Paclitaxel Micelles for Injection in Chinese Patients With Advanced Solid Tumors. | neoplasm | Paclitaxel (NPC208553) | |
| NCT02142738 | Study of Pembrolizumab (MK-3475) Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non-Small Cell Lung Cancer (MK-3475-024/KEYNOTE-024) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01485848 | A Study of MM-121 With Paclitaxel in Platinum Resistant/ Refractory Advanced Ovarian Cancers | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04081389 | Chemokine Modulation Therapy and Standard Chemotherapy Before Surgery for the Treatment of Early Stage Triple Negative Breast Cancer | breast carcinoma in situ | Paclitaxel (NPC208553) | |
| NCT00303628 | Postoperative Chemotherapy With or Without Bevacizumab for Patients With Stage II or III Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00423696 | Bevacizumab and Combination Chemotherapy as First-Line Therapy in Treating Patients With Metastatic Colorectal Cancer That Cannot Be Removed By Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01634555 | A Study to Assess the Pharmacokinetics of Ramucirumab (IMC-1121B) in Combination With FOLFIRI | neoplasm | Fluorouracil (NPC75844) | |
| NCT00798252 | Ascending Multiple-Dose Study of Brivanib Alaninate in Combination With Chemotherapeutic Agents in Subjects With Advanced Cancers | cancer | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT00989651 | Carboplatin, Paclitaxel, Bevacizumab, and Veliparib in Treating Patients With Newly Diagnosed Stage II-IV Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cancer | endometrioid carcinoma;undifferentiated carcinoma | Paclitaxel (NPC208553) | |
| NCT00064116 | Combination Chemotherapy With or Without Rituximab in Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00068757 | Lonafarnib, Trastuzumab, and Paclitaxel in Treating Patients With HER2/Neu-Overexpressing Stage IIIB, Stage IIIC, or Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00609791 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients of Different Ages With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02573493 | Nab-Paclitaxel and Cisplatin or Nab-paclitaxel as Induction Therapy for Locally Advanced Squamous Cell Carcinoma of the Head and Neck (HNSCC) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01750918 | Weekly Paclitaxel, 5FU+Irinotecan or Carboplatin+Paclitaxel in Subjects With Advanced / Metastatic Solid Tumors | cancer | Fluorouracil (NPC75844) | |
| NCT00702572 | Carboplatin, Paclitaxel, Bevacizumab and Vorinostat for Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02215447 | A Feasibility Study Of NAB-Paclitaxel In Combination With Carboplatin As First Line Treatment Of Gastrointestinal Neuroendocrine Carcinomas | neuroendocrine carcinoma | Paclitaxel (NPC208553) | |
| NCT00655876 | Paclitaxel, Cisplatin, and Radiation Therapy With or Without Cetuximab in Treating Patients With Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00787852 | A Study of Dasatinib With Concurrent Chemoradiation for Stage III Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03008512 | A Phase II Study of Weekly Genexol-PM in Patients With Hepatocelluar Carcinoma After Failure of Sorafenib | hepatocellular carcinoma | Paclitaxel (NPC208553) | |
| NCT00003386 | Vaccine Therapy in Treating Patients With Stage III or Stage IV Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04789928 | Conservative Treatment of Catheter - Related Injections With Gentamicine/EDTA | infection | Gentamicin (NPC471628) | |
| NCT04209686 | Paclitaxel, Pembrolizumab and Olaparib in Previously Treated Advanced Gastric Adenocarcinoma | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04895358 | 9-ING-41 in Patients With Advanced Cancers | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00268372 | S0427, Combination Chemotherapy & RT in Treating Patients With Stage III or Stage IV Cancer of the Oropharynx | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00088530 | BBR 2778 for Relapsed, Aggressive Non-Hodgkin's Lymphoma (NHL) | non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT01024101 | Early Phase II Study of Weekly Paclitaxel (BMS-181339) in Patient With Advanced or Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04012827 | Apatinib Mesylate Combined With Doxorubicin and Ifosfamide in Advanced Soft-tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00166465 | Stage IV Colorectal CA ALIMTA | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03013218 | A Study of Evorpacept (ALX148) in Patients With Advanced Solid Tumors and Lymphoma (ASPEN-01) | non-Hodgkins lymphoma | Fluorouracil (NPC75844) | |
| NCT03486314 | A Study to Evaluate the Effects of Rifampin on Pharmacokinetics (PK) of Pevonedistat in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03393507 | ROCKIF Trial: Re-sensitization of Carboplatin-resistant Ovarian Cancer With Kinase Inhibition of FAK | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00094835 | Study to Evaluate Motesanib With or Without Carboplatin/Paclitaxel or Panitumumab in the Treatment of Patients With Advanced Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02115204 | 4 x Epirubicin, Cyclophosphamide, Followed by 4 x Docetaxel Versus 6 x CMF / 6 x CEF | breast cancer | Fluorouracil (NPC75844) | |
| NCT02289768 | Study To Evaluate The Efficacy And Safety Of Actikerall® Solution In Patients With Grade I-II Actinic Keratoses | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT02162719 | Safety and Efficacy Study of Vintafolide and Vintafolide Plus Paclitaxel Compared to Paclitaxel Alone in Participants With Triple Negative Breast Cancer (TNBC) (MK-8109-004) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00399126 | Phase I Trial of Paclitaxel With Perifosine | neoplasm | Paclitaxel (NPC208553) | |
| NCT00286000 | High Dose Simplified Folfiri in Advanced Colorectal Cancer: a Multicentre Phase II Study | colorectal cancer | Fluorouracil (NPC75844) | |
| NCT03617432 | Chidamide Combined With CHOPE Regimen for Peripheral T-cell Lymphoma Patients | neoplasm | Doxorubicin (NPC261012) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | renal cell carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT04083963 | Phase 2 Trial of Neoadjuvant Weekly Carboplatin Plus Paclitaxel in Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01245959 | Induction Chemotherapy in Patients With Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT00923936 | Pilot Study of Liposomal Doxorubicin Combined With Bevacizumab Followed by Bevacizumab Monotherapy in Adults With Advanced Kaposi s Sarcoma | Kaposi's sarcoma | Doxorubicin (NPC261012) | |
| NCT00779129 | A Phase I, Open-label, Study of Pazopanib in Combination With Epirubicin or Doxorubicin for Advanced Solid Tumors | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00057928 | S0227 Cisplatin With Either Paclitaxel or Gemcitabine in Recurrent, Persistent, or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03507491 | A Phase II Randomized Study Comparing the Efficacy and Safety of Targeted Therapy or Cancer Immunotherapy Versus Platinum-Based Chemotherapy in Patients With Cancer of Unknown Primary Site | cancer | Paclitaxel (NPC208553) | |
| NCT01896531 | A Study of Ipatasertib (GDC-0068) in Combination With Fluoropyrimidine Plus Oxaliplatin in Participants With Advanced or Metastatic Gastric or Gastroesophageal Junction Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00246103 | Phase I Trial of Valproic Acid and Epirubicin in Solid Tumor Malignancies | neoplasm | Fluorouracil (NPC75844) | |
| NCT01013805 | Preoperative Radiotherapy and Chemotherapy in Patients With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04943445 | Study of a Pembrolizumab-based Organ Preservation Strategy for Locally Advanced Larynx Cancers | laryngeal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00182715 | Combination Chemotherapy With or Without Cetuximab as First-Line Therapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03694002 | Carboplatin and Paclitaxel With or Without Ramucirumab in Treating Patients With Locally Advanced, Recurrent, or Metastatic Thymic Cancer That Cannot Be Removed by Surgery | Thymic Carcinoma | Paclitaxel (NPC208553) | |
| NCT00289445 | Study With Mitomycin c/5-FU/FA in Pretreated Gastrointestinal Cancer Patients With Metastases (>= Second-line Treatment) | metastasis;neoplasm | Fluorouracil (NPC75844) | |
| NCT04590625 | Study With Afuresertib and Paclitaxel in Platinum Resistant Ovarian | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02617849 | Pembrolizumab With Carboplatin/Paclitaxel in Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00004174 | Combination Chemotherapy Followed By Peripheral Stem Cell Transplantation in Treating Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03678883 | Study of Pembrolizumab (MK-3475) Plus Chemotherapy vs Placebo Plus Chemotherapy as Neoadjuvant Therapy and Pembrolizumab vs Placebo as Adjuvant Therapy in Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-522/KEYNOTE-522) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT04127019 | Adjuvant 6 Cycles of Docetaxel and Cyclophosphamide or 3 Cycles of Cyclophosphamide/Epirubicin/Fluorouracil Followed by 3 Cycles of Docetaxel Versus 4 Cycles of Epirubicin and Cyclophosphamide Followed by Weekly Paclitaxel in Operable Breast Cancer (MASTER) | breast cancer | Fluorouracil (NPC75844) | |
| NCT00288431 | Safety, Tolerability and Maximum Tolerated Dose of Oral AP23573 in Combination With Doxorubicin (8669-015) | sarcoma | Doxorubicin (NPC261012) | |
| NCT00003555 | Paclitaxel Plus Chemoprotection With Amifostine in Treating Patients With Recurrent or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03300544 | Talimogene Laherparepvec, Chemotherapy, and Radiation Therapy Before Surgery in Treating Patients With Locally Advanced or Metastatic Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00323323 | CHOP and Campath-1H in Previously Untreated Aggressive T/NK-Cell Lymphomas | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03704077 | An Investigational Immuno-therapy Study of Relatlimab Plus Nivolumab Compared to Various Standard-of-Care Therapies in Previously Treated Participants With Recurrent, Advanced or Metastatic Gastric Cancer or Gastroesophageal Junction Adenocarcinoma | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02790580 | Dose-dense Doxorubicin/Cyclophosphamide With Intermittent Low-dose Sunitinib in Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT00005028 | Paclitaxel and Bryostatin 1 in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00665392 | Cetuximab and Combination Chemotherapy in Patients With Stage III-IV Resectable Oropharynx Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00254891 | Trial of Paclitaxel/Carboplatin + PF-3512676 vs Paclitaxel/Carboplatin Alone in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01326767 | Central European Society for Anticancer Research (CESAR) Study of Paclitaxel Therapeutic Drug Monitoring | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01418222 | FOLFOX/Bevacizumab With Onartuzumab (MetMAb) Versus Placebo as First-Line Treatment in Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04278287 | Chemoradiotherapy in Unresectable Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003012 | Combination Chemotherapy Following Surgery in Treating Women With Early Stage Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00003992 | Chemotherapy Plus Monoclonal Antibody Therapy in Treating Women With Stage II or Stage IIIA Breast Cancer That Overexpresses HER2 | breast cancer | Paclitaxel (NPC208553) | |
| NCT03085914 | A Study of Epacadostat in Combination With Pembrolizumab and Chemotherapy in Participants With Advanced or Metastatic Solid Tumors (ECHO-207/KEYNOTE-723) | neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01057264 | HAI Abraxane With Gemcitabine and Bevacizumab | cancer | Paclitaxel (NPC208553) | |
| NCT00974324 | Endostar Combined With CHOP Regimen as First Line Chemotherapy for Peripheral T Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00191256 | Efficacy Trial of Gemcitabine Containing Regimens As Preoperative Chemotherapy in Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003664 | Combination Chemotherapy and Biological Therapy In Treating Patients With Kidney Cancer That Is Metastatic or Cannot Be Removed Surgically | kidney cancer | Fluorouracil (NPC75844) | |
| NCT00028587 | PS-341 and Combination Chemotherapy in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00033735 | Irofulven Compared With Fluorouracil in Treating Patients With Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00048893 | Vaccine and Chemotherapy for Previously Untreated Metastatic Breast Cancer | metastasis;breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00807573 | Paclitaxel, Bevacizumab and Pemetrexed in Patients With Untreated, Advanced Non-Small Cell Lung Cancer Using Web-Based Data Collection, Patient Self-Reporting of Adverse Effects and Automated Response Assessment | lung cancer | Paclitaxel (NPC208553) | |
| NCT01852435 | R-CEOP-90/R-CEOP-70 Versus R-CHOP-50 in the Treatment of Diffuse Large B-cell Lymphoma and Follicular Lymphoma Grade 3B | diffuse large B-cell lymphoma;follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02578368 | Chemotherapy Alone vs. Chemotherapy + Surgical Resection in Patients With Limited-metastatic Adenocarcinoma of the Stomach or Esophagogastric Junction | gastric cancer | Fluorouracil (NPC75844) | |
| NCT01836679 | Chidamide in Combination With Carboplatin and Paclitaxel in Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05386524 | Sintilimab and Bevacizumab Biosimilar Combined With PLD in mTNBC | breast cancer | Doxorubicin (NPC261012) | |
| NCT00504504 | Rituximab and ABVD for Hodgkin's Patients | lymphoma | Doxorubicin (NPC261012) | |
| NCT00618826 | A Study of Biweekly Gemcitabine, Paclitaxel, and Avastin in Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03883919 | Liposomal Irinotecan Plus 5-FU / LV Combined With Paricalcitol in Patients With Advanced Pancreatic Cancer Progressed on Gemcitabine-based Therapy | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03314740 | Best Approach in Recurrent-Ovarian-Cancer-with Cediranib-Olaparib | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT01009970 | Study With Rituximab, Cyclophosphamide, Doxorubicin Liposomal (Myocet®), Vincristine, Prednisone, (R-COMP) to Treat Non-Hodgkin's Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02850419 | Heat-Activated Target Therapy of Local-Regional Relapse in Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT00559845 | A Study of Avastin (Bevacizumab) in Patients With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT00002819 | Chemotherapy With or Without Peripheral Stem Cell Transplantation in Treating Patients With Persistent Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01652976 | Phase II Study of 5-FU, Oxaliplatin Plus Dasatinib in Metastatic Pancreatic Adenocarcinoma | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01748851 | XELOX Versus FOLFOX for Advanced Gastric Cancer (AGC) | gastric carcinoma | Fluorouracil (NPC75844) | |
| NCT00711243 | Docetaxel, Oxaliplatin, and Fluorouracil in Treating Patients With Metastatic or Unresectable Stomach Cancer, Gastroesophageal Junction Cancer, or Other Solid Tumor | gastric cancer | Fluorouracil (NPC75844) | |
| NCT03571308 | A Combination of Acalabrutinib With R-CHOP for Patient Diffuse Large B-cell Lymphoma (DLBCL) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04210037 | Study of APG-1252 Plus Paclitaxel in Patients With Relapsed/Refractory Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003595 | Combination Chemotherapy With or Without Monoclonal Antibody Therapy in Treating Patients With Previously Untreated HIV-Associated Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00280176 | Bortezomib, Fluorouracil, and External-Beam Radiation Therapy in Treating Patients With Stage II, Stage III, or Stage IV Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00010257 | Carboplatin Combined With Paclitaxel in Treating Patients With Advanced Thymoma | Thymic Carcinoma;Thymoma | Paclitaxel (NPC208553) | |
| NCT01271725 | Evaluation of an Anti-cancer Immunotherapy Combined With Standard Neoadjuvant Treatment in Patients With WT1-positive Primary Invasive Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03725436 | ALRN-6924 and Paclitaxel in Treating Patients With Advanced, Metastatic, or Unresectable Solid Tumors | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01167712 | Paclitaxel and Carboplatin With or Without Bevacizumab in Treating Patients With Stage II, Stage III, or Stage IV Ovarian Epithelial Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer | endometrioid carcinoma;Fallopian Tube Carcinoma | Paclitaxel (NPC208553) | |
| NCT01678664 | Everolimus After (Chemo)Embolization for Liver Metastases From Digestive Endocrine Tumors | neuroendocrine neoplasm | Doxorubicin (NPC261012) | |
| NCT02181634 | Phase II Trial of Nab-Paclitaxel and Gemcitabine for First-Line Treatment of Patients With Cholangiocarcinoma | cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT00268853 | A Trial in Patients With Diffuse Large-B-cell Lymphoma Comparing Pixantrone Against Doxorubicin | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00004901 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Advanced Mouth Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT03910634 | IMRT With Carboplatin Versus IMRT With Carboplatin and Fluorouracil for Eldly Esophageal Cancer Patients | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT03287271 | Clinical Trial of Combined Fostamatinib and Paclitaxel in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03136406 | QUILT-3.039: NANT Pancreatic Cancer Vaccine: Combination Immunotherapy in Subjects With Pancreatic Cancer Who Have Progressed on or After Standard-of-care Therapy | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00416507 | Gemcitabine With or Without Combination Chemotherapy and Radiation Therapy in Treating Patients With Nonmetastatic Pancreatic Cancer That Cannot Be Removed By Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00311636 | Triptorelin in Preventing Early Menopause in Premenopausal Women Who Are Receiving Chemotherapy for Stage I, Stage II, or Stage III Breast Cancer That Has Been Removed By Surgery | breast cancer | Fluorouracil (NPC75844) | |
| NCT01838538 | Abraxane/Bevacizumab | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02122770 | Effects of Fluconazole and Itraconazole CYP3A-Mediated Inhibition on the Pharmacokinetics, Safety, and Tolerability of MLN4924 in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04979585 | Camrelizumab Plus Amlotinib and Chemotherapy in the First-line Treatment of Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT03117933 | Ribociclib (Ribociclib (LEE-011)) With Platinum-based Chemotherapy in Recurrent Platinum Sensitive Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01621217 | Phase I Study of Cetuximab in Combination With 5-fluoruracil, Mitomycin C and Radiotherapy in Patients With Anal Cancer Stage T2 (>4 cm) - T4 N0-3 M0 or Any T N2-3 M0 | anal neoplasm | Fluorouracil (NPC75844) | |
| NCT02241551 | Phase II Neoadjuvant Chemotheraphy (Gemcitabine and Nab-Paclitaxel vs. mFOLFIRINOX) and Sterotatic Body Radiation Therapy for Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02302807 | A Study of Atezolizumab Compared With Chemotherapy in Participants With Locally Advanced or Metastatic Urothelial Bladder Cancer [IMvigor211] | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT04931342 | Chiauranib Plus Weekly Paclitaxel in Patients With Platinum-refractory or Platinum-resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00113516 | A Study Of SU011248 As Therapy In Patients With Locally Advanced Or Metastatic Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05207514 | Compare the Efficacy and the Safety of Doxorubicin and Cyclophosphamide Followed by Taxotere Versus Doxorubicin and Cyclophosphamide Nanoxel M as Neoadjuvant Chemotherapy in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05449483 | Conversion of Tislelizumab Combined With Chemotherapy in Unresectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00100139 | Comparison of Liposome Entrapped Paclitaxel Easy to Use (LEP-ETU) and Taxol® Pharmacokinetics in Patients With Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT01724021 | A Study of Participant Preference With Subcutaneous Versus Intravenous MabThera/Rituxan in Participants With CD20+ Diffuse Large B-Cell Lymphoma or CD20+ Follicular Non-Hodgkin's Lymphoma Grades 1, 2 or 3a | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04656262 | Low Dose Continuous Cyclophosphamide vs Standard Doxorubicin in Advanced Sarcoma Elderly Patients | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT03952572 | Efficacy and Safety of CDOP vs CHOP for Newly Diagnosed Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00539331 | Phase I/II Study of AZD2171 in Combination With Paclitaxel/Carboplatin in Japanese Non-Small Cell Lung Cancer Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02167321 | Rectal Cancer, Adjuvant Chemotherapy, FOLFOX(5-fluorouracil/Leucovorin/Oxaliplatin), Total Mesorectal Excision | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04009876 | A Study to Evaluate a Chemotherapy Treatment Followed by Chemo and Radiotherapy in Patients With Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03598270 | Platinum-based Chemotherapy With Atezolizumab and Niraparib in Patients With Recurrent Ovarian Cancer | ovarian carcinoma | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT01804127 | Interim PET/CT Guided Cycle Numbers of R-CHOP in DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03912402 | Efficacy and Safety of BCD-100 (Anti-PD-1) in Combination With Platinum-Based Chemotherapy and Bevacizumab in Patients With Recurrent, Persistent or Metastatic Cervical Cancer (CAESURA) | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00499122 | NOV-002, Doxorubicin, Cyclophosphamide, and Docetaxel in Women With Newly Diagnosed Stage II or IIIC Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003972 | Combination Chemotherapy and Peripheral Stem Cell Transplantation in Treating Patients With Stage II or Stage IIIA Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04370418 | A Study of ABT-165 Plus FOLFIRI vs Bevacizumab Plus FOLFIRI in Subjects With Metastatic Colorectal Cancer Previously Treated With Fluoropyrimidine, Oxaliplatin and Bevacizumab | cancer | Fluorouracil (NPC75844) | |
| NCT02187991 | Study to Compare Alisertib With Paclitaxel vs. Paclitaxel Alone in Metastatic or Locally Recurrent Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00113438 | Safety and Effectiveness of Combretastatin A-4 Phosphate Combined With Chemotherapy in Advanced Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT00005800 | Doxorubicin and Docetaxel in Treating Women With Stage III Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00124111 | A Study of Cyclophosphamide/Methotrexate/5-Fluorouracil (CMF) With Pegfilgrastim in Subjects With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02365805 | Randomized CT to Evaluate Efficacy of Neoadjuvant Chemotherapy Customized by Levels of BRCA1-HER2 Negative Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00772798 | Paclitaxel and Carboplatin Combination as 1st Line Treatment in Ovarian Carcinomas | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03554109 | QUILT-3.057: NANT Neoadjuvant Triple- Negative Breast Cancer (TNBC) Vaccine | breast cancer | Fluorouracil (NPC75844) | |
| NCT02755311 | Liver Resection Versus Transarterial Chemoembolization for the Treatment of Intermediate-stage Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT05314998 | Adjuvant Trial in Patients With Resected PDAC Randomized to Allocation of Oxaliplatin- or Gemcitabine-based Chemotherapy by Standard Clinical Criteria or by a Transcriptomic Treatment Specific Stratification Signature | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT04621370 | A Trial of Durvalumab (MEDI 4736) in Combination With Extended Neoadjuvant Regimens in Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00455533 | Study to Assess Effectiveness of Giving Combination of Standard Chemotherapy Drugs Versus Combination of Standard Chemotherapy and New Drug Ixabepilone When Given Before Surgical Removal of Early Stage Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01534663 | Supplementation of Glutamine and Fish Oil Versus Placebo in Patients With Heart Failure | heart failure | Glutamine (NPC197087) | |
| NCT04358341 | Pegliposomal Doxorubicin and 5-fluorouracil as Second Line Therapy for Metastatic Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT03023124 | Study With Trabectedin Versus Adriamycin Plus Dacarbazine, in Patients With Advanced Solitary Fibrous Tumor | neoplasm | Doxorubicin (NPC261012) | |
| NCT05070598 | Camrelizumab Combined With Chemotherapy in First-line Treatment of HER2-positive Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT05168527 | The First Line Treatment of Fruquintinib Combined With Albumin Paclitaxel and Gemcitabine in Pancreatic Cancer Patients | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02918747 | PEG-ASP+Gemoxd vs. PEG-ASP+CHOP as First-line Chemotherapy to Treatment NK/T-cell Lymphoma With Early Stage | lymphoma | Doxorubicin (NPC261012) | |
| NCT02261805 | A Phase I/II Study of Ganetespib in Combination With Doxorubicin | small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT03430882 | Sapanisertib, Carboplatin, and Paclitaxel in Treating Patients With Recurrent or Refractory Malignant Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00025389 | Bevacizumab, Paclitaxel, and Carboplatin Before Surgery in Treating Patients With Stage IB, Stage II, or Stage IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00506155 | Neoadjuvant Chemotherapy With Methotrexate, Vinblastine, Adriamycin and Cisplatin (M-VAC) Plus Avastin in Patients With Urothelial Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT00152178 | The Comparative Trial of UFT + TAM With CMF + TAM in Adjuvant Therapy for Breast Cancer (CUBC) | breast cancer | Fluorouracil (NPC75844) | |
| NCT03042819 | Study of Selinexor and Doxorubicin in Advanced Soft Tissue Sarcomas | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00737243 | Treatment Based on Molecular Profiling Diagnosis Carcinoma of Unknown Primary Site | carcinoma | Paclitaxel (NPC208553) | |
| NCT02842580 | De-escalation Chemotherapies Versus Escalation in Non Pre-treated Unresectable Patients With Metastatic Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT01970722 | Surgery and Chemotherapy With or Without Chemotherapy After Surgery in Treating Patients With Ovarian, Fallopian Tube, Uterine, or Peritoneal Cancer | Fallopian Tube Carcinoma;ovarian carcinoma;uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT05469061 | Tislelizumab in Combination With Chemotherapy for Conversion Therapy of Locally Nonresectable ESCC | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00193011 | Weekly Docetaxel and CMF in the Adjuvant Treatment of Elderly Women With High-Risk Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02626455 | Study of Copanlisib in Combination With Standard Immunochemotherapy in Relapsed Indolent Non-Hodgkin's Lymphoma (iNHL) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04541108 | Phase 0 Master Protocol for CIVO Intratumoral Microdosing of Anti-Cancer Therapies | neoplasm | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03136055 | Pembrolizumab-based Therapy in Previously Treated High Grade Neuroendocrine Carcinomas | neuroendocrine carcinoma | Paclitaxel (NPC208553) | |
| NCT00280150 | Combination Chemotherapy, Bev, RT, and Erlotinib in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04308837 | A Phase II Trial of Neoadjuvant Laparoscopic Hyperthermic Intraperitoneal Chemotherapy (HIPEC) With Chemoradiation | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02520154 | Veliparib in Combination With Carboplatin And Weekly Paclitaxel in Japanese Subjects With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05404945 | Fitness-adapted, Pembrolizumab-based Therapy for Untreated Classical Hodgkin Lymphoma Patients 60 Years of Age and Above | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT03057600 | Study of CB-839 in Combination w/ Paclitaxel in Participants of African Ancestry and Non-African Ancestry With Advanced Triple Negative Breast Cancer (TNBC) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03189719 | First-line Esophageal Carcinoma Study With Chemo vs. Chemo Plus Pembrolizumab (MK-3475-590/KEYNOTE-590) | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT01652482 | Safety and Efficacy Study of MEHD7945A + FOLFIRI Versus Cetuximab + FOLFIRI as Second Line Therapy in Participants With KRAS Wild-Type Metastatic Colorectal Cancer (mCRC) | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00876395 | Everolimus in Combination With Trastuzumab and Paclitaxel in the Treatment of HER2 Positive Locally Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02967289 | IRinotecan and Oxaliplatin for Colon Cancer in Adjuvant Setting | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT01802749 | Bevacizumab Beyond Progression in Platinum Sensitive Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02060253 | Ganetespib, Paclitaxel, Trastuzumab and Pertuzumab for Metastatic Human Epidermal Growth Factor Receptor 2 Positive Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00388076 | A Study Of SU011248 Plus Paclitaxel Versus Bevacizumab Plus Paclitaxel In Patients With Advanced Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00973609 | Optimal Maintenance Therapy With Bevacizumab After Induction in Metastatic Colorectal Cancer (CRC) | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT01818063 | Carboplatin and Combination Chemotherapy With or Without Veliparib in Treating Patients With Stage IIB-IIIC Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT01015339 | Paclitaxel Plus Capecitabine With Capecitabine Maintenance Treatment in Metastatic Adenocarcinoma of the Stomach or Esophagogastric Junction | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01414855 | A Study of Obinutuzumab [RO5072759 (GA101)] in Combination With CHOP Chemotherapy in Patients With Previously Untreated Advanced Diffuse Large B-Cell Lymphoma (GATHER) | lymphoma | Doxorubicin (NPC261012) | |
| NCT01263041 | Effect of L-arginine and Glutamine on Preterm | Sepsis | Glutamine (NPC197087) | |
| NCT00846027 | A Study of Avastin (Bevacizumab) in Combination With Taxane-based Chemotherapy as First Line Treatment in Patients With HER-2 Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02384850 | Phase 1 Trial To Evaluate mFOLFOX6 With Selinexor In Patients With Metastatic Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00096343 | Paclitaxel and Carboplatin in Treating Women Who Are Undergoing Surgery for Newly Diagnosed, Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01494506 | Study of MM-398 With or Without 5-FU/LV, Versus 5-FU/LV in Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00476827 | A Phase II Safety and Tolerability Study of Bevacizumab When Added to Single-agent Chemotherapy to Treat Patient With Breast Cancer Metastatic to Brain | breast cancer | Paclitaxel (NPC208553) | |
| NCT00021060 | Combination Chemotherapy With or Without Bevacizumab in Treating Patients With Advanced, Metastatic, or Recurrent Non-Small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01415765 | MLN4924 Compared With MLN4924 Plus Chemotherapy for Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00654758 | A Phase 1b Study With Volociximab in Combination With Carboplatin and Paclitaxel in First-line, Advanced Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00731861 | Phase I, Pharmacokinetic, Pharmacodynamic Trial of PTK787 and Paclitaxel in Combination for Advanced Solid Tumors | metastasis;carcinoma | Paclitaxel (NPC208553) | |
| NCT01827553 | Pancreatic Carcinoma: Chemoradiation Compared With Chemotherapy Alone After Induction Chemotherapy | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT04506554 | A Study of Risk Enabled Therapy After Neoadjuvant Immunochemotherapy for Bladder Cancer | urinary bladder carcinoma | Doxorubicin (NPC261012) | |
| NCT02688712 | ExIST Study of LY2157299 (Galunisertib) in Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00274937 | Radiation Therapy, Amifostine, and Chemotherapy in Treating Young Patients With Newly Diagnosed Nasopharyngeal Cancer | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT03751761 | GC 2nd Line Durvalumab(MEDI4736)/Tremelimumab Plus Paclitaxel Study | gastric cancer | Paclitaxel (NPC208553) | |
| NCT05422794 | Testing the Addition of Anti-Cancer Drug, ZEN003694 (ZEN-3694) and PD-1 Inhibitor (Pembrolizumab), to Standard Chemotherapy (Nab-Paclitaxel) Treatment in Patients With Advanced Triple-Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04390763 | Study of Efficacy and Safety of NIS793 (With and Without Spartalizumab) in Combination With SOC Chemotherapy in First-line Metastatic Pancreatic Ductal Adenocarcinoma (mPDAC) | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00479765 | A Phase 1 / 2 Dose Escalation Study of Locally-Administered OncoGel™ in Subjects With Recurrent Glioma | brain neoplasm;glioblastoma multiforme | Paclitaxel (NPC208553) | |
| NCT03721744 | A Study of Napabucasin in Combination With Weekly Paclitaxel and Low-dose Gemcitabine in Patients With Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04729387 | Trial on NIraparib-TSR-042 (Dostarlimab) vs Physician's Choice CHEmotherapy in Recurrent, Ovarian, Fallopian Tube or Primary Peritoneal Cancer Patients Not Candidate for Platinum Retreatment | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00005078 | Tirapazamine, Carboplatin, and Paclitaxel in Treating Patients With Advanced Malignant Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00066274 | Comparison of Combination Chemotherapy Regimens in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00049790 | Safety and Efficacy Study of rhAngiostatin Administered in Combination With Paclitaxel and Carboplatin to Patients With Non-Small-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00490711 | Treatment of Paclitaxel Plus Carboplatin Followed by Gemcitabine Plus Carboplatin for Patients With Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00723957 | A Randomized Phase 2 Study of Ixabepilone Plus Carboplatin and Paclitaxel Plus Carboplatin in Advanced Nonsmall-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01415336 | AX Versus AC as Adjuvant Treatment for Node-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00052559 | Bevacizumab, Fluorouracil, and External-Beam Radiation Therapy in Treating Patients With Stage II or Stage III Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00020787 | Vaccine Therapy Plus Chemotherapy in Treating Patients With Metastatic or Locally Recurrent Stomach Cancer or Esophageal Cancer | esophageal cancer;gastric cancer | Fluorouracil (NPC75844) | |
| NCT00034190 | Safety and Efficacy of S-8184 in Second Line Treatment of Stage III or IV Colorectal Adenocarcinoma | colorectal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00016406 | S0012 Doxorubicin, Cyclophosphamide, and Paclitaxel With or Without Filgrastim in Treating Women With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01968915 | Safety and Tolerability of Oral LCL161 in Japanese Adult Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02641691 | Non-Operative Radiation Management of Adenocarcinoma of the Lower Rectum | rectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00542191 | Phase II Trial of Neoadjuvant Metronomic Chemotherapy in Triple-Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00970580 | A Study of BIIB022 in Combination With Paclitaxel and Carboplatin in Subjects With Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03542266 | CC486-CHOP in Patients With Previously Untreated Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02835586 | STREAMER : STent Restenosis And MEdicaments Release | ischemia | Paclitaxel (NPC208553) | |
| NCT02820857 | Assessment of the Efficacy of Bevacizumab in Combination With Folfiri as Second-line Treatment in Patients Suffering From an Advanced Inoperable Poorly Differentiated Neuroendocrine Carcinoma of an Unknown or Gastroentero-pancreatic Primary Cancer | neuroendocrine carcinoma | Fluorouracil (NPC75844) | |
| NCT02551432 | Pembrolizumab and Paclitaxel in Refractory Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00321828 | Combination Chemotherapy and Bevacizumab in Treating Patients With Stage IV Colon Cancer That Cannot Be Removed By Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT05210374 | Disulfiram With Copper Gluconate and Liposomal Doxorubicin in Treatment-Refractory Sarcomas | sarcoma | Doxorubicin (NPC261012) | |
| NCT01476787 | Combined Rituximab and Lenalidomide Treatment for Untreated Patients With Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02255110 | A Japanese Trial of TH-302 in Subjects With Locally Advanced Unresectable or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01935492 | 8 Continuous vs 8 Intermittent Cycles in First and Second Line in HER2/Neu Neg Metastatic Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT01277406 | 4SC-201 (Resminostat) in Advanced Colorectal Carcinoma | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00361621 | Ph II CHOP+Velcade in Mediastinal LBCL | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03837977 | Second-line Therapy for Patients With Progressive Poorly Differentiated Extra-pulmonary Neuroendocrine Carcinoma | neuroendocrine carcinoma | Fluorouracil (NPC75844) | |
| NCT02494973 | Postoperative Hepatic Arterial Chemotherapy in High-risk Patients as Adjuvant Treatment After Resection of Colorectal Liver Metastases | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00337376 | A Study of the mTOR Inhibitor Rapamycin (Rapamune, Sirolimus) in Combination With Abraxane in Advanced Solid Cancers | cancer | Paclitaxel (NPC208553) | |
| NCT00647530 | Fluorouracil and Oxaliplatin With or Without Panitumumab In Treating Patients With High-Risk Colon Cancer That Can Be Removed by Surgery | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00807911 | Adjuvant Chemotherapy After Preoperative Chemoradiotherapy to Treat Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT02023593 | FOLFIRI as Second Line Chemotherapy for Metastatic Esophageal Carcinoma | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT00003614 | Paclitaxel Plus Estramustine in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT05446870 | Pembrolizumab With Chemotherapy and MK-4830 for Treating Participants With Ovarian Cancer (MK-4830-002) | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT01009801 | Transarterial Chemoembolization With Doxorubicin With or Without Everolimus in Treating Patients With Liver Cancer | liver cancer | Doxorubicin (NPC261012) | |
| NCT00568464 | Study on VCD/IE in the Patients With Ewing's Sarcoma Family of Tumors (ESFT) | Ewing sarcoma | Doxorubicin (NPC261012) | |
| NCT02941562 | A Prospective Phase II Randomized Clinical Trial of Preoperative Chemotherapy Combined With Short-course Radiotherapy Versus Conventional Neo-adjuvant Therapy for Locally Advanced Rectal Cancer Implemented by MDT | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03932409 | Frontline Immunotherapy Combined With Radiation and Chemotherapy in High Risk Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01216111 | Adjuvant Platinum and Taxane in Triple-negative Breast Cancer (PATTERN) | breast cancer | Fluorouracil (NPC75844) | |
| NCT04148885 | A Trial of Paclitaxel (Albumin-binding) for Castration-resistant Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00005021 | Combination Chemotherapy With or Without Biological Therapy in Treating Patients With Refractory Solid Tumor or Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00052936 | Combination Chemotherapy With or Without Rituximab in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01121848 | Randomized Study With Oxaliplatin in 2nd Line Pancreatic Cancer | pancreatic neoplasm | Fluorouracil (NPC75844) | |
| NCT00129922 | Fluorouracil, Epirubicin, and Cyclophosphamide Alone or Followed by Paclitaxel for Early Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00658957 | Prevention of Infection Using a Topical Gentamicin-Collagen Sponge in Diabetic Patients With An Uninfected Foot Ulcer | diabetic foot | Gentamicin (NPC471628) | |
| NCT04589234 | Saltikva for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00004242 | Combination Chemotherapy in Treating Patients With Metastatic or Unresectable Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT00559455 | Phase II Study of Eloxatin+5-FU/LV in Patients With Unresectable Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00193193 | Weekly Paclitaxel, Low-Dose Estramustine, and Carboplatin in the Treatment of Hormone Refractory Prostate Carcinoma | prostate cancer | Paclitaxel (NPC208553) | |
| NCT01642771 | Comparative Study on Two Post-operative Adjuvant Chemotherapy Regimens for Treating Triple-negative Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01036087 | Panitumumab, Nab-paclitaxel and Carboplatin for HER2 Negative Inflammatory Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT03977233 | Tumor Subtypes in Subjects on FOLFIRINOX With Non-Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01662869 | A Study of Onartuzumab in Combination With mFOLFOX6 in Participants With Metastatic HER2-Negative and MET-Positive Gastroesophageal Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT04767295 | A Study of Camrelizumab Combined With Chemotherapy as Neoadjuvant Therapy in Adcanced Esophageal Squamous Cell Carcinoma (ESCC) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00820547 | Efficacy and Tolerance Study of Bevacizumab in Her2- Inflammatory Breast Cancer Patients | breast cancer | Fluorouracil (NPC75844) | |
| NCT04371224 | NaliCap (Irinotecan Liposome (Nal-IRI)/Capecitabine) vs. NAPOLI (Nal-IRI/5-FU/LV) ) in Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00226746 | Efficacy Study of Gemcitabine, Paclitaxel, and Irradiation in the Treatment of Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03498521 | Study of Paclitaxel in Combination With BOS172722 in Patients With Advanced Nonhaematologic Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00265018 | HD10 for Early Stages | lymphoma | Doxorubicin (NPC261012) | |
| NCT00020488 | Combination Chemotherapy in Treating Patients Who Have Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT02256436 | A Study of Pembrolizumab (MK-3475) Versus Paclitaxel, Docetaxel, or Vinflunine for Participants With Advanced Urothelial Cancer (MK-3475-045/KEYNOTE-045) | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT03678883 | 9-ING-41 in Patients With Advanced Cancers | Malignant Bone Neoplasm | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT05214976 | A Trial of Camrelizumab for Injection in Combination With Famitinib Malate Capsule and Paclitaxel For Injection(Albumin Bound) in Advanced Solid Tumors of Patients | neoplasm | Paclitaxel (NPC208553) | |
| NCT00583349 | Phase I & II Trial of Intravesicular Abraxane for Treatment-refractory Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01497470 | A Clinical Study in Cancer Patients to Investigate the Potential Impact of Custirsen, on the Blood Levels of the Chemotherapeutic Drug, Paclitaxel, When Given Together as Part of a Treatment Regimen | cancer | Paclitaxel (NPC208553) | |
| NCT00211185 | A Study of ONTAK and CHOP in Newly Diagnosed, Peripheral T-Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01985841 | Bevacizumab in Combination With Chemotherapy in the Neo-adjuvant Setting for HER2 (-) Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00005046 | Paclitaxel in Treating Patients With Recurrent or Persistent Ovarian or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01487226 | Adjuvant Chemotherapy in Patients With Lymph Node Metastasis After Radical Surgery in Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04475016 | TIP (Paclitaxel + Ifosfamide + Cisplatin) Combined With Nimotuzumab & Triprilimab as Neoadjuvant Treatment in Locally Advanced Penile Cancer | penile cancer | Paclitaxel (NPC208553) | |
| NCT03777462 | Borderline Resectable Pancreatic Cancer Neoadjuvant Chemoradiotherapy Clinicaltrial-1 | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01882816 | Intensity-Modulated Radiation Therapy (IMRT) in the Treatment of Non-Anaplastic Non-Medullary Thyroid Cancer | thyroid cancer | Doxorubicin (NPC261012) | |
| NCT00423293 | Intensity-Modulated Radiation Therapy, Fluorouracil, and Mitomycin C in Treating Patients With Invasive Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT01274962 | A Study on the Timing of FOLFOX for Patients With Operable, Node Positive Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03564691 | Study of MK-4830 as Monotherapy and in Combination With Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-4830-001) | neoplasm | Paclitaxel (NPC208553) | |
| NCT04341883 | Study of Anti-PD-1 Combined With Albumin-Bound Paclitaxel in Patients With Recurrent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04776525 | Sequential Neoadjuvant Chemotherapy in Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT05485766 | Novel Neoadjuvant and Adjuvant Strategy for Germline BRCA 1/2 Mutated Triple Negative Breast Cancer | Hereditary breast and ovarian cancer syndrome | Paclitaxel (NPC208553) | |
| NCT00003029 | Fluorouracil With or Without Cisplatin in Treating Patients With Advanced or Metastatic Cancer of the Pancreas | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03037541 | Cryosurgery and Cream Combination for Actinic Keratosis | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT00002582 | Tamoxifen, Ovarian Ablation, and/or Chemotherapy in Treating Women With Stage I, Stage II, or Stage IIIA Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02400567 | Efficacy of Letrozole + Palbociclib Combination as Neoadjuvant Treatment of Stage II-IIIA PAM 50 ROR-defined Low or Intermediate Risk Luminal Breast Cancer, in Postmenopausal Women | breast cancer | Fluorouracil (NPC75844) | |
| NCT00016913 | Chemotherapy, Hormone Therapy, and Radiation Therapy in Treating Patients With Locally Advanced Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03071926 | Metronomic PLD in Patients With Primary Endocrine Resistant ABC | breast cancer | Doxorubicin (NPC261012) | |
| NCT04527900 | The UPPROACH (Upfront Intensity Modulated Proton Beam Therapy) Approach | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01836653 | Achievement of Improved Survival by Molecular Targeted Chemotherapy and Liver Resection for Not Optimally Resectable Colorectal Liver Metastases | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02812654 | Ifosfamide, Doxorubicin and Hypofractionated Radiotherapy in Neoadjuvant Sarcoma Treatment | sarcoma | Doxorubicin (NPC261012) | |
| NCT05298020 | Envafolimab Combined With Endostar and Chemotherapy for First-line Treatment of Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00541125 | G-CSF in Preventing Neutropenia During First-Line Treatment With Chemotherapy and Bevacizumab in Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03197584 | Neo-adjuvant Pembrolizumab in Primary Stage IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00733889 | A Study to Evaluate the Combination of Cetuximab and Chemotherapy as Neoadjuvant Therapy Followed Concomitant Chemoradiotherapy Plus Cetuximab in Locoregional Esophageal Carcinoma | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT01004991 | Phase I/II Trial of R-CHOP + Azacytidine in Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01514188 | Preliminary Efficacy and Safety of INNO-206 Compared to Doxorubicin in Advanced Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT04481204 | New and Emerging Therapies for the Treatment of Resectable, Borderline Resectable, or Locally Advanced Pancreatic Cancer, PIONEER-Panc Study | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | cutaneous melanoma;breast carcinoma | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT00971841 | Rollover Study of Weekly Paclitaxel (BMS-181339) in Patients With Advanced or Recurrent Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04269200 | Durvalumab With or Without Olaparib as Maintenance Therapy After First-Line Treatment of Advanced and Recurrent Endometrial Cancer | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT05193292 | Camrelizumab Combined With Trastuzumab and Chemotherapy in Patients With HER2-positive Advanced Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT03278015 | Phase 2 Trial of Gemcitabine vs S-1 vs Gemcitabine Plus Nab-paclitaxel as Adjuvant Chemotherapy of Post-operative Pancreatic Cancer Patients | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00314977 | Sequential vs Upfront Intensified Neoadjuvant Chemotherapy in Patients With Large Resectable or Locally Advanced Breast Cancer. | breast cancer | Doxorubicin (NPC261012) | |
| NCT04443348 | Pre-op Pembro + Radiation Therapy in Breast Cancer (P-RAD) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00001450 | Phase II Trial of a 96-Hour Continuous Infusion of Paclitaxel Followed by Cisplatin for Patients With Stage III/IV and Relapsed NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01893294 | Gemcitabine Hydrochloride in Treating Patients With Locally Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00003150 | Combination Chemotherapy With or Without Monoclonal Antibody Therapy in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003957 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Relapsed Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT03002064 | Docetaxol Plus Cisplatin Versus 5-Fu Plus Cisplatin as 1st-line Chemotherapy in Advanced ESCC Patients | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00948675 | Study of Participants With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02718417 | Phase I Low Dose WART in Combination With Weekly Paclitaxel for Platinum Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03725059 | Study of Pembrolizumab (MK-3475) Versus Placebo in Combination With Neoadjuvant Chemotherapy & Adjuvant Endocrine Therapy in the Treatment of Early-Stage Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative (ER+/HER2-) Breast Cancer (MK-3475-756/KEYNOTE-756) | breast cancer | Doxorubicin (NPC261012) | |
| NCT03416153 | Individualized Adaptive De-escalated Radiotherapy for HPV-related Oropharynx Cancer | oropharynx cancer | Paclitaxel (NPC208553) | |
| NCT00809133 | Trial Exploring Afatinib (BIBW 2992) + Paclitaxel (Part A), Afatinib + Paclitaxel + Bevacizumab (Part B), Afatinib + Carboplatin (Part C) and Afatinib+ Paclitaxel +Carboplatin(Part D) in Patients With Advanced Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT00966914 | Phase 3 Study of Tavocept Versus Placebo in Patients With Newly Diagnosed or Relapsed Advanced Primary Adenocarcinoma of the Lung Treated With Docetaxel or Paclitaxel Plus Cisplatin | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05093920 | Role of DEB-TACE Versus c-TACE in Treatment of HCC | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT01783535 | Protocol for the Study and Treatment of Participants With Intraocular Retinoblastoma | retinoblastoma | Doxorubicin (NPC261012) | |
| NCT04077983 | Nab-Paclitaxel Combined With Gemcitabine Adjuvant Chemotherapy After Radical Resection of Intrahepatic Cholangiocarcinoma | cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT02581215 | Phase II Randomized Trial of mFOLFIRINOX +/- Ramucirumab in Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT04675983 | A Study of Sintilimab Plus Ramucirumab as First-line Treatment for G/EGJ Adenocarcinoma (ORIENT-106) | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00226915 | Trial of Tri-weekly TJ Versus Weekly TJ for Stage II-IV Mullerian Carcinoma | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00195013 | Randomized Placebo-Controlled Trial of Glutamine for Breast Cancer Patients With Peripheral Neuropathy | peripheral neuropathy | Glutamine (NPC197087) | |
| NCT00006929 | Suramin, Paclitaxel, and Carboplatin in Treating Patients With Stage IIIB or Stage IV Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01060904 | A Phase 1 Study of Brentuximab Vedotin Combined With Multi-Agent Chemotherapy for Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02710734 | Risk Enabled Therapy After Initiating Neoadjuvant Chemotherapy for Bladder Cancer (RETAIN) | urinary bladder carcinoma | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT00370760 | Oral Colchicine Combined With Intravitreal Infusion of Dexamethasone, LMW Heparin and 5-FU for Management of Proliferative Vitreoretinopathy (PVR) | proliferative vitreoretinopathy | Fluorouracil (NPC75844) | |
| NCT02013453 | A Phase II Trial of Proton Pump Inhibitors With Chemotherapy in Patients With Metastatic Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01993810 | Comparing Photon Therapy To Proton Therapy To Treat Patients With Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05125055 | Neoadjuvant Anti-PD-1 and TP Versus TPF on Pathological Response in OSCC | oral squamous cell carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT01696032 | SGI-110 in Combination With Carboplatin in Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03344172 | Pre-Operative Trial (PGHA vs. PGH) for Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01959490 | Trastuzumab and Pertuzumab or Bevacizumab With Combination Chemotherapy in Treating Patients With Stage II-III Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00393068 | Chemotherapy, Radiation Therapy and Immunotherapy Prior to Surgery in Operable Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00539617 | Chemotherapy & Erlotinib in Treating Patients w/ Esophageal or Gastroesophageal Cancer That Cannot Be Removed by Surgery | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT02021422 | A Pilot, Prospective, Non-randomized Evaluation of the Safety of Anakinra Plus Standard Chemotherapy | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02229058 | Albumin Bound Paclitaxel Plus S-1 as the First Line Chemotherapy in Advanced or Recurrent Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00338221 | Clinical Trial of Alanyl-Glutamine or Glycine in Children With Persistent Diarrhea or Malnutrition | malnutrition | Glutamine (NPC197087) | |
| NCT01183780 | A Study in Second Line Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00084448 | Paclitaxel and Celecoxib in Treating Patients With Recurrent or Persistent Platinum-Resistant Ovarian Epithelial or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01301716 | A Study of the Safety and Pharmacology of GDC-0980 in Combination With Either Paclitaxel and Carboplatin (With or Without Bevacizumab) or Pemetrexed and Cisplatin in Patients With Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT00030368 | Bortezomib and Paclitaxel in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03428529 | Capecitabine Versus Bolus 5-Fu Associated to Radiotherapy as Neoadjuvant Treatment for Rectal Cancer. | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03417921 | A Study of ABTL0812 in Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00005644 | Combination Chemotherapy in Treating Patients With Advanced Cancer of the Urothelium and Decreased Kidney Function | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT04134468 | MRI Effects of Pegvorhyaluronidase Alfa (PEGPH20) in Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04634539 | Trial of First-line L-glutamine With Gemcitabine and Nab-paclitaxel in Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Glutamine (NPC197087) | |
| NCT01003158 | Study Assessing Safety and Tolerability of AZD8931 Alone or in Combination With Paclitaxel in Japanese Patients. | breast cancer | Paclitaxel (NPC208553) | |
| NCT02391662 | Nab-paclitaxel and Gemcitabine, in Elderly Patients Untreated Metastatic Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02069041 | A Study of Ramucirumab (LY3009806) in Participants With Advanced Liver Cancer | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02125513 | Neoadjuvant Chemotherapy in Epithelial Ovarian Cancer | Fallopian Tube Carcinoma;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT02887313 | FOLFOX6 Totally Neoadjuvant Chemoradiation Therapy in Locally Advanced Rectal Cancer: A Real World Study | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03596281 | Pembrolizumab in Combination With Bevacizumab and Pegylated Liposomal Doxorubicin in Patients With Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01244256 | Efficacy and Comparative of the Association Beclomethasone Clotrimzaol + Gentamicin in Patients With Acne Contaminated | folliculitis | Gentamicin (NPC471628) | |
| NCT01321008 | Stage I/II Nasal NK Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03524508 | Nal-IRI(Nanoliposomal Irinotecan) Plus 5-FU/LV in Metastatic Biliary Tract Cancer | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT02715804 | A Study of PEGylated Recombinant Human Hyaluronidase in Combination With Nab-Paclitaxel Plus Gemcitabine Compared With Placebo Plus Nab-Paclitaxel and Gemcitabine in Participants With Hyaluronan-High Stage IV Previously Untreated Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01183559 | A Trial of ZD6474, Paclitaxel, Carboplatin, 5-Fluorouracil, and Radiation Therapy Followed by Surgery | esophageal carcinoma;stomach neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02152137 | Inolitazone Dihydrochloride and Paclitaxel in Treating Patients With Advanced Anaplastic Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT04675528 | Food Effect on Pharmacokinetics and SafEty of DHP107 (Liporaxel®) FEEL Study | neoplasm | Paclitaxel (NPC208553) | |
| NCT00209716 | Phase I/II Study of Taxotere,CDDP and 5-FU(TPF) in Pre-treated Pts With Metastatic Esophageal Cancer. | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT01887587 | Vincristine, Doxorubicin, And Dexamethasone + Ixazomib in Acute Lymphoblastic Leukemia (ALL), Lymphoblastic Lymphoma Or Mixed Phenotype Acute Leukemia | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00137111 | Therapy for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01366131 | Study Evaluating the Safety and Efficacy of MEGF0444A in Combination With Carboplatin, Paclitaxel and Bevacizumab in Patients With Advanced or Recurrent Non-Squamous Non-Small Cell Lung Cancer Who Have Not Received Prior Chemotherapy for Advanced Disease (NILE) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00057382 | T138067 Versus Doxorubicin in Chemotherapy-Naive, Unresectable, Hepatocellular Carcinoma Patients | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00461344 | Docetaxel + Doxorubicin as Neoadjuvant Chemotherapy in Patients With Breast Cancer | breast ductal carcinoma in situ | Doxorubicin (NPC261012) | |
| NCT00295789 | Trial of TP Versus TC Regimens for Stage IVb, Persistent, or Recurrent Cervical Cancer (JCOG0505, CC-TPTC-P3) | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00054548 | Combination Chemotherapy Plus Oblimersen in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01605526 | A Study of RO5045337 in Combination With Doxorubicin in Patients With Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT01046864 | Combination of Brivanib With 5-Fluorouracil/Leucovorin (5FU/LV) and 5-Fluorouracil/Leucovorin/Irinotecan (FOLFIRI) | cancer | Fluorouracil (NPC75844) | |
| NCT00894569 | Paclitaxel/Carboplatin With or Without Cetuximab in CUP | carcinoma | Paclitaxel (NPC208553) | |
| NCT00191672 | A Trial Of Gemcitabine Plus Paclitaxel And Gemcitabine Plus Docetaxel In Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04723875 | Postoperative Adjuvant Chemotherapy in Early-stage Cervical Cancer That Not Meet Criteria of Adjuvant Therapeutic According to NCCN Guideline | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00016341 | Combination Chemotherapy Compared With Hormone Therapy in Treating Patients With Recurrent, Stage III, or Stage IV Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01181401 | InductionChemo-Radio-Antibody-Treatment | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT04762901 | LCI-BRE-MTN-NIR-001:Ph I Study of Niraparib in Combo With Standard Chemo in Metastatic Trip Neg Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03396445 | Study to Compare Neoadjuvant Combination of Trastuzumab and Pertuzumab With Concurrent Taxane Chemotherapy or Endocrine Therapy and Quality of Life Assessment Under Adjuvant Therapy in Operable HER2+/HR+ Breast Cancer Patients | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00617591 | Pegylated Liposomal Doxorubicin, Low Freq Dexamethasone & Revlimid (Dd-R) in Newly Diagnosed Multiple Myeloma (MM) | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01641939 | A Study of Trastuzumab Emtansine Versus Taxane in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01227707 | A Study of Avastin (Bevacizumab) Plus Xeloda (Capecitabine) in Patients With Locally Advanced Rectal Cancer. | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01821859 | A Study of MEK162 and Paclitaxel in Patients With Epithelial Ovarian, Fallopian Tube or Peritoneal Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00533585 | BAY 43-9006 in Previously Untreated Patients With Non-Small Cell Lung Cancer (NSCLC) | lung cancer | Paclitaxel (NPC208553) | |
| NCT01060007 | Five Fractions of Radiotherapy Followed by Full Dose FOLFOX Chemotherapy as Preoperative Treatment for Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03771846 | HAIC Versus Systemic Chemotherapy for Unresectable ICC | intrahepatic cholangiocarcinoma | Fluorouracil (NPC75844) | |
| NCT02283372 | Nab-Paclitaxel Plus Gemcitabine With Concurrent MR-Guided IMRT in Patients With Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00002999 | S9715, Chemotherapy and Radiation Therapy in Treating Patients With Advanced Cancer of the Nasopharynx | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT01131364 | Combination Therapy of F16IL2 and Doxorubicin in Solid Tumour Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT04019769 | Glutamine Supplementation in People With Immune Dysregulation | Eczema | Glutamine (NPC197087) | |
| NCT00011986 | Combination Chemotherapy in Treating Patients With Stage III or Stage IV Ovarian Epithelial Cancer or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02892123 | Vevorisertib (ARQ 751) as a Single Agent or in Combination With Other Anti-Cancer Agents, in Solid Tumors With PIK3CA / AKT / PTEN Mutations (MK-4440-001) | cancer | Paclitaxel (NPC208553) | |
| NCT00003326 | Paclitaxel in Treating Patients With Metastatic, Recurrent, or Unresectable Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00755118 | Lohp, 5-Fu/Lv and Bevacizumab, Alternative With Cpt-11, 5-Fu/Lv and Cetuximab In Metastatic Crc | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01937507 | Hepatic Artery Infusion With FOLFOX in Liver Metastasis From Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02016209 | Neoadjuvant Chemotherapy of Nanoparticle Albumin-bound Paclitaxel in Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01865110 | R-CHOP + R-HAD vs R-CHOP Followed by Maintenance Lenalidomide + Rituximab vs Rituximab for Older Patients With MCL | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00544011 | Bevacizumab and Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03935256 | Adjuvant Sequential & Concurrent CarboTaxol + Radiotherapy for High Risk Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00563953 | Caelyx as Primary Treatment for Patients With Breast Cancer and a History of Heart Disease and/or Age Over 65 Years | breast cancer | Doxorubicin (NPC261012) | |
| NCT00005068 | Combination Chemotherapy In Treating Patients With Metastatic or Unresectable Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT00666692 | A Phase 1b Study With Volociximab in Combination With Carboplatin, Paclitaxel, and Bevacizumab in First-line, Advanced Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03693677 | First Line Metastatic Pancreatic Cancer : 5FU/LV+Nal-IRI, Gemcitabine+Nab-paclitaxel or a Sequential Regimen of 2 Months 5FU/LV+Nal-IRI | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT01688336 | FOLFIRINOX for Unresectable Locally Advanced and Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01649336 | Weekly Paclitaxel With or Without Pazopanib in Platinum Resistant or Refractory Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02125513 | Dose-Escalation Study of Intraperitoneal (IP) Cisplatin, IV/IP Paclitaxel, IV Bevacizumab, and Oral Olaparib for Newly Diagnosed Ovarian, Primary Peritoneal, and Fallopian Tube Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00004924 | Paclitaxel and Irinotecan in Treating Patients With Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00455897 | CHOP-Rituximab Augmented With GM-CSF in Patients With Previously Untreated Diffuse Large B Cell Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03087864 | PDL-1 Targeting in Resectable Oesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00021255 | Combination Chemotherapy With or Without Trastuzumab in Treating Women With Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT03921684 | Trial of Nivolumab With FOLFOX After Chemoradiation in Rectal Cancer Patients | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00005849 | Bryostatin 1 Plus Paclitaxel in Treating Patients With Stage IIIB, Stage IV, or Recurrent Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04151277 | PRIMUS 001: A Study Looking at Two Different Chemotherapy Regimens in Patients With Metastatic Pancreatic Cancer | neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01843049 | Dose Escalation by Boosting Radiation Dose Within the Primary Tumor Using 18FDG-PET/CT for Unresectable Thoracic Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00005871 | Nitrocamptothecin or Fluorouracil in Treating Patients With Recurrent or Refractory Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00003742 | Combination Chemotherapy in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00129389 | FAC Versus FAC Plus Weekly Paclitaxel as Adjuvant Treatment of Node Negative High Risk Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT00147537 | Combination Study Of CP-751,871 With Paclitaxel And Carboplatin In Advanced Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | cutaneous melanoma;metastatic melanoma;breast carcinoma;non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00578864 | Protracted Etoposide During Induction Therapy for High Risk Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT01205217 | Phase I/II Study of Lapatinib in Combination With Paclitaxel as 1L Chemotherapy for ErbB2-positive MBC | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01251107 | Study Comparing ABVD vs BEACOPP in Advanced Hodgkin's Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00003930 | Radiation Therapy and Combination Chemotherapy in Treating Patients With Stage II or Stage III Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00005087 | Paclitaxel, Cisplatin, and Filgrastim Combined With Radiation Therapy in Treating Patients With Locally Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04929041 | Testing the Addition of Radiation Therapy to the Usual Treatment (Immunotherapy With or Without Chemotherapy) for Stage IV Non-Small Cell Lung Cancer Patients Who Are PD-L1 Negative | lung adenocarcinoma;squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01769391 | A Study of Necitumumab and Chemotherapy in Participants With Stage IV Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00301925 | Combination Chemotherapy in Treating Patients With Early Stage Breast Cancer That Has Been Removed By Surgery | breast cancer | Fluorouracil (NPC75844) | |
| NCT03949634 | Cardiac Safety and Efficacy for Early-stage Breast Cancer Patients Treated With Pegylated Liposomal Doxorubicin(PLD) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00104676 | Combination Chemotherapy in Treating Patients With Stage II or Stage III Germ Cell Tumors | teratoma;testicular neoplasm | Paclitaxel (NPC208553) | |
| NCT03830385 | Paclitaxel (Albumin Bound),Bleomycin And Cisplatin Or Carboplatin for Recurrent Or Metastatic Squamous Cell Carcinoma Of The Head And Neck | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00322400 | Phase1b to Evaluate Safety of AMG706 in Combination With Paclitaxel or Docetaxel for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00278694 | Oxaliplatin and 5-FU Based Preoperative Chemoradiation | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01701349 | Fosbretabulin or Placebo in Combination With Carboplatin/Paclitaxel in Anaplastic Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT02038621 | The Maintenance Therapy of Capecitabine of Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT05024513 | Biliary Drainage Plus HAIC in Locally Advanced pCCA | cholangiocarcinoma | Fluorouracil (NPC75844) | |
| NCT01454102 | Study of Nivolumab (BMS-936558) in Combination With Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab Maintenance, Erlotinib, Ipilimumab or as Monotherapy in Subjects With Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC) (CheckMate 012) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02279732 | Phase 3 Trial in Squamous Non Small Cell Lung Cancer Subjects Comparing Ipilimumab Plus Paclitaxel and Carboplatin Versus Placebo Plus Paclitaxel and Carboplatin | lung cancer | Paclitaxel (NPC208553) | |
| NCT00209690 | Phase I/II Study of TPF as First-line Chemotherapy in Patients With Metastatic Esophageal Cancer. | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT04015661 | Induction Chemotherapy Followed by Concurrent Chemoradiotherapy in Patients With LA-NPC | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00002711 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03563248 | Losartan and Nivolumab in Combination With FOLFIRINOX and SBRT in Localized Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02020707 | Abraxane/Bevacizumab | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00544362 | Fluorouracil, Cisplatin, Cetuximab, and Radiation Therapy in Treating Patients With Esophageal Cancer That Can Be Removed by Surgery | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00003944 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Stage III or Stage IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02297217 | Chemoradiotherapy for Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02865811 | Pembrolizumab Combined With PLD For Recurrent Platinum Resistant Ovarian, Fallopian Tube Or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Doxorubicin (NPC261012) | |
| NCT00005026 | Combination Chemotherapy in Treating Patients With Stage III or Stage IV Ovarian Epithelial or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00124943 | Use of Nanoparticle Paclitaxel (ABI-007) for the Prevention of In-Stent Restenosis | Coronary Restenosis | Paclitaxel (NPC208553) | |
| NCT01694576 | NPC Staged N2-3M0:Adjuvant Chemotherapy or Just Observation After Concurrent Chemoradiation | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT01227408 | Neoadjuvant Doxorubicin, Polyglutamate Paclitaxel, Capecitabine and Metronomic Chemotherapy in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01796002 | Efficacy and Safety of Romidepsin CHOP vs CHOP in Patients With Untreated Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02177695 | S1314, Co-expression Extrapolation (COXEN) Program to Predict Chemotherapy Response in Patients With Bladder Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT00615056 | A Study Combining FOLFOX or FOLFIRI With AG-013736 or Bevacizumab (Avastin) in Patients With Metastatic Colorectal Cancer After Failure Of One First Line Regimen | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT03541252 | Topical Laser-assisted Combination Chemotherapy for Basal Cell Carcinoma- a Clinical Study | basal cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00091377 | Phenoxodiol Combined With Either Cisplatin or Paclitaxel in Patients With Recurrent Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01774786 | A Study of Pertuzumab in Combination With Trastuzumab and Chemotherapy in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Metastatic Gastroesophageal Junction or Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02133612 | Adjuvant Chemotherapy With Paclitaxel and Cisplatin in Lymph Node-Positive Thoracic Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01796197 | Paclitaxel + Trastuzumab + Pertuzumab as Pre-Op for Inflammatory BrCa | breast cancer | Doxorubicin (NPC261012) | |
| NCT02135822 | Nab-paclitaxel Plus Gemcitabine in Chinese Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03606928 | mFLOT Chemotherapy as First-line Treatment in GC | gastric cancer | Fluorouracil (NPC75844) | |
| NCT05201248 | A Study to Evaluate Adverse Events and Change in Disease Activity of Subcutaneous (SC) Epcoritamab As Monotherapy or Combined With Standard of Care Therapies in Adult Participants in China With B-Cell Non-Hodgkin Lymphoma | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT03004833 | Nivolumab and AVD in Early-stage Unfavorable Classical Hodgkin Lymphoma | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT04483076 | Oxaliplatin Combined With S-1(SOX) Neoadjuvant Chemotherapy for Different Cycles in Patients With Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01034189 | Cetuximab/Paclitaxel/Cisplatin Concurrent Chemoradiotherapy Followed by Esophagectomy for Loco-regional Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00256243 | Neoadjuvant Biweekly Treatment Followed by Weekly Treatment of Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03697239 | High Dose Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) in Patients Who Have Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00866944 | Study of Adecatumumab Relative to FOLFOX After R0 Resection of Colorectal Liver Metastases | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01636622 | Study of Vemurafenib, Carboplatin, and Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT02506842 | Second-Line Adjuvant Therapy With Nab-Paclitaxel Plus Gemcitabine Versus Oxaliplatin Plus Folinic Acid and Fluorouracil for Gemcitabine-Refractory Pancreatic Cancer After Curative Resection | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT02632305 | A Study to See the Effects That a New Combination of the Three Drugs, Nab-paclitaxel, Gemcitabine, and Cisplatin Has on Biliary Tract Cancer | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT00009802 | Calcitriol Plus Paclitaxel in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01348217 | Radiochemotherapy With and Without Dose Escalation in Patients Presenting Locally Advanced or Inoperable Carcinoma of the Oesophagus | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00003078 | Radiation Therapy With or Without Cisplatin or Fluorouracil in Treating Patients With Cancer of the Cervix | cervical cancer | Fluorouracil (NPC75844) | |
| NCT00365365 | Safety & Efficacy of Three Docetaxel-Based Chemotherapy Regimens Plus Bevacizumab With or Without Trastuzumab for Adjuvant Treatment of Patients With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02639650 | Study of Paclitaxel Plus Cisplatin as the First-line Chemotherapy in High Risk Gestational Trophoblastic Tumor | gestational trophoblastic neoplasm | Paclitaxel (NPC208553) | |
| NCT03273595 | Study of Pembrolizumab (MK-3475) Plus Chemotherapy vs Placebo Plus Chemotherapy as Neoadjuvant Therapy and Pembrolizumab vs Placebo as Adjuvant Therapy in Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-522/KEYNOTE-522) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02063022 | Efficacy of Dose Intensification in Patients With Non-metastatic Ewing Sarcoma | Ewing sarcoma | Doxorubicin (NPC261012) | |
| NCT00945724 | Safety and Feasibility Study of Combination of State of Art Chemoimmunotherapy, Intensive Central Nervous System Prophylaxis and Scrotal Irradiation to Treat Primary Diffuse Large B-cell Lymphoma of Testis | lymphoma | Doxorubicin (NPC261012) | |
| NCT05108870 | TheraT® Vectors (Vaccines) Combined With Chemotherapy to Treat HPV16 Head and Neck Cancers | oropharynx squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03345810 | Durvalumab (MEDI4736) in Frail and Elder Patients With Metastatic NSCLC (DURATION) | large cell lung carcinoma;lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02591030 | Safety and Efficacy of Modified Folfirinox Versus Gemcis in Bile Duct Tumours | bile duct cancer | Fluorouracil (NPC75844) | |
| NCT01358071 | Phase II Study of NGR-hTNF in Combination With Doxorubicin in Platinum-resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02607982 | CCRT for Esophageal Cancer. | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01861951 | A Trial Comparing Two Medications as First Treatment in Elderly Patients With Metastatic or Advanced Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00453323 | Paclitaxel and Capecitabine in Patients With Metastatic/Recurrence Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01253681 | A Study of the Addition of Avastin (Bevacizumab) to Carboplatin and Paclitaxel Therapy in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04807972 | Study to Evaluate Adverse Events and Change in Disease Activity When Intravenous (IV) Infusion of ABBV-927 is Administered in Combination With IV Modified FOLFIRINOX (mFFX) With or Without IV Budigalimab Compared to mFFX in Adult Participants With Untreated Pancreatic Cancer Metastasis | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00841945 | Treatment of Aggressive Localized Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01206881 | Neoadjuvant Pegylated Liposomal Doxorubicin and Cyclophosphamide +/- Trastuzumab Followed by Docetaxel in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03574324 | TPF Induction Chemotherapy vs PF Adjuvant Chemotherapy Combined With Concurrent Chemoradiotherapy in the Treatment of Locally Advanced NPC | nasopharyngeal neoplasm | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00691912 | Therapy of Metastatic Breast Cancer With Paclitaxel and Liposomal Doxorubicin | breast cancer | Doxorubicin (NPC261012) | |
| NCT05263219 | DEB-TACE+HAIC vs. HAIC for Large HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02429700 | TC or BEP in Treating Patients With Ovarian Malignant Sex Cord-Stromal Tumors | ovarian sex cord-stromal tumor | Paclitaxel (NPC208553) | |
| NCT05344742 | A Study of Mitoxantrone Hydrochloride Liposome Injection Combination Therapy in Chinese Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01186328 | EZN-3042 Administered With Re-induction Chemotherapy in Children With Relapsed Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT04753879 | Multi-agent Low Dose Chemotherapy GAX-CI Followed by Olaparib and Pembro in Metastatic Pancreatic Ductal Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03145558 | TATE Versus TACE in Intermediate Stage HCC | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT04875026 | Frequency and Intensity of Local Reactions in Patients Treated With 4% 5-FU vs 4% 5-FU Associated With an Emollient Cream: a Randomised, Controlled Clinical Trial | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT00051311 | Modified Stem Cell Transplant Procedure to Treat Patients With Blood and Immune System Cancers | hematopoietic and lymphoid cell neoplasm | Doxorubicin (NPC261012) | |
| NCT00353717 | Study In Women And Men With Metastatic Breast Cancer That Have Overexpression Of ErbB2 | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03164993 | Atezolizumab Combined With Immunogenic Chemotherapy in Patients With Metastatic Triple-negative Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT00003322 | Combination Chemotherapy in Treating Patients With Primary Peritoneal or Stage III Epithelial Ovarian Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00215943 | Phase III Randomized Trial of Thalidomide/Dexamethasone Versus Vincristine+Adriamycin+Dexamethasone (VAD) | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00004055 | Topotecan, Paclitaxel, and Filgrastim in Treating Patients With Previously Untreated Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | Fallopian Tube Carcinoma;ovarian carcinoma | Fluorouracil (NPC75844) | |
| NCT01503372 | FLO +/- Pazopanib as First-line Treatment in Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00060385 | Combination Chemotherapy With or Without Etoposide in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04603846 | A Study of Anti-PD-L1 Antibody ZKAB001 Combined With Albumin-bound Paclitaxel in Advanced Urothelial Carcinoma | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT00608205 | Radiation Therapy Cisplatin With or Without Fluorouracil Patients With Stage III or Stage IV Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT02101021 | Gemcitabine and Nab-paclitaxel Combined With Momelotinib in Participants With Previously Untreated Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01210768 | A Study of Pegylated Liposomal Doxorubicin and Cyclophosphamide in Her2-negative Stage I and II Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT03606967 | Testing the Addition of an Individualized Vaccine to Nab-Paclitaxel, Durvalumab and Tremelimumab and Chemotherapy in Patients With Metastatic Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00610948 | Everolimus, Fluorouracil, Leucovorin, Panitumumab, and Oxaliplatin in Treating Patients With Tumors That Did Not Respond to Treatment | neoplasm | Fluorouracil (NPC75844) | |
| NCT03143491 | Study of SOR007 Ointment for Cervical Intraepithelial Neoplasia (CIN) | cervical intraepithelial neoplasia | Paclitaxel (NPC208553) | |
| NCT00817583 | Induction Chemotherapy Followed by Concurrent Chemoradiotherapy for Nasopharyngeal Carcinoma (NPC) | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT01851174 | Study to Evaluate Bi-weekly Dosing of Gemcitabine Plus Nab-Paclitaxel to Treat Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00862784 | A Study of IMC-1121B (Ramucirumab) in Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00934895 | Phase I/II Study of Weekly Abraxane and RAD001 in Women With Locally Adv. or Metastatic Breast Ca | breast cancer | Paclitaxel (NPC208553) | |
| NCT01285557 | Diffuse Gastric and Esophagogastric Junction Cancer S-1 Trial | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00174707 | Study of Docetaxel in Breast Cancer Patients | breast neoplasm | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT03348904 | Nivolumab and Epacadostat With Platinum Doublet Chemotherapy Versus Platinum Doublet Chemotherapy in Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00434252 | A Study of Bevacizumab With Carboplatin and Paclitaxel Chemotherapy for the First-Line Treatment of Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT01172223 | Primary Chemotherapy in Patients With HER2-positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03003546 | Nab-paclitaxel/Rituximab-coated Nanoparticle AR160 in Treating Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma, LS1681 Trial | chronic lymphocytic leukemia | Paclitaxel (NPC208553) | |
| NCT00582725 | R-CHOP + GM-CSF for Previously Untreated LCL in Elderly | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00129922 | Fluorouracil, Epirubicin, and Cyclophosphamide Alone or Followed by Paclitaxel for Early Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003421 | Combination Chemotherapy in Treating Patients With Advanced Hodgkin's Disease | lymphoma | Doxorubicin (NPC261012) | |
| NCT00201396 | A Multicenter Trial Comparing Induction C/T Followed by CCRT v.s. CCRT Alone in Stage IV Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT02630199 | A Study of BBI503 in Combination With Selected Anti-Cancer Therapeutics in Adult Patients With Advanced Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT00075738 | Fluorouracil, Irinotecan, Leucovorin, and Cisplatin as First-Line Therapy in Treating Patients With Metastatic Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT01953445 | A Study of Pertuzumab in Combination With Trastuzumab (Herceptin) and a Taxane in First-Line Treatment in Participants With Human Epidermal Growth Factor 2 (HER2)-Positive Advanced Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04843098 | Study of TL117 in Patients With Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00028938 | Chemotherapy and Radiation Therapy With or Without Epoetin Alfa in Treating Patients With Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01966133 | TACE as an Adjuvant Therapy After Hepatectomy for HCC | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT04902261 | Tislelizumab Combined With Nab-paclitaxel and Gemcitabine for Recurrent Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02134067 | Dose-escalating, Safety, Tolerability and PK Study of TAS-119 in Combination With Paclitaxel in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04005170 | Combination of Toripalimab and Chemoradiotherapy in Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00981656 | Radiation Therapy and Chemotherapy in Treating Patients With Stage I Bladder Cancer | urinary bladder cancer | Fluorouracil (NPC75844) | |
| NCT00145002 | A Study for Aggressive Adult T-cell Leukemia-lymphoma (ATLL) | adult T-cell leukemia/lymphoma | Doxorubicin (NPC261012) | |
| NCT04266249 | CompassHER2-pCR: Decreasing Chemotherapy for Breast Cancer Patients After Pre-surgery Chemo and Targeted Therapy | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00891605 | Safety Study of ABT-263 in Combination With Paclitaxel in Subjects With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02047500 | Phase I TH-302 Plus Gemcitabine Plus Nab-Paclitaxel in Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05209074 | Ivosidenib + mFOLFIRINOX in Patients With Resectable Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT04054362 | Paricalcitol Addition to Chemotherapy in Patients With Previously Untreated Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02328105 | LCI-LUN-ABR-001: Carbo With Nab-Paclitaxel in Patients With Advanced NSCL Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01118026 | Response-Based Therapy Assessed By PET Scan in Treating Patients With Bulky Stage I and Stage II Classical Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02413320 | Neoadjuvant Study of Two Platinum Regimens in Triple Negative Breast Cancer | triple-negative breast cancer | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT03806049 | Study of Relacorilant in Combination With Nab-Paclitaxel for Patients With Recurrent Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00187161 | Treatment of Burkitt Lymphoma/Leukemia and B Large Cell NHL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03428425 | A Study of HIPEC Plus Apatinib and S-1 Conversion Therapy for Gastric Cancer With Positive Exfoliative Cancer Cells | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03675737 | Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy in Participants Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (MK-3475-859/KEYNOTE-859) | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT02681003 | 6xFU/Epirubicin/Cyclophosphamide (FEC) Compared to 3xFEC-3xDocetaxel in High-risk Node-negative Breast Cancer Patients | breast cancer | Fluorouracil (NPC75844) | |
| NCT01057069 | Neo Adjuvant Chemotherapy in Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05290935 | Immunotherapy for Recurrent Cervical Cancer Refractory to Platinum-based Chemotherapy: Multi-Center Trial | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT01650376 | A Study of MEK162 and Paclitaxel in Patients With Epithelial Ovarian, Fallopian Tube or Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00454649 | Investigational Agent AG-013736 In Combinations With Standard Of Care Treatments For Patient's With Advanced Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT00316888 | Cetuximab, Cisplatin, Fluorouracil, and Radiation Therapy in Treating Patients With Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT01104298 | Doxorubicin vs. Trabectedin Plus Doxorubicin in Non Operable and/or Metastatic STS | sarcoma | Doxorubicin (NPC261012) | |
| NCT03780049 | HAIC Plus H101 vs HAIC Alone for Unresectable HCC at BCLC A-B | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT02911142 | Lenalidomide Combined With Modified DA-EPOCH and Rituximab (EPOCH-R2) in Primary Effusion Lymphoma or KSHV-associated Large Cell Lymphoma | B-cell neoplasm | Doxorubicin (NPC261012) | |
| NCT00043108 | Combination Chemotherapy, Surgery, and Radiation Therapy in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01137994 | A Randomized Trial Comparing EC-wPversus EP-wP as Adjuvant Therapy for Operable Breast Cancer Patients Less Than 40 Years Old | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00002105 | Randomized, Comparative Trial of DOX-SL (Stealth Liposomal Doxorubicin Hydrochloride) Versus Bleomycin and Vincristine in the Treatment of AIDS-Related Kaposi's Sarcoma | Kaposi's sarcoma | Doxorubicin (NPC261012) | |
| NCT00462397 | Paclitaxel and Carboplatin Followed by Cisplatin and Radiation Therapy in Treating Patients With Stage IB, Stage II, Stage III, or Stage IVA Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00039039 | Combination Chemotherapy Followed by Radiation Therapy With or Without Paclitaxel in Treating Patients With Unresectable Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01388621 | Carboplatin-based Chemotherapy With or Without Panitumumab in Platinum-sensitive Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02648425 | Safety and Tolerability of ASLAN001 in Combination With Cisplatin and 5-FU or Cisplatin and Capecitabine | neoplasm | Fluorouracil (NPC75844) | |
| NCT05476432 | HAIC With One-day FOLFOX vs. HAIC With Two-day FOLFOX for Unresectable HCC: a Non-inferiority Study | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT01493310 | Nab-paclitaxel (Abraxane) With or Without Mifepristone in Patients With Advanced Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04581876 | The Safety and Efficacy of the Combination of Raltitrexed for Injection and Nab-Paclitaxel in Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00136565 | Study of Bortezomib Combined With ACVBP in Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00466986 | Abraxane Plus Carboplatin for Recurrent Platinum-Sensitive Ovarian Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01159418 | LBH589 Oral in Combination With Carboplatin and Paclitaxel in Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04937738 | Perioperative Chemotherapy in Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00148681 | Preoperative Herceptin and Navelbine for Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01524094 | Cytoreduction and Intraperitoneal Chemotherapy Versus Systemic Chemotherapy in Colorectal Peritoneal Carcinomatosis | carcinoma | Fluorouracil (NPC75844) | |
| NCT00023907 | Paclitaxel in Treating Patients With Ovarian Epithelial Cancer or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00020007 | Paclitaxel and Hyperthermic Perfusion in Treating Patients With Lung Cancer or Lung Metastases That Cannot Be Removed By Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT03671252 | Prospectively Randomized Control Clinical Trial of FOLFOXIRI Preoperative Chemotherapy Alone on Rectal Cancer in Local Advance Comparing to Oral Capecitabine Combined With Long-term Radiation | rectum cancer | Fluorouracil (NPC75844) | |
| NCT02359968 | PReoperative Chemoradiation (Paclitaxel-carboplatin or FOLFOX) for Resectable Esophageal and Junctional Cancer | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT00379574 | Bortezomib Plus CHOP Every 2 Weeks for Advanced Stage DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00079287 | Vinorelbine, Gemcitabine, and Docetaxel Compared With Paclitaxel and Carboplatin in Treating Patients With Advanced Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00268450 | Cisplatin, Bevacizumab, and Gemcitabine Followed by Surgery, Bevacizumab, and Paclitaxel in Treating Patients With Locally Advanced Nonmetastatic Bladder Cancer That Can Be Removed By Surgery | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00002949 | Vinorelbine and Paclitaxel Plus Radiation Therapy in Treating Patients With Advanced Cancer Arising in the Pelvis | vaginal cancer;cervical cancer;endometrial cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00970385 | Study About Treatment of Newly Diagnosed Non Cutaneous Peripheral T Cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04046887 | Study of Lonsurf in Combination With Gemcitabine and Nab-Paclitaxel in Patients With Advanced (PDAC) | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04540692 | Evaluation of Sequencing of Anthracyclines and Taxanes for Locally Advanced HER2-negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01219777 | TRINOVA-1: A Study of AMG 386 or Placebo, in Combination With Weekly Paclitaxel Chemotherapy, as Treatment for Ovarian Cancer, Primary Peritoneal Cancer and Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02596971 | A Study of Atezolizumab in Combination With Either Obinutuzumab Plus Bendamustine or Obinutuzumab Plus (+) Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) in Participants With Follicular Lymphoma (FL) or Rituximab + CHOP in Participants With Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03941093 | Evaluation of Efficacy and Safety of Neoadjuvant Treatment With Pamrevlumab in Combination With Chemotherapy (Either Gemcitabine Plus Nab-paclitaxel or FOLFIRINOX) in Participants With Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT03764553 | Liposomal iRInotecan, Carboplatin or oXaliplatin for Esophagogastric Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT01920932 | Adcetris (Brentuximab Vedotin), Combination Chemotherapy, and Radiation Therapy in Treating Younger Patients With Stage IIB, IIIB and IV Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT05459129 | A Study Evaluating Efficacy and Safety of Multiple Treatment Combinations in Patients With Locally Advanced Squamous Cell Carcinoma of the Head and Neck (Morpheus-Head and Neck Cancer) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02048943 | Dovitinib Lactate, Gemcitabine Hydrochloride, and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Solid Tumors or Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02032277 | A Study Evaluating Safety and Efficacy of the Addition of ABT-888 Plus Carboplatin Versus the Addition of Carboplatin to Standard Chemotherapy Versus Standard Chemotherapy in Subjects With Early Stage Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03595592 | Neoadjuvant Treatment of HER2 Positive Early High-risk and Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003230 | Paclitaxel in Treating Patients With Refractory or Recurrent Acute Leukemia or Chronic Myelogenous Leukemia | leukemia | Paclitaxel (NPC208553) | |
| NCT01935492 | 8 Continuous vs 8 Intermittent Cycles in First and Second Line in HER2/Neu Neg Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02315196 | Pegylated Liposomal Doxorubicin Hydrochloride and Carboplatin Followed by Surgery and Paclitaxel in Treating Patients With Triple Negative Stage II-III Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT02073968 | PET-Adjusted Intensity Modulated Radiation Therapy and Combination Chemotherapy in Treating Patients With Stage II-IV Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00011921 | Chemotherapy Followed by Peripheral Stem Cell or Bone Marrow Transplant Compared With Chemotherapy Alone in Treating Patients With Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03563170 | QUILT-3.072: NANT Hepatocellular Carcinoma (HCC) Vaccine | hepatocellular carcinoma | Paclitaxel (NPC208553) | |
| NCT04770272 | Study to Compare a Mono Atezolizumab Window Followed by a Atezolizumab - CTX Therapy With Atezolizumab - CTX Therapy | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00004077 | Paclitaxel, Ifosfamide, and Cisplatin in Treating Patients With Metastatic Testicular Cancer | testicular neoplasm | Paclitaxel (NPC208553) | |
| NCT01170663 | A Study of Paclitaxel With or Without Ramucirumab (IMC-1211B) in Metastatic Gastric Adenocarcinoma | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00402545 | C-TPF in Patients With Newly Diagnosed Locally Advanced Squamous Cell Carcinoma of the Head and Neck | upper aerodigestive tract neoplasm | Fluorouracil (NPC75844) | |
| NCT03691090 | Study of SHR-1210 in Combination With Chemotherapy in Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01542931 | TPF Induction Chemotherapy for Locally Advanced and Resectable Oral Squamous Cell Carcinoma | oral squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00773695 | A Study of Bevacizumab (Avastin) in Combination With Neoadjuvant Treatment Regimens in Participants With Primary Human Epidermal Growth Factor Receptor 2 (HER2) Negative Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00003084 | Combination Chemotherapy With Ketoconazole in Treating Patients With Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00003637 | Radiation Therapy Alone Compared to Radiation Therapy Plus Chemotherapy in Treating Patients With Previously Untreated Cancer of the Nasopharynx | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00003117 | Paclitaxel With or Without Carboplatin in Treating Patients With Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00838656 | First Line Tx Stage III Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00691236 | Evaluation of Zoledronic Acid as a Single Agent or as an Adjuvant to Chemotherapy in High Grade Osteosarcoma | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT00005574 | Gentamicin Treatment of Muscular Dystrophy | Becker muscular dystrophy;Duchenne muscular dystrophy | Gentamicin (NPC471628) | |
| NCT00151034 | Trastuzumab (Herceptin), Paclitaxel, Carboplatin and Gemcitabine in Advanced Urothelial Cancer | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT01888978 | Molecularly Tailored Therapy for Pancreas Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03487939 | Neoadjuvant FOLFOXIRI Chemotherapy in Resectable Liver Metastasis of Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT04267549 | Conversion Therapy of Sintilimab in Combination With Apatinib and Chemotherapy in Unresectable Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00210379 | Phase II Study of Combined Modality Treatment in Primary Testicular Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00002755 | Standard Chemotherapy Compared With High-Dose Combination Chemotherapy and Peripheral Stem Cell Transplantation in Treating Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02451943 | A Study of Doxorubicin Plus Olaratumab (LY3012207) in Participants With Advanced or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00499369 | Irinotecan and Cetuximab With or Without Bevacizumab in Treating Patients With Metastatic Colorectal Cancer That Progressed During First-Line Therapy | malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT03219762 | Study of Nab-paclitaxel in Sensitive and Refractory Relapsed SCLC | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01419548 | ABT-888, Carboplatin, and Paclitaxel for Cancer With Liver or Kidney Problems | neoplasm | Paclitaxel (NPC208553) | |
| NCT00183911 | Study of 5-Fluorouracil and Leucovorin and Intra-abdominal Floxuridine Chemoradiation in Patients With Fully Resected Locally Advanced Gastric Adenocarcinoma | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT04003792 | Treatment With Hepatic Arterial Infusion of Oxaliplatin in Combination With Systemic FOLFIRI Chemotherapy and Bevacuzimab in Patients With Liver-only Colorectal Liver Metastases (CRLM): Conversion to Complete Resection in Patients With Initially Inoperable Liver-only CRLM. | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT04460066 | A Study of Anti-PD-L1 Antibody in Neoadjuvant Chemotherapy of Esophageal Squamous Cell Carcinoma. | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00022620 | Paclitaxel in Treating Patients With Refractory or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00096278 | Fluorouracil, Leucovorin, and Oxaliplatin With or Without Bevacizumab in Treating Patients Who Have Undergone Surgery for Stage II or Stage III Colon Cancer | colon adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT05221658 | A Phase II Clinical Study to Evaluate the Efficacy and Safety of HLX07 Combination Therapy or Monotherapy in Patients With Advanced ESCC | esophageal squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT03492047 | N-acetyl Cysteine Effect in Peripheral Neuropathy in Cancer Patients | peripheral neuropathy | Paclitaxel (NPC208553) | |
| NCT02580253 | Adjuvant Chemotherapy Based on the Adenosine Triphosphate Tumor Chemosensitivity Assay for Hepatocellular Carcinoma After Liver Transplantation | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00169130 | ACVBP Followed by ASCT in Patients With BCL-2 Positive Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00111787 | Study Of Lapatinib In Combination With Paclitaxel In The Treatment Of Newly Diagnosed Inflammatory Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00392392 | Preoperative Bevacizumab and Trastuzumab With ABI-007 and Carboplatin in HER2+ Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01087658 | Oral Glutamine in the Prevention of Oxaliplatin-induced Neurotoxicity | colorectal neoplasm | Glutamine (NPC197087) | |
| NCT00832494 | Phase II Study of DMXAA (ASA404) in Combination With Chemotherapy in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00503750 | Phase II Neoadjuvant Trial of Trastuzumab in Combination With Dose-Dense ABI-007 (Abraxane™) | breast cancer | Paclitaxel (NPC208553) | |
| NCT04336098 | Study of SRF617 in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00002955 | Combination Chemotherapy in Treating Patients With Advanced Cancer of the Pancreas | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00004206 | Oxaliplatin and Fluorouracil in Treating Patients With Recurrent Ovarian Cancer | ovarian cancer | Fluorouracil (NPC75844) | |
| NCT02004093 | EWOC-1 Trial: Carboplatin +/- Paclitaxel in Vulnerable Elderly Patients With Stage III-IV Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03415802 | Efficacy and Safety of Nab-Paclitaxel Plus S-1 in the First-line Treatment of Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04927780 | Perioperative or Adjuvant mFOLFIRINOX for Resectable Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00511459 | Phase 2 Study of AMG 386 Plus Paclitaxel With or Without Bevacizumab as First Line Therapy in Her2-Negative Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT03600090 | Phase I Study of EOC202 Plus Paclitaxel in Chinese Patients With Metastatic Breast Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT02784288 | Phase II Treatment Stratification Trial Using Neck Dissection-Driven Selection to Improve Quality of Life for Low Risk Patients With HPV+ Oropharyngeal Squamous Cell Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02846428 | To Evaluate the Efficacy and Safety of Capecitabine Prior to Surgery in Women With Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00476190 | ALL Adult Consortium Trial: Adult ALL Trial | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01309789 | A Phase 1 Study of Brentuximab Vedotin Given Sequentially and Combined With Multi-Agent Chemotherapy for CD30-Positive Mature T-Cell and NK-Cell Neoplasms | anaplastic large cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04292743 | Eryaspase With Modified FOLFIRINOX in Advanced Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT04765228 | Pegylated Liposomal Doxorubicin Combined With Anlotinib for Neoadjuvant Treatment of Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00131027 | High-Dose Methotrexate (MTX) for Adult Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03299660 | Avelumab With Chemoradiation in Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT02545010 | Pembrolizumab, Carboplatin, and Paclitaxel in Treating Patients With Stage III-IV Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01171170 | Paclitaxel-Carboplatin-Bevacizumab +/- Nitroglycerin in Metastatic Non-Squamous-Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02355379 | Randomised Study Evaluating Adjuvant Chemotherapy After Resection of Stage III Colonic Adenocarcinoma in Patients of 70 and Over | colon adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00873119 | Belinostat, Carboplatin and Paclitaxel (BelCaP) Compared to Carboplatin and Paclitaxel in Patients With Cancer of Unknown Primary | carcinoma | Paclitaxel (NPC208553) | |
| NCT00855764 | Late Phase II Study of Weekly Paclitaxel (BMS-181339) in Patients With Advanced or Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02562378 | T-DM1 and Non-pegylated Liposomal Doxorubicin in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00652119 | Intraperitoneal Paclitaxel and Carboplatin With IV Avastin Therapy in Patients With Carcinomas of Mullerian Origin | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02182232 | A Dose Escalation Study of BIBF 1120 Together With Paclitaxel and Carboplatin in Patients With Advanced Stage Non-small-cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00993655 | AURELIA: A Study of Avastin (Bevacizumab) Added to Chemotherapy in Patients With Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00590785 | Phase III Comparison of Adjuvant Chemotherapy W/High-Dose Cyclophosphamide Plus Doxorubicin (AC) vs Sequential Doxorubicin Fol by Cyclophosphamide (A-C) in High Risk Breast Cancer Patients With 0-3 Positive Nodes (Intergroup, CALGB 9394) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00209612 | Phase I/II Study of Paclitaxel Plus CPT-11 in Pts. With 2nd Line Chemotherapy of Inoperable or Recurrent GC. | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04661696 | Dose Escalation of Lapatinib With Paclitaxel in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00933387 | A Study of Neoadjuvant Bio-C/T Followed by Concurrent Bio-R/T in High-risk Locally Advanced Oral Squamous Cell Carcinoma | mouth neoplasm | Paclitaxel (NPC208553) | |
| NCT00003172 | Comparison of Combination Chemotherapy Regimens in Treating Patients With Advanced Stomach Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT03618550 | Phase II Study of Second- Line Pembrolizumab Plus GVD for Relapsed or Refractory Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03387085 | QUILT-3.067: NANT Triple Negative Breast Cancer (TNBC) Vaccine: Molecularly Informed Integrated Immunotherapy in Subjects With TNBC Who Have Progressed on or After Standard-of-care Therapy. | breast cancer | Fluorouracil (NPC75844) | |
| NCT01914900 | Preoperative TPF Chemotherapy in a Population of Molecularly Selected Locally Advanced Resectable Oral Cavity Squamous Cell Cancer | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT02605694 | Duvelisib With Rituximab vs R-CHOP in Subjects With Relapsed/Refractory Follicular Lymphoma (FRESCO) | lymphoma | Doxorubicin (NPC261012) | |
| NCT04374630 | Evaluation of the Sensitivity to Different Chemotherapy Regimens in Platinum-Partial Sensitive Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00921531 | Adjuvant Therapy With Thalidomide for Chemoembolization in Advanced Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT01847001 | Study of Propranolol in Newly Diagnosed Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT00083226 | Doxorubicin and Bortezomib in Treating Patients With Liver Cancer | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00015886 | Combination Chemotherapy in Treating Women With Breast Cancer That is Metastatic or Cannot be Treated With Surgery | breast cancer | Fluorouracil (NPC75844) | |
| NCT01740648 | Trametinib, Fluorouracil, and Radiation Therapy Before Surgery in Treating Patients With Stage II-III Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01770171 | Carboplatin-Paclitaxel ± Bevacizumab in Advanced (Stage III-IV) or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00002692 | Intravenous Compared With Intrahepatic Chemotherapy in Treating Patients With Colorectal Cancer Metastatic to the Liver | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00022178 | Combination Chemotherapy in Treating Patients With Metastatic Cancer of an Unknown Site of Origin | carcinoma | Fluorouracil (NPC75844) | |
| NCT00002519 | Paclitaxel Plus Radiation Therapy in Treating Patients With Untreated Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03519308 | A Pilot Study of Perioperative Nivolumab and Paricalcitol to Target the Micoenvironment in Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01099358 | Study in Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT04808687 | Neoadjuvant Nab-Paclitaxel and S-1 in Resectable Pancreatic Cancer | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT01472016 | Study of ABT-700 in Subjects With Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT01289821 | First Line Treatment of Metastatic Colorectal Cancer With mFOLFOX6 in Combination With Regorafenib | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT02124317 | Nab-paclitaxel Plus S-1 in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00309569 | Randomized Study Comparing Pre- and Postoperative vs. Conventional Adjuvant Treatment in Receptor-negative Patients | breast cancer | Fluorouracil (NPC75844) | |
| NCT02923921 | Study of Pegilodecakin (LY3500518) With FOLFOX Compared to FOLFOX Alone Second-line Tx in Participants With Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00952029 | Combination Chemotherapy and Bevacizumab With or Without Bevacizumab Maintenance Therapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02482441 | A Phase 1a/b Dose Escalation Study of the Safety, Pharmacokinetics, and Pharmacodynamics of OMP-131R10 | neoplasm | Fluorouracil (NPC75844) | |
| NCT00838656 | Intraperitoneal Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Cancer of the Peritoneal Cavity | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00959387 | Induction Chemotherapy for Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT03085992 | Folfoxiri Plus Bevacizumab Followed by Chemoradiotherapy Plus Bevacizumab in Patients With Resectable Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00912444 | Neoadjuvant Treatment of Docetaxel, Anthracycline and Cyclophosphamide (TAC) Versus Docetaxel and Cyclophosphamide (TC) in Triple-Negative or Her2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02625623 | Avelumab in Third-Line Gastric Cancer (JAVELIN Gastric 300) | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02720068 | Study of Favezelimab (MK-4280) as Monotherapy and in Combination With Pembrolizumab (MK-3475) With or Without Chemotherapy or Lenvatinib (MK-7902) AND Favezelimab/Pembrolizumab (MK-4280A) as Monotherapy in Adults With Advanced Solid Tumors (MK-4280-001) | neoplasm | Fluorouracil (NPC75844) | |
| NCT00004031 | SWOG-9704 Chemoradiotherapy and Peripheral Stem Cell Transplantation Compared With Combination Chemotherapy in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03130790 | Varlitinib in Combination With mFOLFOX6 for Advanced or Metastatic Gastric Cancer (First Line) | gastric cancer | Fluorouracil (NPC75844) | |
| NCT01855750 | A Study of the Bruton's Tyrosine Kinase Inhibitor, PCI-32765 (Ibrutinib), in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Non-Germinal Center B-Cell Subtype of Diffuse Large B-Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00443677 | Treatment of Advanced Hodgkin's Disease (Stages IIB-III-IV) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00003622 | Vinorelbine Plus Paclitaxel in Treating Patients With Metastatic Prostate Cancer That Is Refractory to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03020329 | Induction Chemotherapy in Young Patients With Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02979522 | A Study of Brentuximab Vedotin + Adriamycin, Vinblastine, and Dacarbazine in Pediatric Participants With Advanced Stage Newly Diagnosed Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00093444 | Heat Activated Liposomal Doxorubicin and Radiofrequency Ablation in Treating Patients With Primary or Metastatic Liver Tumors | liver cancer | Doxorubicin (NPC261012) | |
| NCT02884128 | A Study of PEP02 in Combination With 5-fluorouracil (5-FU) and Leucovorin (LV) in Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT02412670 | Chemotherapy Before Surgery in Treating Patients With High Grade Upper Urinary Tract Cancer | urothelial carcinoma | Doxorubicin (NPC261012) | |
| NCT04608409 | Apatinib Combined With Abraxane and Carboplatin or Cisplatinum as First-line Treatment for Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00971932 | Study of Cetuximab in Combination With Chemotherapy in Patients With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck (SCCHN) | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT04480099 | Targeted Drug Combined With CHOP in the Treatment of Newly Diagnosed Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00191854 | Gemcitabine Combinations in Metastatic Breast Cancer (MBC), 1st Line | breast cancer | Paclitaxel (NPC208553) | |
| NCT00070213 | Leucovorin and Fluorouracil With or Without Oxaliplatin Compared to Capecitabine With or Without Oxaliplatin in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02039791 | Study of Nimotuzumab in Combination With Neoadjuvant Chemotherapy for Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04685616 | Brentuximab Vedotin in Early Stage Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00022217 | Cisplatin-Epinephrine Injectable Gel Plus Paclitaxel and Carboplatin in Treating Patients With Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00079430 | Paclitaxel, Bevacizumab And Adjuvant Intraperitoneal Carboplatin in Treating Patients Who Had Initial Debulking Surgery for Stage II, Stage III, or Stage IV Ovarian Epithelial, Primary Peritoneal, or Fallopian Tube Cancer | ovarian serous cystadenocarcinoma;endometrioid carcinoma;fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01583426 | Nanoparticle-based Paclitaxel vs Solvent-based Paclitaxel as Part of Neoadjuvant Chemotherapy for Early Breast Cancer (GeparSepto) | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00501410 | FOLFOX Chemotherapy Regimen (5-FU, Leucovorin, Oxaliplatin) in Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01461915 | Phase 2 Study to Assess the Efficacy & Safety of Gemcitabine + Nab Paclitaxel With or Without Dociparstat in Metastatic Pancreatic Cancer Patients | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00038246 | Study of Paclitaxel, Estramustine Phosphate and Thalidomide for Patients With Metastatic Androgen-Independent Prostate Carcinoma | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03609047 | Adjuvant Palbociclib in Elderly Patients With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00148317 | Phase II Study of Velcade, Decadron, and Doxil Followed by Cyclophosphamide in Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00447967 | Combination of CPT-11 and LoHP vs Combination of 5-FU, Leucovorin and LoHP as 1st Line Treatment in Gastric Patients | gastric cancer | Fluorouracil (NPC75844) | |
| NCT03503604 | A Study of Anti-VEGF Monoclonal Antibody hPV19 in Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00068575 | Chemotherapy, Interferon Alfa, and Radiation Therapy in Treating Patients Who Have Undergone Surgery For Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT04221035 | High-Risk Neuroblastoma Study 2 of SIOP-Europa-Neuroblastoma (SIOPEN) | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT00376428 | Interest of Gentamicin-induced Readthrough in Cystic Fibrosis Patients | cystic fibrosis | Gentamicin (NPC471628) | |
| NCT05422066 | Selinexor Plus R-CHOP in High-risk GCB-subtype Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01374620 | Weekly Paclitaxel and Cyclophosphamide in Metronomic Administration : Dose Escalation Study of Weekly Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT01929941 | An Open-Label Study of a Novel JAK-inhibitor, INCB047986, Given in Patients With Advanced Malignancies | Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT03164382 | Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil/Leucovorin Versus Sorafenib in Advanced Hepatocellular Carcinoma | carcinoma;liver cancer | Fluorouracil (NPC75844) | |
| NCT02659930 | Pomalidomide in Combination With Liposomal Doxorubicin in People With Advanced or Refractory Kaposi Sarcoma | Kaposi's sarcoma | Doxorubicin (NPC261012) | |
| NCT00431080 | Randomized Phase III Trial Comparing Sequential Administration of FE75C Followed by Docetaxel Versus Paclitaxel as Adjuvant Chemotherapy in Axillary Lymph Node (+) Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003591 | Radiation Therapy Plus Paclitaxel in Treating Patients With Nonmetastatic, Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00629499 | Nanoparticle Albumin-Bound (Nab) Paclitaxel/Cyclophosphamide in Early-Stage Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01872403 | Neoadjuvant Chemotherapy of Nanoparticle Albumin-bound Paclitaxel/Carboplatin vs. Paclitaxel /Carboplatin in Stage Ⅱ B and IIIA Squamous Cell Carcinoma of the Lung | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003691 | Combination Chemotherapy With or Without G-CSF in Treating Patients With Stage III, Stage IV, or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00527111 | Pre-op Rectal ChemoRad +/- Cetuximab | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00003317 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Stage IIIA Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01127555 | Salvage mFOLFOX in BTC After Failure of Gemcitabine | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT00747799 | Study of Combination of Sorafenib With Cisplatin and 5-fluorouracil as First-line Treatment of Recurrence After Radiotherapy Patients Who Failed With Radiotherapy in Recurrent or Metastatic Nasopharyngeal Carcinoma (NPC) | nasopharyngeal neoplasm | Fluorouracil (NPC75844) | |
| NCT02698735 | Gentamicin Therapy for Recessive Dystrophic Epidermolysis Bullosa (RDEB) Nonsense Mutation Patients | recessive dystrophic epidermolysis bullosa | Gentamicin (NPC471628) | |
| NCT01240655 | A Study of LCL161 in Combination With Weekly Paclitaxel in Adult Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01084083 | Induction Chemotherapy Followed By Cetuximab and Radiation in HPV-Associated Resectable Stage III/IV Oropharynx Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00667251 | Chemotherapy and Lapatinib or Trastuzumab in Treating Women With HER2/Neu-Positive Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00919880 | Comparison of Neo-adjuvant Weekly Paclitaxel With or Without Carboplatin in Early Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03127007 | Safety and Efficacy of Atezolizumab Combined to Preoperative Radio-chemotherapy in Localized Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT01471132 | Sequential HIPEC of Oxaliplatin and Paclitaxel for Gastric Cancer Patients With Peritoneum Metastasis | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01858207 | Combine TACE and RFA Versus RFA Monotherapy in Unilobar HCC of 3.1 to 7 cm Patient | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00708812 | Paclitaxel Plus Carboplatin With or Without Endostar in Patients With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01240629 | Doxorubicin-GnRH Agonist Conjugate AEZS-108 in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer | prostate cancer | Doxorubicin (NPC261012) | |
| NCT02075021 | Phase I/II Trial of the Combination of Lenalidomide (Revlimid) and Nab-paclitaxel (Abraxane) in the Treatment of Relapsed/Refractory Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT03167177 | QUILT-3.046: NANT Melanoma Vaccine: Combination Immunotherapy in Subjects With Melanoma Who Have Progressed On or After Chemotherapy and PD-1/PD-L1 Therapy | melanoma | Paclitaxel (NPC208553) | |
| NCT00312650 | Doxil and Gemcitabine in Recurrent Ovarian Cancer | ovarian carcinoma | Doxorubicin (NPC261012) | |
| NCT00003587 | S9806: Combination Chemotherapy in Treating Patients With Stage IIIB or Stage IV Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01121406 | BI 6727 (Volasertib) Randomised Trial in Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT00003652 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Stage II or Stage III Anal Cancer | anus cancer | Fluorouracil (NPC75844) | |
| NCT00005050 | Eniluracil, Fluorouracil, and Oxaliplatin in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00042302 | A Double-Blind, Randomized, Placebo-Controlled, Multicenter, Phase III Study of Tariquidar + Paclitaxel/Carboplatin as First-Line Therapy in Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00217737 | Oxaliplatin, Leucovorin Calcium, and Fluorouracil With or Without Bevacizumab in Treating Patients Who Have Undergone Surgery for Stage II Colon Cancer | Lynch syndrome;signet ring cell carcinoma | Fluorouracil (NPC75844) | |
| NCT04060459 | Paclitaxel-binding Albumin and Cisplatin as Neoadjuvant Chemotherapy in Patients With Muscle-Invasive Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT02427620 | Ibrutinib, Rituximab, and Consolidation Chemotherapy in Treating Young Patients With Newly Diagnosed Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01253681 | Study of AMG 386 in Combination With Paclitaxel and Carboplatin in Subjects With Ovarian Cancer | carcinoma | Paclitaxel (NPC208553) | |
| NCT00054665 | PS-341 Alone and PS-341 Plus EPOCH Chemotherapy to Treat Non-Hodgkin's Lymphoma | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT03498716 | A Study Comparing Atezolizumab (Anti PD-L1 Antibody) In Combination With Adjuvant Anthracycline/Taxane-Based Chemotherapy Versus Chemotherapy Alone In Patients With Operable Triple-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04301739 | to Evaluate Efficacy and Safety of HLX10 in Combination With Chemotherapy Versus Placebo in Combination With Chemotherapy as Neoadjuvant Therapy and HLX10 Versus Placebo as Adjuvant Therapy in Patients With Triple Negative Breast Cancer (TNBC) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00992199 | Randomized Controlled Trials on Adjuvant Intraperitoneal Chemotherapy for Resectable Local Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00002580 | Tamoxifen, Ovarian Ablation, and/or Combination Chemotherapy in Treating Premenopausal Women With Stage I, Stage II, or Stage IIIA Invasive Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00004089 | Chemotherapy Plus Radiation Therapy in Treating Patients With Previously Untreated Thyroid Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00950300 | A Study to Compare Subcutaneous (SC) Versus Intravenous (IV) Administration of Herceptin (Trastuzumab) in Women With Human Epidermal Growth Factor Receptor (HER) 2-Positive Early Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00448136 | A Study of Avastin (Bevacizumab) in Combination With Chemotherapy in Patients With Endocrine Tumors of the Gastrointestinal Tract. | neoplasm | Fluorouracil (NPC75844) | |
| NCT03881111 | First-line Esophageal Carcinoma Study With Chemo vs. Chemo Plus Pembrolizumab (MK-3475-590/KEYNOTE-590)-China Extension Study | esophageal carcinoma | Fluorouracil (NPC75844) | |
| NCT00002922 | Paclitaxel in Treating Patients With Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00124956 | Doxorubicin Pharmacokinetic (PK) Study | cancer | Doxorubicin (NPC261012) | |
| NCT00025298 | Chemotherapy Plus Radiation Therapy With or Without Amifostine in Treating Patients With Locally Advanced Cancer of the Nasopharynx | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00499850 | Phase I FOLFOX Combination | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01670500 | Cisplatin vs. Doxorubicin/Cyclophosphamide in BrCa | breast cancer | Doxorubicin (NPC261012) | |
| NCT02300935 | Study of Trametinib and Nab-paclitaxel in Patients With Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00486759 | A Study of Bevacizumab (Avastin) in Combination With Rituximab (MabThera) and CHOP (Cyclophosphamide, Hydroxydaunorubicin [Doxorubicin], Oncovin [Vincristine], Prednisone) Chemotherapy in Patients With Diffuse Large B-cell Lymphoma | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT02978495 | Neoadjuvant Carboplatin in Triple Negative Breast Cancer | Hereditary breast and ovarian cancer syndrome | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT01891357 | Phase II Trial to Validate Markers for a Response Evaluation of a Combined Therapy in Patients With HER2+ Breast Cancer | breast ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04393584 | FOLFIRINOX vs FLOT Chemotherapy for Resectable Gastric or Esophagogastric Junction Adenocarcinoma | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT00199758 | Study of an Early Change of a Chemotherapeutic Doublet Versus Four Cycles of Chemotherapy in Advanced Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00551759 | Chemotherapy, Radiation Therapy, and Cetuximab Followed by Surgery, Docetaxel, and Cetuximab in Treating Patients With Esophageal Cancer or Gastroesophageal Junction Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT03029611 | Two Therapeutic Strategies in First-line in Patients With Epithelial Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02973685 | HAIC Versus TACE for Large Hepatocellular Carcinoma Staged BCLC A/B. | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT03942068 | Effect of Tumor Treating Fields (TTFields, 200 kHz) Concomitant With Weekly Paclitaxel for the Treatment of Recurrent Ovarian Cancer (ENGOT-ov50 / GOG-3029 / INNOVATE-3) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00502567 | A Study to Assess Safety, Tolerability and PK of AZD2171 and Chemotherapy on Patients With Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT02393833 | IBCSG Trial 22-00 Serum Substudy | breast cancer | Fluorouracil (NPC75844) | |
| NCT01009515 | Carboplatin, Paclitaxel, and Temozolomide for Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT01113476 | Nab-paclitaxel, Gemcitabine, and Bevacizumab in Advanced Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT04793932 | Short-course Versus Long-course Pre-operative Chemotherapy With mFOLFIRINOX or PAXG (CASSANDRA TRIAL) | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00662597 | ASA404 or Placebo in Combination With Paclitaxel and Carboplatin as First-Line Treatment for Stage IIIb/IV Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003063 | Biological Therapy With Combination Chemotherapy in Patients With Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00210184 | Evaluation of Efficacy and Safety of FOLFIRI Association Treatment in Patients 70 Years of Age and Older With Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT03947879 | The Effect of Pharmaceutical Grade L-glutamine (Endari) on Glycemic Control in Patients With Diabetes Mellitus Type II | type 2 diabetes mellitus | Glutamine (NPC197087) | |
| NCT02050178 | Dose Escalation Study of OMP-54F28 in Combination With Nab-Paclitaxel and Gemcitabine in Patients With Previously Untreated Stage IV Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02777437 | Laparoscopic Surgery VS Laparoscopic Surgery + Neoadjuvant Chemotherapy for T4 Tumor of the Colon Cancer | colonic neoplasm | Fluorouracil (NPC75844) | |
| NCT02608229 | BVD-523 Plus Nab-paclitaxel and Gemcitabine in Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04495088 | Preoperative FOLFOX Versus Postoperative Risk-adapted Chemotherapy in Patients With Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00281788 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Esophageal Cancer or Gastroesophageal Junction Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00002747 | Surgery and Radiation Therapy With or Without Chemotherapy in Treating Patients With Mouth Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT02505269 | Brentuximab Vedotin Plus AD in Non-bulky Limited Stage Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00000122 | Fluorouracil Filtering Surgery Study (FFSS) | glaucoma | Fluorouracil (NPC75844) | |
| NCT04674956 | A Prospective, Randomized, Double-blinded, Multi-center Clinical Trial to Evaluate the Efficiency and Safety of Anti-PD1 Antibody (Camrelizumab) Combined With Paclitaxel(Albumin Bound) and Gemcitabine as First-line Therapy in Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00002662 | Paclitaxel or Docetaxel in Treating Women With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00206518 | Taxotere and Adriamycin/Cytoxan (AC) Validation in Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT00194753 | Adjuvant Therapy for High-Risk Breast Cancer With Wkly Adriamycin & Oral Cytoxan With G-CSF for 12 Wks | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00991796 | CS-1008 With Carboplatin/Paclitaxel in Chemotherapy naïve Subjects With Metastatic or Unresectable Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003235 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer That Can Not Be Surgically Removed | lung cancer | Paclitaxel (NPC208553) | |
| NCT00469443 | Randomized Phase III Study of Folfiri+Avastin Versus Xeliri+Avastin as 1st Line Treatment of CRC | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00730353 | Sutent + Taxol for Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01204996 | A Study of the Safety and Efficacy of CNTO 888 in Combination With SoC (Standard of Care) Chemotherapy in Patients With Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT00003612 | Combination Chemotherapy and Trastuzumab in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03981796 | A Study to Evaluate Dostarlimab Plus Carboplatin-paclitaxel Versus Placebo Plus Carboplatin-paclitaxel in Participants With Recurrent or Primary Advanced Endometrial Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT02525653 | Albumin-Bound Paclitaxel and Gemcitabine in Patients With Untreated Stage IV or Recurrent Squamous Cell Lung Cancers | lung cancer | Paclitaxel (NPC208553) | |
| NCT04180384 | A Safety Study of Oraxol (HM30181 + Oral Paclitaxel) in Cancer Patients | neoplasm | Paclitaxel (NPC208553) | |
| NCT03801668 | Albumin-bound Paclitaxel Plus S-1 Versus SOX as First-line Treatment in Advanced or Recurrent Gastric Adenocarcinoma | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02268695 | Platinum-Cetuximab Combined With Docetaxel or With 5FU in Patients With Recurrent/Metastatic HNSCC | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00217607 | Paclitaxel in Treating Patients With Locally Advanced or Metastatic Soft Tissue Angiosarcoma or Lymphangiosarcoma That Cannot Be Removed By Surgery | sarcoma | Paclitaxel (NPC208553) | |
| NCT00291785 | Phase I/II CT 2106 and 5-FU/FA in Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04769414 | Flouro-Gem in Adenocarcinoma of the Pancreas (GEFLUPAN) | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03989336 | Apatinib With Albumin-bound Paclitaxel in Patients With Platinum-resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00925821 | Lenalidomide-Adriamycin-Dexamethasone (RAD) Induction Followed by Stem Cell Transplant in Newly Diagnosed Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00507962 | Cisplatin HAI Study in Patients With Advanced Cancer and Dominant Liver Involvement | cancer | Doxorubicin (NPC261012) | |
| NCT02742051 | A Trial of Neoadjuvant Everolimus Plus Letrozole Versus FEC in Women With ER-positive, HER2-negative Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01467115 | Induction Chemotherapy Followed by Cetuximab and Radiation Therapy for Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00035152 | Study Comparing Weekly Taxol and Carboplatin vs Standard Taxol and Carboplatin Regimen for Stage IIIB or IV Non-Small-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03808480 | Nivolumab After Cyclophosphamide and Doxorubicin Induction Therapy in NSCLC With PD-L1<10% | non-small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT00003215 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Newly Diagnosed Aggressive Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00050960 | Evaluation of Efficacy, Safety and Tolerability of Targretin Capsules in Patients With Advanced or Metastatic Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02317991 | Study of Nab-Paclitaxel and Ramucirumab as Second-line Treatment for Patients With Metastatic Gastroesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003389 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00049595 | Comparison of Two Combination Chemotherapy Regimens in Treating Patients With Stage III or Stage IV Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00087217 | 17-N-Allylamino-17-Demethoxygeldanamycin and Paclitaxel in Treating Patients With Metastatic or Unresectable Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT00499018 | Dose Dense Chemotherapy + Rituximab +/-Intensified High Dose Chemoimmunotherapy With Support of Peripheral Autologous Stem Cell in Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01063517 | Efficacy Study of Olaparib With Paclitaxel Versus Paclitaxel in Gastric Cancer Patients | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03515798 | Study of Immunotherapy in Combination With Chemotherapy in HER2-negative Inflammatory Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02412371 | A Study Evaluating the Efficacy and Tolerability of Veliparib in Combination With Paclitaxel/Carboplatin-Based Chemoradiotherapy Followed by Veliparib and Paclitaxel/Carboplatin Consolidation in Adults With Stage III Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00193115 | Docetaxel Followed by Doxorubicin Plus Cyclophosphamide for Node Positive or High-Risk Primary Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01966471 | A Study of Trastuzumab Emtansine (Kadcyla) Plus Pertuzumab (Perjeta) Following Anthracyclines in Comparison With Trastuzumab (Herceptin) Plus Pertuzumab and a Taxane Following Anthracyclines as Adjuvant Therapy in Participants With Operable HER2-Positive Primary Breast Cancer | breast cancer | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT02432365 | Study of Weekly Paclitaxel and Cisplatin in FIGO IB2 and IIA2 Cervical Cancer Followed by Radical Hysterectomy | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02109341 | Phase I/II Study to Evaluate Nab-paclitaxel in Substitution of CPT11 or Oxaliplatin in FOLFIRINOX Schedule as First Line Treatment in Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03116152 | Study of IBI308 With Advanced/Metastatic Esophageal Squamous Cell Carcinoma After Failure of First-line Treatment | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00009932 | Combination Chemotherapy in Treating Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003782 | Combination Chemotherapy in Treating Women With Stage I, Stage II, or Stage IIIA (cT1-3, N0-1, M0) Breast Cancer and Positive Axillary Lymph Nodes | breast cancer | Doxorubicin (NPC261012) | |
| NCT00262808 | GM-CSF and Combination Chemotherapy in Treating Patients Who Are Undergoing Surgery for Stage II or Stage III Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03915444 | Nab-Paclitaxel + Cisplatin + Gemcitabine in Untreated Metastatic Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01402271 | Alisertib (MLN8237) in Participants With Ovarian, Fallopian Tube or Peritoneal Cancer Preceded by Phase 1 Study of MLN8237 Plus Paclitaxel Treatment of Ovary or Breast Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00191646 | An Ovarian, Primary Peritoneal or Fallopian Tube Cancer Study for Patients That Have Not Received Prior Chemotherapy | Fallopian Tube Carcinoma;ovarian neoplasm;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT02951728 | Decitabine Plus R-CHOP in Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00006103 | Combination Chemotherapy in Treating Patients With Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01136031 | Paclitaxel and Irinotecan in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01138046 | A Phase II, Randomized, Open-label Study of Lapatinib Plus Chemotherapy Versus Trastuzumab Plus Chemotherapy in HER2-positive and p95HER2-positive Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03527628 | OPTmizing Advanced Stage HodgkIn LymphoMa patIentS Therapy | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT02027428 | Safety and Efficacy Study of Abraxane as Maintenance Treatment After Abraxane Plus Carboplatin in 1st Line Stage IIIB / IV Squamous Cell Non-small Cell Lung Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00133406 | Long-term Impact and Intervention for Diarrhea in Brazil | diarrheal disease | Glutamine (NPC197087) | |
| NCT01032382 | Safety, Efficacy and Pharmacokinetics (PK) Study of WR 279,396 Versus Paromomycin for Treatment of Cutaneous Leishmaniasis (Peru-PK) | Leishmaniasis | Gentamicin (NPC471628) | |
| NCT00201318 | A Randomized Study in Non-Hodgkin's Lymphoma Patients Carrying Hepatitis B Surface Antigen | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01383707 | A Study of Bevacizumab and Modified FOLFOX-6 (mFOLFOX-6) in Participants With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00020449 | Liposomal Doxorubicin and Interleukin-12 in Treating Patients With AIDS-Related Kaposi's Sarcoma | sarcoma | Paclitaxel (NPC208553) | |
| NCT00544414 | Chemotherapy Followed by Surgery, Chemotherapy, and Radiation Therapy in Treating Patients With Locally Advanced Head And Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT02276560 | Cisplatin and Nab-paclitaxel for (N2) Defined NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03694522 | A Study of Bemarituzumab (FPA144) Combined With Modified FOLFOX6 (mFOLFOX6) in Gastric/Gastroesophageal Junction Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT05193604 | A Clinical Trial to Evaluate Tolerability and Security of TQB2858 Injection in Subjects With Advanced Pancreatic Carcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04572542 | Camrelizumab Combined With Apatinib Mesylate Tablets and Nab-paclitaxel in the Second-line Treatment of Advanced Gastric Cancer | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00673894 | Effects of Glutamine on GLP-1 and Insulin Secretion in Man | type 2 diabetes mellitus | Glutamine (NPC197087) | |
| NCT03015675 | IV Ascorbic Acid in Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT05081687 | Brazilian Total Neoadjuvant Therapy Trial | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT01632306 | A Study of LY2090314 and Chemotherapy in Participants With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT02959879 | Neo-adjuvant FOLF(IRIN)OX for Resectable Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00110019 | Carboplatin and Paclitaxel With or Without Sorafenib Tosylate in Treating Patients With Stage III or Stage IV Melanoma That Cannot Be Removed by Surgery | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT00815308 | Erbitux Combined With Chemo-radiotherapy in Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01946074 | A Study of ABT-165 in Subjects With Solid Tumors | neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00591851 | Phase II Study of Dose-Dense Doxurubicin and Cyclophosphamide (AC) Followed By Paclitaxel With Trastuzumab in HER2/ NEU-Amplified Breast Cancer: Feasibility | breast cancer | Doxorubicin (NPC261012) | |
| NCT04356170 | Trial Evaluating Adapted Chemotherapy in Patients With Squamous Carcinoma | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00973752 | Treatment of Older Adults With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT04182724 | Camrelizumab, Apatinib and Nab-paclitaxel as Second-line Treatment in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01091428 | Trial of Best Supportive Care and Either Cisplatin or Paclitaxel to Treat Patients With Primary Ovarian Cancer, Primary Peritoneal Cancer or Fallopian Tube Cancer and Inoperable Malignant Bowel Obstruction | fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04540211 | A Study of Atezolizumab Plus Tiragolumab in Combination With Paclitaxel and Cisplatin Compared With Paclitaxel and Cisplatin as First-Line Treatment in Participants With Unresectable Locally Advanced, Unresectable Recurrent, or Metastatic Esophageal Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00109837 | S0333 Combination Chemotherapy in Treating Patients With Newly Diagnosed Acute Lymphoblastic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT02250599 | Efficacy and Safety of TC+AVASTIN Versus TC in Patients With Metastatic Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT02713386 | Pembrolizumab, Carboplatin, and Paclitaxel in Treating Patients With Stage III-IV Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00002888 | Combination Chemotherapy in Treating Patients With Advanced Head and Neck Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00543829 | Study of Suitable Schedule of Docetaxel,Anthracycline and Cyclophosphamide in Adjuvant Therapy of Beast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00268333 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Recurrent Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01840592 | Sorafenib Plus Doxorubicin in Patients With Advanced Hepatocellular Carcinoma With Disease Progression on Sorafenib | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT02980965 | Neoadjuvant Chemo-endocrine Therapy Versus Chemotherapy Alone in ER-positive, HER2-negative Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02934464 | Assessment of Ramucirumab Plus Paclitaxel as Switch MANteInance Versus Continuation of First-line Chemotherapy in Patients With Advanced HER-2 Negative Gastric or Gastroesophageal Junction Cancers | stomach neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT00608803 | Phase 1 Study of ZIO-201-T in Combination With Doxorubicin in Solid Tumors | cancer | Doxorubicin (NPC261012) | |
| NCT01868984 | Paclitaxel for the Treatment of Upper-Extremity Arteriovenous Access Fistula Stenosis | stricture | Paclitaxel (NPC208553) | |
| NCT00144807 | ACVBP Plus Rituximab in Patients Aged From 18 to 59 Years With High-risk Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00006012 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Limited-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00006378 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00038142 | Vincristine, Doxorubicin, Cyclophosphamide and Dexrazoxane (VACdxr) in High Risk Ewing's Sarcoma Patients | Ewing sarcoma | Doxorubicin (NPC261012) | |
| NCT00944801 | Pegylated Liposomal Doxorubicine and Prolonged Temozolomide in Addition to Radiotherapy in Newly Diagnosed Glioblastoma | glioblastoma multiforme | Doxorubicin (NPC261012) | |
| NCT00851045 | Ph II Trial of a Novel Anti-angiogenic Agent in Combination With Chemotherapy for the Second-line Treatment of Metastatic Colorectal Cancer | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00154700 | Twice Weekly TP-HDFL for Recurrent or Metastatic Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04910126 | Camrelizumab Plus Doxorubicin for the First Line Treatment of Adcanced Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01226719 | FOLFOXIRI Plus Panitumumab Patients With Metastatic KRAS Wild-Type Colorectal Cancer With Liver Metastases Only | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00373256 | Combination Of Lapatinib With Carboplatin, Paclitaxel, and With or Without Trastuzumab In Metastatic Breast Cancer. | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01653912 | Phase Ib Study of Olaparib Plus Weekly Carboplatin and Paclitaxel in Relapsed Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04258644 | Camrelizumab in Combination With Apatinib Mesylate, Paclitaxel-albumin and S-1 for Translational Treatment of Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04755543 | A Study of LP002 for the Treatment of Patients With Malignant Digestive System Neoplasms | digestive system neoplasm | Fluorouracil (NPC75844) | |
| NCT01871571 | Bevacizumab, Fluorouracil, Leucovorin Calcium, and Oxaliplatin Before Surgery in Treating Patients With Stage II-III Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00062010 | Interferon Alfa, Isotretinoin, and Paclitaxel in Treating Patients With Recurrent Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01014767 | Intercontinental Multidisciplinary Registry and Treatment Optimization Study for Choroid Plexus Tumors | brain cancer;choroid plexus neoplasm | Doxorubicin (NPC261012) | |
| NCT00429299 | Pazopanib (VOTRIENT) Plus Paclitaxel (TAXOL), Pazopanib Plus Paclitaxel (TAXOL) Plus Carboplatin (PARAPLATIN), and Pazopanib Plus Paclitaxel (TAXOL) Plus Lapatinib (TYKERB) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02897700 | A Randomized Trial of Chemotherapy in Surgical Patients With Infiltrating Ductal Carcinoma of Breast | breast cancer | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT01779050 | Afatinib in HER2 (Human Epidermal Growth Factor Receptor)-Treatment Failures | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00280787 | Induction Chemotherapy Using Paclitaxel, Carboplatin, CPT-11 With Pegfilgrastim | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00246571 | Weekly Taxol Plus Xeloda® vs Taxotere q3wk Plus Xeloda® in the Treatment of Metastatic BC | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01498783 | Phase I Study of 5-Fluorouracil in Children and Young Adults With Recurrent Ependymoma | central nervous system cancer;ependymoma | Fluorouracil (NPC75844) | |
| NCT00997906 | Cisplatin and RT With or Without Gemcitabine, Carboplatin, and Paclitaxel in Treating Patients With Locally Advanced NPC | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02389751 | Ganetespib in Combination With Paclitaxel, Carboplatin, and Radiation Therapy in Treating Patients With Stage II-III Esophageal Cancer | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT02311439 | Induction Chemotherapy, FOLFIRINOX Followed With Concurrent Capecitabine and Radiation Therapy in Inoperable Locally Advanced Cancer of the Pancreas | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT01204437 | Adjuvant Chemotherapy for Elderly Non Frail Patients With an Increased Risk for Relapse of a Primary Carcinoma of the Breast | breast cancer | Fluorouracil (NPC75844) | |
| NCT02285062 | Efficacy and Safety Study of Lenalidomide Plus R-CHOP Chemotherapy Versus Placebo Plus R-CHOP Chemotherapy in Untreated ABC Type Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01379989 | INOVATYON STUDY -International, Randomized Study in Patients With Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00024102 | Comparison of Combination Chemotherapy Regimens in Treating Older Women Who Have Undergone Surgery for Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05000892 | Neoadjuvant Sintilimab in Combination With Carboplatin and Nab-paclitaxel in Untreated Salivary Gland Malignant Neoplasms | salivary gland cancer | Paclitaxel (NPC208553) | |
| NCT00625898 | BETH Study: Treatment of HER2 Positive Breast Cancer With Chemotherapy Plus Trastuzumab vs Chemotherapy Plus Trastuzumab Plus Bevacizumab | breast cancer | Fluorouracil (NPC75844) | |
| NCT02733250 | Pembrolizumab With Nab-Paclitaxel in Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00941499 | Hepatic Arterial Infusion Oxaliplatin + 5FU, Leucovorin, and Bevacizumab +/- Cetuximab | cancer | Fluorouracil (NPC75844) | |
| NCT01770301 | Efficacy and Safety of Bevacizumab (Avastin®) Combined to Weekly Paclitaxel Followed by Bevacizumab (Avastin®) Alone in Patients With Relapsed Ovarian Sex-cord Stromal Tumours (ALIENOR) | sex cord-gonadal stromal tumor | Paclitaxel (NPC208553) | |
| NCT02024009 | Systemic Therapy and Chemoradiation in Advanced Localised Pancreatic Cancer - 2 | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT04259944 | Post-surgical Liquid Biopsy-guided Treatment of Stage III and High-risk Stage II Colon Cancer Patients: the PEGASUS Trial | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00118261 | Erlotinib, Modified FOLFOX6, and Bevacizumab as First-Line Therapy Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00072553 | Celecoxib, Leucovorin, Fluorouracil, and Oxaliplatin in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00002774 | Radiation Therapy and Combination Chemotherapy in Treating Patients With Stage III or Stage IV Head and Neck Cancer | head and neck squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT00526890 | Carboplatin, Paclitaxel, Selenomethionine, and Radiation Therapy in Treating Patients With Stage III Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00602797 | Vinorelbine Tartrate and Paclitaxel in Treating Older Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05006664 | Brentuximab Vedotin in Combination With CHEP in Patient With PTCL | lymphoma | Doxorubicin (NPC261012) | |
| NCT02024607 | BRAF/MEK/EGFR Inhibitor Combination Study in Colorectal Cancer (CRC) | cancer | Fluorouracil (NPC75844) | |
| NCT02997332 | Durvalumab in Combination With Docetaxel, Cisplatin and 5-FU for Locally Advanced Head and Neck Squamous Cell Carcinoma | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT00006027 | Comparison of Radiation Therapy With or Without Combination Chemotherapy Following Surgery in Treating Patients With Stage I or Stage II Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT04980222 | A Study to Evaluate the Safety and Efficacy of Glofitamab in Combination With Rituximab (R) Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) in Circulating Tumor (ct)DNA High-Risk Patients With Untreated Diffuse Large B-Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04973904 | Toripalimab Combined With Chemotherapy and Bevacizumab as First-Line Treatment in Patients With Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03154294 | Evaluation of the Safety and Tolerability of TAK-228 With TAK-117 and Paclitaxel in Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01357161 | Study of Ombrabulin in Patients With Platinum-Sensitive Recurrent Ovarian Cancer Treated With Carboplatin/Paclitaxel | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02392637 | Gemcitabine Hydrochloride, Cisplatin, and Nab-Paclitaxel in Treating Patients With Advanced or Metastatic Biliary Cancers | gallbladder cancer;intrahepatic cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT00919984 | Efficacy Study of Additional Intraperitoneal Chemotherapy to Treat Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00004252 | Leucovorin and Fluorouracil With or Without SU5416 in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00326456 | MITO-2: A Study Comparing 2 Chemotherapy Regimens (Carboplatin/Liposomal Doxorubicin vs Carboplatin/Paclitaxel) in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT02741570 | Study of Nivolumab in Combination With Ipilimumab Compared to the Standard of Care (Extreme Regimen) as First Line Treatment in Patients With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT02282839 | Oral Glutamine and Mucositis of Head and Neck Cancer Patients Undergoing Radiation | mucositis | Glutamine (NPC197087) | |
| NCT00053807 | Interleukin-2, Interferon Alfa, and Fluorouracil Compared With Observation in Treating Patients Who Have Undergone Surgery for Kidney Cancer | kidney cancer | Fluorouracil (NPC75844) | |
| NCT02421588 | Clinical Trial of Lurbinectedin (PM01183) in Platinum Resistant Ovarian Cancer Patients | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00470301 | Tipifarnib and Combination Chemotherapy in Treating Patients With Stage II or Stage III Breast Cancer | male breast carcinoma | Doxorubicin (NPC261012) | |
| NCT04046575 | Radiation Dose Intensification With Accelerated Hypofractionated Intensity Modulated Radiation Therapy and Concurrent Carboplatin and Paclitaxel for Inoperable Esophageal Cancer | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT02574663 | TGR-1202 Alone and in Combination With Either Nab-paclitaxel + Gemcitabine or With FOLFOX in Patients With Select Relapsed or Refractory Solid Tumors | rectum cancer;colorectal carcinoma;gastrointestinal stromal tumor | Paclitaxel (NPC208553) | |
| NCT00069095 | A Study of Capecitabine (Xeloda) and Bevacizumab as a First-line Therapy in Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00753909 | Paclitaxel, Carboplatin Plus Bevacizumab in Pretreated, Advanced or Metastatic Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01319981 | Hyper-CVAD With Liposomal Vincristine in Acute Lymphoblastic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT00687687 | Evaluation of Paclitaxel (Taxol, NSC | Uterine Carcinosarcoma | Paclitaxel (NPC208553) | |
| NCT00525915 | Oxaliplatin-Based Chemotherapy and Chemoradiotherapy or Chemoradiotherapy in Esophageal or Gastroesophageal Carcinoma | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT05113251 | Trastuzumab Deruxtecan (T-DXd) Alone or in Sequence With THP, Versus Standard Treatment (ddAC-THP), in HER2-positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03504423 | Study Evaluating Efficacy and Safety of FFX Versus Combination of CPI-613 With mFFX in Patients With Metastatic Adenocarcinoma of the Pancreas | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT02633137 | Sequential Chemotherapy and Lenalidomide Followed by Rituximab and Lenalidomide Maintenance for Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02559674 | QUILT-2.001: ALT-803 in Patients With Advanced Pancreatic Cancer in Conjunction With Gemcitabine and Nab-Paclitaxel | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00657878 | Efficacy Study of Chemotherapy to Treat Ovarian Cancer Recurrence by Prolonging the Platinum Free Interval | ovarian cancer | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT05002686 | Safety and Efficacy of Sintilimab in Combination With Chemoradiothrapy Followed by D2 Surgical Resection in Patients With Advanced Gastric Cancer With Retroperitoneal Lymph Node Metastasis | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03805399 | FUSCC Refractory TNBC Umbrella (FUTURE) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03361319 | Combination Nab-paclitaxel (N-P) and Nintedanib or N-P and Placebo in Relapsed NSCLC Adenocarcinoma | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02873598 | A Dose Escalation Trial of SBRT After Induction Chemotherapy for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02422563 | NeoAdjuvant Chemotherapy Followed by Radical Hysterectomy (OP) Versus Primary Chemo-RADiation in Cervical Cancer FIGO Stage IB2 and IIB | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00542191 | Phase II Trial of Neoadjuvant Metronomic Chemotherapy in Triple-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00687440 | Phase II Trial to Compare the Safety of Two Chemotherapy Plus Trastuzumab Regimens as Adjuvant Therapy for HER2-positive Breast Cancer (Study P05048) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00465907 | Study of Weekly Paclitaxel, Carboplatin and Irinotecan to Treat Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05135364 | HAIC Combined With Camrelizumab and TKI for Unresectable Hepatocellular Carcinoma After TACE Failure | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT03399747 | Abbreviated 3 Cycles of Rituximab Plus CHOP(Cyclophosphamide, Adriamycin, Vincristine, and Prednisolone) Immunochemotherapy in Patients With Completely Excised LocalizedGastrointestinal CD(Cluster of Differentiation Antigen)20(+) Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05174156 | Study on the Biomarker of First-line Immunotherapy Combined With Chemotherapy in Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00544648 | Ph I/II Nab-Paclitaxel & Carboplatin w/Concurrent Radiation Therapy for Unresectable Stg III NSCLC | lung cancer | Paclitaxel (NPC208553) | |
| NCT05394415 | Chemoradiation Plus Tislelizumab for Conversion Therapy of Locally Nonresectable ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03126708 | Cetuximab in Combination With Paclitaxel Plus Cisplatin Versus Paclitaxel Plus Cisplatin Alone for the First-line Treatment of Metastatic Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01411410 | Phase I Study of PI3(Phosphoinositol 3)-Kinase Inhibitor BAY80-6946 With Paclitaxel in Patients With Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT03467373 | A Study of Glofitamab in Combination With Rituximab or Obinutuzumab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP), or Polatuzumab Vedotin Plus Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (CHP) in Participants With Non-Hodgkin Lymphomas or With DLBCL | neoplasm of mature B-cells;non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00688740 | Docetaxel in Node Positive Adjuvant Breast Cancer | breast cancer | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT04447092 | Pembrolizumab Plus Chemotherapy in 1st Line Treatment of Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01404936 | Study of a-Interferon With Adriamycin, Bleomycin, Velban, and Dacarbazine (ABVD) With Hodgkin's Disease | lymphoma | Doxorubicin (NPC261012) | |
| NCT05328336 | Perioperative Tislelizumab Combined With Nab-Paclitaxel for Muscle-invasive Urothelial Bladder Cancer: A Multicenter Study | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT02212015 | Evaluation of Votrient in Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT00385021 | ChronoFOLFOX Plus Avastin for Patients With Metastatic Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00003004 | Combination Chemotherapy in Treating Patients With Refractory or Recurrent Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00278148 | Erlotinib, Paclitaxel, and Carboplatin Combined With Radiation Therapy for Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00609765 | Avastin, Fluorouracil, Doxorubicin and Streptozocin in Locally Advanced and Metastatic Pancreatic Endocrine Tumors | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03283696 | A Study of Olaratumab (LY3012207), Doxorubicin, and Ifosfamide in Participants With Advanced or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT05342636 | A Study of Combination Therapies With Pembrolizumab (MK-3475) in Participants With Advanced Esophageal Cancer (MK-3475-06A) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00284752 | Phase II Trial of Abraxane in Front Line Therapy of Hormone Refractory Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00202969 | Study of S-1, S-1/CDDP, and 5-FU/CDDP for Advanced Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00915603 | Trial of Paclitaxel/Bevacizumab +/- Everolimus for Patients With HER2-Negative Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00343239 | Docetaxel in Locally Advanced Gastric Adenocarcinoma | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT02879513 | Trial of Adjuvant Chemotherapy in Breast Cancer Patients With Pathological Partial Response and Complete Response to Neoadjuvant Chemotherapy | inflammatory breast carcinoma | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT01985724 | Sequential Administration of FE75C and Docetaxel Versus Docetaxel/Cyclophosphamide in HER-2 Negative, Node Positive Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT01607879 | Use of β-hydroxy-β-methylbutyrate to Counteract Muscle Loss in Men With Prostate Cancer on Androgen Ablation | prostate cancer | Glutamine (NPC197087) | |
| NCT00288041 | Bortezomib, Paclitaxel, and Carboplatin in Treating Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00004126 | Paclitaxel and Oxaliplatin in Treating Patients With Recurrent or Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03740165 | Durvalumab Treatment in Combination With Chemotherapy and Bevacizumab, Followed by Maintenance Durvalumab, Bevacizumab and Olaparib Treatment in Advanced Ovarian Cancer Patients | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00777673 | Preoperative Chemotherapy in Triple Negative Invasive Breast Cancer That Can be Removed by Surgery. | breast cancer | Paclitaxel (NPC208553) | |
| NCT00408070 | Bevacizumab Study With Carboplatin & Paclitaxel in Ovarian, Fallopian Tube or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03057366 | A Study of [14 C]-Pevonedistat in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00024349 | Radiation Therapy With or Without Chemotherapy in Treating Patients With Stage II or Stage III Bladder Cancer | urinary bladder cancer | Fluorouracil (NPC75844) | |
| NCT04761601 | First in Human Study of UCT-01-097 in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00680758 | Cisplatin, Paclitaxel, and Everolimus in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00043121 | Combination Chemotherapy in Treating Patients With Advanced Cancer That is Metastatic or Cannot Be Removed By Surgery | neoplasm | Fluorouracil (NPC75844) | |
| NCT01004978 | Chemoembolization With or Without Sorafenib Tosylate in Treating Patients With Liver Cancer That Cannot Be Removed by Surgery | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT02399137 | A Phase 2 Study of MM-141 Plus Nab-paclitaxel and Gemcitabine in Front-line Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00001579 | A Phase I Trial of 5-Fluorouracil Given With 776C85 (GW776) and Low-Dose Leucovorin in Adult Patients With Solid Tumors | lymphoma | Fluorouracil (NPC75844) | |
| NCT01822756 | An Open-Label Study of Ruxolitinib Given With Chemotherapy in Patients With Advanced Solid Tumors | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01275677 | Chemotherapy With or Without Trastuzumab After Surgery in Treating Women With Invasive Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT00889382 | Paclitaxel-Loaded Polymeric Micelle and Carboplatin as First-Line Therapy in Treating Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | metastatic melanoma;non-small cell lung carcinoma | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT04592861 | Carbon Ion RT for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00355199 | Comparison of HD Chemotherapy Followed by Auto-transplant and R-CHOP in High Risk Patients With DLBCL. | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00041210 | Combination Chemotherapy in Treating Patients With Previously Untreated Advanced Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04380337 | Organ Preservation Program Using Short-Course Radiation & FOLFOXIRI in Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00154882 | Paclitaxel (Phyxol) and Cisplatin as First-line Chemotherapy for Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04148911 | A Study of Atezolizumab Plus Nab-Paclitaxel in the Treatment of Unresectable Locally Advanced or Metastatic PD-L1-Positive Triple-Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03412799 | Study of SBP-101 Combined With Nab-Paclitaxel and Gemcitabine in Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01227941 | MK-4827 in Combination With Pegylated Liposomal Doxorubicin in Participants With Advanced Solid Tumors and Ovarian Cancer (MK-4827-011) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03126812 | Pembrolizumab, Paclitaxel, and Carboplatin in Patients With Advanced Stage Epithelial Ovarian Cancer (EOC). | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02470585 | Dose Dense Paclitaxel With Pembrolizumab (MK-3475) in Platinum Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04994236 | Hepatic Artery Infusion Chemotherapy for Unresectable Hepatocelluar Carcinoma Who Failed to Systemic Therapy | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00502411 | Post-Operative Chemoradiation for Extremity & Trunk Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02114502 | Carfilzomib/SAHA Combined With High-Dose Gemcitabine/Busulfan/Melphalan With Autologous Stem Cell Transplant in Myeloma | multiple myeloma | Glutamine (NPC197087) | |
| NCT01027910 | PCI-24781 in Combination With Doxorubicin to Treat Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT02861690 | Liposomal Paclitaxel Combined Nedaplatin in Treatment of Advanced or Recurrent Esophageal Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT04683315 | PurIST Classification-Guided Adaptive Neoadjuvant Chemotherapy by RNA Expression Profiling of EUS Aspiration Samples | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00750386 | An Efficacy and Safety Study of MORAb-003 in Platinum-Resistant or Refractory Relapsed Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003127 | S9720 Combination Chemotherapy in Treating Patients With Metastatic, Recurrent, or Refractory Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00003214 | Chemosensitivity Testing to Assign Treatment for Patients With Stage III or Stage IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00071188 | ZD6474 Alone or in Combination With Paclitaxel and Carboplatin in Subjects With Previously Untreated Locally Advanced or Metastatic Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00873275 | Ursodiol, Combination Chemotherapy, and Bevacizumab in Treating Patients With Stage IV Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03069950 | Study of Chemotherapy With or Without Hepatic Arterial Infusion for Patients With Unresectable Metastatic Colorectal Cancer to the Liver | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00183742 | Trial of Liposomal Doxorubicin (Doxil) and Weekly Docetaxel (Taxotere) | carcinoma | Doxorubicin (NPC261012) | |
| NCT04602117 | ISPY-P1.01:Evaluating the Safety of Weekly Paclitaxel With Trastuzumab Duocarmazine (SYD985) in Patients With Metastatic Cancer | carcinoid tumor;endometrium neoplasm;gastric cancer;ovarian carcinoma;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00425152 | Fluorouracil and Leucovorin and/or Levamisole After Surgery In Treating Patients With Dukes' B Or Dukes' C Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00354978 | Study of FOLFIRI Plus Bevacizumab in Colorectal Cancer Patients | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT01224652 | Efficacy Study of Paclitaxel Versus Irinotecan in Patients With Recurrent or Metastatic Gastric Cancer Who Progress Following First-line Therapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04031703 | Study Comparing Paclitaxel Plus Carboplatin Versus Anthracyclines Followed by Docetaxel as Adjuvant Chemotherapy for Triple Negative Breast Cancer (PATTERN) | breast cancer | Fluorouracil (NPC75844) | |
| NCT02514031 | ARQ-761 Treatment With Gemcitabine/Nab-Paclitaxel Chemotherapy In Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04185883 | Sotorasib Activity in Subjects With Advanced Solid Tumors With KRAS p.G12C Mutation (CodeBreak 101) | neoplasm | Fluorouracil (NPC75844) | |
| NCT04383743 | Pembrolizumab and Combination Chemotherapy Before Surgery for the Treatment of Muscle-Invasive Bladder Cancer | urinary bladder carcinoma | Doxorubicin (NPC261012) | |
| NCT02436993 | Phase II Breast Ca Carboplatin + Paclitaxel With Pertuzumab + Trastuzumab or Bevacizumab | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01931592 | Controling Intestinal Colonization With Extended Spectrum ß-Lactamase Producing Enterobacteriaceae ESBL-E | cancer | Gentamicin (NPC471628) | |
| NCT00112658 | Combination Chemotherapy as First-Line Therapy in Treating Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00002734 | Radiolabeled Monoclonal Antibody, Paclitaxel, and Interferon Alfa in Treating Patients With Recurrent Ovarian Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04888663 | Study of Ramucirumab, Trastuzumab and Paclitaxel in Patients With HER2-positive Recurrent/Metastatic Gastric Cancer (HER-RAM Study) | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04876313 | An Open Label, Single-arm, Phase 2 Study of Neoadjuvant Nivolumab and Nab-paclitaxel Before Radical Cystectomy for Patients With Muscle-invasive Bladder Cancer (NURE-Combo) | urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT05253651 | A Study of Tucatinib With Trastuzumab and mFOLFOX6 Versus Standard of Care Treatment in First-line HER2+ Metastatic Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00011999 | Chemotherapy and Radiation Therapy Following Surgery in Treating Patients With Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04314895 | Trial of NanoPac Intratumoral Injection in Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00052429 | High-Dose Radiation Therapy Plus Chemotherapy in Treating Patients With Advanced Nose or Throat Cancer | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT01358877 | A Study of Pertuzumab in Addition to Chemotherapy and Trastuzumab as Adjuvant Therapy in Participants With Human Epidermal Growth Receptor 2 (HER2)-Positive Primary Breast Cancer | breast cancer | Fluorouracil (NPC75844);Doxorubicin (NPC261012) | |
| NCT01146795 | Alisertib (MLN8237) in Participants With Ovarian, Fallopian Tube or Peritoneal Cancer Preceded by Phase 1 Study of MLN8237 Plus Paclitaxel Treatment of Ovary or Breast Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT04566887 | Acalabrutinib With R-CHOP in Previously Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00022230 | Combination Chemotherapy Plus Biological Therapy in Treating Patients With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00600977 | Liposomal Anthracyclin in the Treatment of Elderly ALL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00004099 | Surgery With or Without Combination Chemotherapy in Treating Patients With Stomach Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT02446652 | Evaluation of TRANSKRIP ® Plus Chemotherapy in Recurrent-Persistent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00436267 | Internal Radiation Therapy With Y-90 Microspheres, External Radiation Therapy With Tomotherapy, and Fluorouracil in Treating Patients With Newly Diagnosed or Recurrent Pancreatic Cancer and Liver Metastases That Cannot Be Removed By Surgery | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00691054 | Abraxane Therapy in Patients With Pancreatic Cancer Who Failed First-Line Gemcitabine Therapy | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00001300 | A Randomized Study of the Effect of Adjuvant Chemotherapy With Doxorubicin and Ifosfamide With Mesna in the Treatment of High-Grade Adult Extremity Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT02199418 | Addition of Cisplatin to Neoadjuvant Therapy for T Locally Advanced Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03668730 | Reduced-dose Radiotherapy for Low-risk Stage III Patients With Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553);Fluorouracil (NPC75844) | |
| NCT04480190 | Neoadjuvant Therapy in Biliary Adenocarcinoma | cholangiocarcinoma | Fluorouracil (NPC75844) | |
| NCT03487016 | First-line Therapy in Metastatic PDAC | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT00004102 | Combination Chemotherapy in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00413322 | Study of PXD101 Alone and in Combination With 5-Fluorouracil (5-FU) in Patients With Advanced Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT03193918 | Study of Crenolanib With Ramucirumab and Paclitaxel for Advanced Esophagogastric Adenocarcinoma | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01286766 | Neoadjuvant Combination Chemotherapy of DCS (Cisplatin + Docetaxel + S-1) and DCF (Docetaxel + Cisplatin + 5-FU) in Patients With Locally Advanced Gastric Adenocarcinoma | gastric cancer | Fluorouracil (NPC75844) | |
| NCT04757337 | Comparison of Oral Cyclophosphamide vs Doxorubicin in ≥65 Years Old Advanced or Metastatic Soft Tissue Sarcoma Patients | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02204345 | A Study Evaluating RO5479599 in Combination With Carboplatin and Paclitaxel in Participants With Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) of Squamous Histology | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00431080 | Randomized Phase III Trial Comparing Sequential Administration of FE75C Followed by Docetaxel Versus Paclitaxel as Adjuvant Chemotherapy in Axillary Lymph Node (+) Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT02651727 | Ph 1 Study of VS-4718, a FAK Inhibitor, in Combination With Nab-paclitaxel and Gemcitabine in Advanced Cancer Subjects | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01285765 | Evaluate a Treatment Adapted to the PET Response Compared to a Standard Treatment, for Low Risk DLBCL CD 20+ Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03132116 | Intra-nodal Injection of Gentamicin for the Treatment of Suppurated Cat Scratch Disease's Lymphadenitis | cat-scratch disease | Gentamicin (NPC471628) | |
| NCT01055028 | Paclitaxel + Bevacizumab (Avastin) for the Treatment of Metastatic or Unresectable Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT03690739 | Evaluation of Symptom Benefit Rate of Trabectedin/PLD in Patients With Recurrent Ovarian Cancer | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT02476955 | Veliparib With Carboplatin and Paclitaxel and as Continuation Maintenance Therapy in Adults With Newly Diagnosed Stage III or IV, High-grade Serous, Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00004159 | Gemcitabine Plus Paclitaxel in Treating Patients With Advanced Non-small Cell Lung Cancer or Other Solid Tumor | lung cancer | Paclitaxel (NPC208553) | |
| NCT03430843 | A Study of Tislelizumab (BGB-A317) Versus Chemotherapy as Second Line Treatment in Participants With Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02640365 | A Study of BBI608 in Combination With Standard Chemotherapies in Adult Patients With Advanced Gastrointestinal Cancer | cancer | Fluorouracil (NPC75844) | |
| NCT00316199 | Efficacy Study of Gemcitabine-Paclitaxel to Treat Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00611507 | A Phase II Study of Oxaliplatin in Association With 5FU and Folinic Acid in the Treatment of Subjects With Non-Surgical or Advanced Metastatic Gastric Cancer | stomach neoplasm | Fluorouracil (NPC75844) | |
| NCT00566540 | Ph 2 Intensification Regimen for Previously Untreated, Resectable, Advanced Squamous Cell Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01009983 | Paclitaxel, Carboplatin, and Panitumumab in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00408408 | Chemotherapy With or Without Bevacizumab in Treating Women With Stage I, Stage II, or Stage IIIA Breast Cancer That Can Be Removed By Surgery | breast cancer | Doxorubicin (NPC261012) | |
| NCT03517176 | CEND-1 in Combination With Nabpaclitaxel and Gemcitabine in Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05065801 | Efficacy of Gembrax Followed by Folfirinox Versus Folfirinox Alone in First Metastatic Line Pancreatic Cancer Patients | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT00024375 | DHA-Paclitaxel in Treating Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00630032 | Safety and Efficacy Comparison of Docetaxel and Ixabepilone in Non Metastatic Poor Prognosis Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00707889 | Phase 2 Study of ABT-869 in Combination With mFOLFOX6 Versus Bevacizumab in Combination With mFOLFOX6 to Treat Advanced Colorectal Cancer | colorectal adenocarcinoma;rectum cancer | Fluorouracil (NPC75844) | |
| NCT01704703 | Study of FOLFIRI + Panitumumab Using Ultra-selection Technology of Patients With Stage IV Colorectal Cancer Refractory to Irinotecan Without Any Mutation on KRAS, PIK3Ca, BRAF and NRAS Genes Detected With Highly Sensitive Techniques | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00629278 | Combination Chemotherapy and Trastuzumab in Treating Women With Stage I, Stage II, or Stage III HER2-Positive Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT00005586 | Leucovorin and Fluorouracil Compared With Observation in Treating Patients With Colorectal Cancer That Has Been Surgically Removed | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00073723 | Trial of ABI-007 Administered Weekly in Chemotherapy Naive Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00081289 | Neoadjuvant Chemoradiotherapy and Adjuvant Chemotherapy in Treating Patients Who Are Undergoing Surgical Resection for Locally Advanced Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT04625907 | FaR-RMS: An Overarching Study for Children and Adults With Frontline and Relapsed RhabdoMyoSarcoma | rhabdomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00635726 | Methotrexate, Vinblastine, Doxorubicin and Cisplatin (MVAC) Followed by Gemcitabine Plus Cisplatin (GEM+CDDP) in Locally Advanced or Metastatic Bladder Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT00003299 | Cisplatin Plus Etoposide With or Without Paclitaxel in Treating Patients With Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04632992 | A Study to Determine the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of ABBV-368 Plus Tilsotolimod and Other Therapy Combinations in Participants With Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma | cancer | Paclitaxel (NPC208553) | |
| NCT00335816 | Radiation Therapy and Fluorouracil With or Without Combination Chemotherapy Followed by Surgery in Treating Patients With Stage II or Stage III Rectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00933374 | Trial to Evaluate Paclitaxel Plus RAD001 in Urothelial Carcinoma | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00066846 | Bevacizumab Plus Fluorouracil and Leucovorin in Treating Patients With Locally Advanced or Metastatic Stage IV Colorectal Cancer That Has Progressed After Standard Chemotherapy | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00003549 | S9716: Combination Chemotherapy in Treating Patients With Merkel Cell Cancer | Merkel cell skin cancer | Fluorouracil (NPC75844) | |
| NCT00825149 | A Study of Obinutuzumab in Combination With Chemotherapy in Participants With CD20+ B-Cell Follicular Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00008281 | Comparison of Three Chemotherapy Regimens in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03018626 | R-ACVBP and DA-EPOCH-R in Patients With Non-GCB DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01721746 | A Study to Compare BMS-936558 to the Physician's Choice of Either Dacarbazine or Carboplatin and Paclitaxel in Advanced Melanoma Patients That Have Progressed Following Anti-CTLA-4 Therapy (CheckMate 037) | metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT00970996 | Cisplatin, Temozolomide, Abraxane, With Interleukin-2 and Interferon for Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00523380 | Efficacy Study of Recombinant Interleukin-21 in the Treatment of Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01930292 | Debio 1143 in Combination With Carboplatin and Paclitaxel in Patient With Advanced Solid Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT03762564 | Study With Paclitaxel +/- Ramucirumab in Patients With Squamous-cell Carcinoma of the Esophagus After Prior Therapy | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00313768 | Randomized Ph 2 Trial Of Paclitaxel/Carboplatin /Bevacizumab + PF-3512676 And P/C/B Alone In Advanced Nonsquamous NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00717340 | A Phase 1b/2a, Open-Label, Multi-Center Study of Tivozanib (AV-951) in Combination With Paclitaxel in Subjects With Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00091312 | Fluorouracil and Irinotecan With or Without Leucovorin Compared With Observation in Treating Patients Who Have Undergone Surgery for Stage II Colon Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02358031 | A Study of Pembrolizumab (MK-3475) for First Line Treatment of Recurrent or Metastatic Squamous Cell Cancer of the Head and Neck (MK-3475-048/KEYNOTE-048) | head and neck malignant neoplasia | Fluorouracil (NPC75844) | |
| NCT01787006 | Definitive Radiochemotherapy Plus/Minus Cetuximab in Unresectable Locally Advanced Esophageal Cancer | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00110721 | GM-CT-01 Plus 5-Fluorouracil as Third- or Fourth-Line Therapy for Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03462212 | Study of GEN-1 With NACT for Treatment of Ovarian Cancer (OVATION 2) | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00002587 | Paclitaxel Plus Topotecan in Treating Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04799639 | Efficacy and Toxicity of Paclitaxel, Cisplatin Combined With Sindilimab in NACT for Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT05241249 | Stimulation With Bethanechol in Combination With Gemcitabine and Nab-paclitaxel in Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00209625 | Phase I / II Study of Irinotecan (CPT-11) Combined With l-Leucovorin (l-LV) and 5-FU in Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00002883 | Surgery With or Without Combination Chemotherapy in Treating Patients With Cancer of the Esophagus | esophageal cancer;gastric cancer | Fluorouracil (NPC75844) | |
| NCT04498793 | Study of Tislelizumab Plus Chemotherapy vs Chemotherapy as Perioperative Treatment in Participants With HER2 Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00818090 | Paclitaxel and Cisplatin for Thymic Neoplasm | Thymic Carcinoma;Thymoma | Paclitaxel (NPC208553) | |
| NCT00049101 | Erlotinib and Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03588039 | Study of Oraxol and Pembrolizumab in Subjects With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT05132504 | Neoadjuvant Folfirinox Combined With Pembrolizumab Followed by Surgery for Patients With Resectable Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT03394885 | Study of GEN-1 With NACT for Treatment of Ovarian Cancer (OVATION 2) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00038402 | Evaluation of the Addition of Herceptin to Standard Chemotherapy in the Neoadjuvant Setting for Operable Breast Cancer | breast cancer | Fluorouracil (NPC75844) | |
| NCT04249167 | Cryoablation, Atezolizumab/Nab-paclitaxel for Locally Advanced or Metastatic Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00336791 | Randomized Clinical Trial to Evaluate the Predictive Accuracy of a Gene Expression for Stage I-II Breast Cancer | breast cancer | Doxorubicin (NPC261012);Fluorouracil (NPC75844) | |
| NCT00006454 | Paclitaxel Plus Carboplatin With or Without Topotecan in Treating Patients With Stage IIB, Stage III, or Stage IV Ovarian Epithelial Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00181701 | Intraperitoneal Paclitaxel and Carboplatin in the Treatment of Women With Carcinoma of Mullerian Origin | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT03853707 | Ipatasertib in Combination With Carboplatin, Carboplatin/Paclitaxel, or Capecitabine/Atezolizumab in Treating Patients With Metastatic Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03233347 | Doxorubicin, Vinblastine, Dacarbazine, Brentuximab Vedotin, and Nivolumab in Treating Patients With Stage I-II Hodgkin Lymphoma | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT03860844 | Isatuximab in Combination With Chemotherapy in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia or Acute Myeloid Leukemia | acute myeloid leukemia;acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00915850 | Trial of Docetaxel, Cisplatin, Fluorouracil (5-FU) for Unresectable Advanced Esophageal Squamous Cell Carcinoma (ESCC) | esophageal cancer | Fluorouracil (NPC75844) | |
| NCT00795340 | Cediranib, Paclitaxel, and Carboplatin in Treating Patients With Stage IIIB or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00196872 | A Study to Compare ETC vs. EC-TX and Ibandronate vs. Observation in Patients With Node-positive Primary Breast Cancer (GAIN) | breast cancer | Paclitaxel (NPC208553) | |
| NCT01171482 | Comparison Study of Sorafenib and 5-fluorouracil/Mitomycin for Metastatic Hepatocellular Carcinoma | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT01293630 | A Dose-escalation Study of Ombrabulin in Combination With Paclitaxel and Carboplatin in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00840450 | Two Different Schedules of Carboplatin, Paclitaxel, Gemcitabine, and Surgery in Treating Patients With Newly Diagnosed Stage IIIC or Stage IV Primary Epithelial Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01031030 | Cyclophosphamide, Methotrexate, and Fluorouracil, With or Without Epirubicin Hydrochloride, in Treating Women Who Have Undergone Surgery for Breast Cancer (Group III Closed to New Patients as of 12/7/2009) | breast cancer | Fluorouracil (NPC75844) | |
| NCT01272557 | Sorafenib Plus Doxorubicin Versus Sorafenib Alone for the Treatment of Advanced Hepatocellular Carcinoma: a Randomized Phase II Trial | carcinoma of liver and intrahepatic biliary tract | Doxorubicin (NPC261012) | |
| NCT02776917 | Adjuvant Treatment of EC Followed by Taxane +/- Carboplatin in Triple-Negative Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03252808 | Phase I Study of TBI-1401(HF10) Plus Chemotherapy in Patients With Unresectable Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01885013 | Myocet + Cyclophosphamide + Metformin Vs Myocet + Cyclophosphamide in 1st Line Treatment of HER2 Neg. Metastatic Breast Cancer Patients | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT05052931 | Nab-paclitaxel Combined With Oxaliplatin and S-1 for Perioperative Treatment of Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01641783 | Efficacy and Safety Study of ABI-007 Plus Capecitabine as First-line Chemotherapy for Advanced Gastric Cancer Patients | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04390958 | Neoadjuvant Chemotherapy With Nab-paclitaxel Plus Cisplatin and Capecitabine for Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00178256 | Pulsed Paclitaxel And Daily Thoracic Radiotherapy For Inoperable (Stage I/II) Or Unresectable Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04908787 | PD-1 Antibody Combined Neoadjuvant Chemotherapy for Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01016054 | A Study of the Safety and Pharmacokinetics of AGS-8M4 Given in Combination With Chemotherapy in Women With Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00515073 | Papillary Serous Carcinoma of the Endometrium | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01204749 | Saracatinib and Paclitaxel in Platinum-resistant Ovarian Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03490292 | Avelumab With Chemoradiation for Stage II/III Resectable Esophageal and Gastroesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00309530 | Randomized Study on Adjuvant Chemotherapy and Adjuvant Chemo-Immunotherapy in Colon Carcinoma Dukes C | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT01672671 | BRCA1-associated DNA Repair Dysfunction in Patients With Early Triple Negative Breast Cancer Treated With Neoadjuvant Platinum-based Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT03880695 | Anlotinib Hydrochloride Combined With Liposomal Doxorubicin in the Treatment of Locally Advanced or Metastatic Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT02019355 | Actinic Keratosis Study | actinic keratosis | Fluorouracil (NPC75844) | |
| NCT02351323 | Novel Type 2 Diabetes Mellitus Preventive Therapies | diabetes mellitus | Glutamine (NPC197087) | |
| NCT00439296 | ABT-751 With Chemotherapy for Relapsed Pediatric ALL | childhood acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT05204173 | Efficacy and Safety of Sintilimab Combined Intraperitoneal and Intravenous Paclitaxel Plus Oral S-1 in Gastric Cancer Patients With Peritoneal Metastasis | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00025688 | Paclitaxel With or Without Carboplatin in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02607332 | Paclitaxel in Patients With Metastatic or Advanced Gastrointestinal Stromal Tumors (GIST) After Failure to Imatinib and Sunitinib | gastrointestinal stromal tumor | Paclitaxel (NPC208553) | |
| NCT01113957 | A Trial of ABT-888 in Combination With Temozolomide Versus Pegylated Liposomal Doxorubicin Alone in Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03959293 | Clinical Trial Evaluating FOLFIRI + Durvalumab vs FOLFIRI + Durvalumab and Tremelimumab in Second-line Treatment of Patients With Advanced Gastric or Gastro-oesophageal Junction Adenocarcinoma | gastric adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00004092 | Combination Chemotherapy in Treating Patients With High-Risk Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00254592 | Neoadjuvant Treatment of Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02389985 | Paclitaxel/Pazopanib for Platinum Resistant/Refractory Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01088815 | Hedgehog Inhibitors for Metastatic Adenocarcinoma of the Pancreas | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04868708 | A Study of AK104( an Anti-PD-1 and Anti-CTLA-4 Bispecific Antibody) in Recurrent or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00752115 | Combination Chemotherapy With Sildenafil Plus Carboplatin and Paclitaxel in Patients With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00070122 | Combination Chemotherapy and Bevacizumab in Treating Patients With Locally Advanced, Metastatic, or Recurrent Colorectal Cancer | colorectal adenocarcinoma;malignant colon neoplasm;rectum cancer | Fluorouracil (NPC75844) | |
| NCT01314833 | Minus Anthracycline or Short-Term Versus Epirubicin and Cyclophosphamide Followed by Paclitaxel Regimen for Adjuvant Breast Cancer Therapy | breast cancer | Fluorouracil (NPC75844) | |
| NCT01746238 | Bevacizumab/Doxorubicin/Radiation for Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT00352755 | A Phase II Study of Surgical Debulking With Peritonectomy and Biweekly Intraperitoneal 5FU With Systemic Oxaliplatin/5FU/Leucovorin in Patients With Pseudomyxoma Peritonei or Peritoneal Carcinomatosis | peritoneal neoplasm | Fluorouracil (NPC75844) | |
| NCT00004013 | Paclitaxel With or Without Trastuzumab Following Peripheral Stem Cell Transplantation in Treating Patients With Refractory Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01547741 | Docetaxel and Cyclophosphamide Compared to Anthracycline-Based Chemotherapy in Treating Women With HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03802071 | A Study of Durvalumab in Combination With Doxorubicin for Advanced Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT01276496 | Weekly Doses of Cilengitide and Paclitaxel in Treating Patients With Advanced Solid Tumors That Cannot Be Removed by Surgery | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00003880 | Paclitaxel Plus Carboplatin With or Without SCH-58500 in Treating Patients With Newly Diagnosed Stage III Ovarian or Stage III Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01617928 | A Study of Veliparib in Combination With Carboplatin and Paclitaxel in Japanese Subjects With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02475772 | A Study With Intraperitoneal Cisplatin and Doxorubicin in Recurrent Ovarian Cancer and Peritoneal Carcinomatosis | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT05346107 | PLD-cyclophosphamide-Nab-P Continuously Combined With Dual HER2 Blockage for HER2-positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01206465 | Pralatrexate and Fluorouracil in Treating Patients With Recurrent Solid Tumors | neoplasm | Fluorouracil (NPC75844) | |
| NCT01746771 | A Phase I-II Study of HM781-36B Combined With Paclitaxel and Trastuzumab in HER-2 Positive Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04584112 | A Study of the Safety, Efficacy, and Pharmacokinetics of Tiragolumab in Combination With Atezolizumab and Chemotherapy in Participants With Triple-Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553);Doxorubicin (NPC261012) | |
| NCT02413853 | Combination Chemotherapy and Bevacizumab With or Without PRI-724 in Treating Patients With Newly Diagnosed Metastatic Colorectal Cancer | colorectal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT00006469 | Combination Chemotherapy and Radiation Therapy Followed By Surgery in Treating Patients With Stage IIB or Stage IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00321685 | Bevacizumab, Radiation Therapy, and Combination Chemotherapy in Treating Patients Who Are Undergoing Surgery for Locally Advanced Nonmetastatic Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT02967887 | Evaluation of Hepatic Arterial Infusion of Cisplatin and 5-FU in Biomarker Stratified HCC | hepatocellular carcinoma | Fluorouracil (NPC75844) | |
| NCT00001383 | A Phase I Study of Infusional Paclitaxel With the P-Glycoprotein Antagonist PSC 833 | renal cell carcinoma;lymphoma;ovarian cancer;breast cancer | Paclitaxel (NPC208553) | |
| NCT00080418 | Liposome Entrapped Paclitaxel Easy to Use (LEP-ETU) in Patients With Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT01688700 | Study of Nimotuzumab in Combination With Neoadjuvant Chemotherapy for Resectable Esophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01303497 | Efficacity of Weekly Paclitaxel in Association or Not With Bevacizumab in Metastatic or Locally Advanced Angiosarcomas | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT00070434 | S0304 Induct Chemo Then Chemo-RT in Pts w/Locally Advanced Adenocarcinoma of the Rectum | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02822157 | Circulating Tumor DNA Guiding (Olaparib) Lynparza® Treatment in Ovarian Cancer | malignant epithelial tumor of ovary | Doxorubicin (NPC261012);Paclitaxel (NPC208553) | |
| NCT00628810 | Combination Chemotherapy and Bevacizumab in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00512096 | Neoadjuvant Chemotherapy for Patients With Squamous Cell Carcinoma of the Penis | penile cancer | Paclitaxel (NPC208553) | |
| NCT03975049 | Triplet Combination or Doublet Regimen Versus Chemoradiation as Neoadjuvant Therapy for Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT03370406 | Intralesional 5-Fluorouracil (5FU), Topical Imiquimod Treatment for SCC | squamous cell carcinoma | Fluorouracil (NPC75844) | |
| NCT02314806 | Gentamicin Versus Clindamycin Versus Normal Saline Lavage in Axilla | breast cancer | Gentamicin (NPC471628) | |
| NCT00186849 | Therapy for Children With Advanced Stage High Risk Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT03475680 | Mechanical Bowel Preparation and Oral Antibiotics Before Colon Cancer Surgery | malignant colon neoplasm | Gentamicin (NPC471628) | |
| NCT04815408 | Batiraxcept (AVB-S6-500)/Placebo in Combination With Paclitaxel in Patients With Platinum-Resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03539328 | Study on MK-3475 Plus Chemotherapy Versus Chemotherapy Alone in Recurrent, Platinum-resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01468740 | Prospective Study on HIV-related Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00021281 | Combination Chemotherapy With or Without SU5416 in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT00193219 | Bevacizumab and Cetuximab in Combination With FOLFOX6 in Patients With Metastatic Colorectal Cancer | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00347412 | Study of NOV-002 in Combination With Chemotherapy to Treat Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004067 | Doxorubicin and Cyclophosphamide Plus Paclitaxel With or Without Trastuzumab in Treating Women With Node-Positive Breast Cancer That Overexpresses HER2 | breast cancer | Paclitaxel (NPC208553) | |
| NCT00170664 | Taxol/ Carboplatin as First Line Therapy in Patients With Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00762034 | A Study of Pemetrexed, Carboplatin and Bevacizumab in Participants With Nonsquamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02217020 | Neoadjuvant FOLFOXIRI Chemotherapy Alone for Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT04718415 | Neoadjuvant Sintilimab in Combination With Carboplatin and Nab-paclitaxel in Resectable Oral Cavity or Oropharyngeal Squamous Cell Carcinoma | oral squamous cell carcinoma;oropharynx squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03885219 | Nab-paclitaxel and S-1 in Patients With Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00005635 | Trastuzumab Plus Paclitaxel in Treating Women With Metastatic Breast Cancer That Overexpresses HER2 | breast cancer | Paclitaxel (NPC208553) | |
| NCT04358354 | Triplet Combination of Cytotoxics as First-line Treatment for Metastatic Gastric Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00721747 | Taxotere®, Followed by Myocet® and Cyclophosphamide First Line Treatment in HER2 Neg Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00002722 | High-Dose Chemotherapy in Treating Patients With Advanced Stomach Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00583830 | A Study of Mapatumumab in Combination With Paclitaxel and Carboplatin in Subjects With Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02615730 | PI3Kβ Selective Inhibitor With Paclitaxel, Advanced Gastric Adenocarcinoma | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04672005 | Modified FOLFIRINOX Alternated With Biweekly Gemcitabine Plus Nab-Paclitaxel Untreated Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844);Paclitaxel (NPC208553) | |
| NCT04023916 | Sintilimab Plus R-CHOP as the First-line Treatment in Patients With Diffuse Large B-Cell Lymphoma. | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00003170 | Glutamine in Preventing Acute Diarrhea in Patients With Pelvic Cancer | cancer | Glutamine (NPC197087) | |
| NCT02531308 | Metformin in Combination With Standard Induction Therapy for Large B-cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00003543 | Monoclonal Antibody Therapy Plus Combination Chemotherapy in Treating Patients With Advanced Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT02630264 | E10A for the Treatment of Squamous Cell Carcinoma of the Head and Neck | upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT00642759 | Carboplatin, Abraxane, and Bevacizumab in Previously Untreated Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03781323 | Neoadjuvant FOLFOX Therapy With Short Course Radiation and Active Surveillance in Locally Advanced Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00002972 | Paclitaxel in Treating Patients With Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00002615 | Surgery With or Without Combination Chemotherapy in Treating Patients With Stomach Cancer | gastric cancer | Fluorouracil (NPC75844) | |
| NCT00231868 | A Study of Radiation Therapy and Paclitaxel and Carboplatin in Patients With Uterine Papillary Serous Carcinoma | uterine cancer | Paclitaxel (NPC208553) | |
| NCT03884556 | TTX-030 Single Agent and in Combination With Immunotherapy or Chemotherapy for Patients With Advanced Cancers | lymphoma | Paclitaxel (NPC208553) | |
| NCT00006994 | S9908: Glutamine in Treating Mucositis Caused by Radiation Therapy in Patients With Newly Diagnosed Cancer of the Mouth or Throat | oral mucositis | Glutamine (NPC197087) | |
| NCT03117751 | Total Therapy XVII for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia and Lymphoma | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00005646 | Paclitaxel in Treating Patients With Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02510118 | Synergistic Anti-tumor Effect of ChangTai Keli for Colon Cancer Patients | malignant colon neoplasm | Fluorouracil (NPC75844) | |
| NCT00320541 | A Trial of Paclitaxel and Bevacizumab vs. Gemcitabine, Paclitaxel, and Bevacizumab in Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00719797 | Combination Chemotherapy and Bevacizumab as First-Line Therapy in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Fluorouracil (NPC75844) | |
| NCT03537690 | FID-007 in Treating Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT05218499 | Brightline-1: A Study to Compare BI 907828 With Doxorubicin in People With a Type of Cancer Called Dedifferentiated Liposarcoma | dedifferentiated liposarcoma | Doxorubicin (NPC261012) | |
| NCT02244632 | Modufolin (Arfolitixorin) in Combination With 5-Fluorouracil Alone or Together With Oxaliplatin or Irinotecan in Colorectal Cancer | colorectal neoplasm | Fluorouracil (NPC75844) | |
| NCT00307723 | Study of Bevacizumab in Combination With 5-FU, Oxaliplatin and External Beam Radiation Followed by Gemcitabine and Bevacizumab for Locally Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT01649947 | Modulation of Autophagy in Patients With Advanced/Recurrent Non-small Cell Lung Cancer - Phase II | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04638790 | First Line Chemotherapy for Classical Hodgkin Lymphoma in Russia (HL-Russia-1) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00004011 | S9900: Surgery With or Without Combination Chemotherapy in Treating Patients With Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03038100 | IGFBP-2 Vaccine and Combination Chemotherapy in Treating Patients With Stage III-IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Undergoing Surgery | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03110510 | FOLFIRI as Salvage Treatment in Metastatic Biliary Tract Cancer (BTC) Patients Who Were Failed After Gemcitabine Containing Chemotherapy: A Phase II Single Arm Prospective Study | biliary tract cancer | Fluorouracil (NPC75844) | |
| NCT00547443 | Sorafenib and High-Dose Carboplatin, Paclitaxel, and External-Beam Radiation Therapy in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03806309 | Maintenance With OSE2101 Plus FOLFIRI, or FOLFIRI After FOLFIRINOX-based Induction Therapy in Locally Advanced or Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Fluorouracil (NPC75844) | |
| NCT02881125 | Paclitaxel and Nortriptyline Hydrochloride in Treating Patients With Relapsed Small Cell Carcinoma | small cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01101594 | A Study of hLL1-DOX (Milatuzumab-Doxorubicin Antibody-Drug Conjugate) in Patients With Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01500993 | Capecitabine in the Perioperative Treatment of Rectal Cancer | rectum cancer | Fluorouracil (NPC75844) | |
| NCT00001569 | Phase I Trial of Continuous Hyperthermic Peritoneal Perfusion (CHPP) With Cisplatin Plus Early Postoperative Intraperitoneal Paclitaxel and 5-FU for Peritoneal Carcinomatosis | peritoneal neoplasm;carcinoma | Paclitaxel (NPC208553) | |
| NCT00059826 | Adjuvant Chemoradiotherapy and Interferon Alfa in Treating Patients With Resected Pancreatic Cancer | pancreatic carcinoma | Fluorouracil (NPC75844) | |
| NCT03030287 | A Phase 1b Study of OMP-305B83 Plus Paclitaxel in Subjects With Ovarian, Peritoneal or Fallopian Tube Cancer | Fallopian Tube Carcinoma;ovarian carcinoma;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT02772822 | A Study Comparing the Efficiency and Safety of S-CHOP(Cyclophosphamide, Hydroxydaunomycin, Oncovin, and Prednisone) Versus R-CHOP in Untreated CD20(Cluster of Differentiation Antigen 20)-Positive DLBCL Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05177796 | Panitumumab and Pembrolizumab in Combination With Neoadjuvant Chemotherapy for the Treatment of Stage III-IV Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00277043 | A Phase III Randomized Trial Assessing the Utility of a Test Dose Program With Taxanes | allergic disease | Paclitaxel (NPC208553) | |
| NCT04472429 | Carboplatin-paclitaxel With Retifanlimab or Placebo in Participants With Locally Advanced or Metastatic Squamous Cell Anal Carcinoma (POD1UM-303/InterAACT 2). | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04258657 | Neoadjuvant Chomotherapy With Paclitaxel-albumin and S-1 for Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT05318794 | Neoadjuvant Systemic and Peritoneal Chemotherapy for Advanced Gastric Cancer | gastric cancer | Doxorubicin (NPC261012) | |
| NCT04652076 | GYNecological Cancers Treated With NETrin mAbs in Combination With Chemotherapy and /or Pembrolizumab | cervical carcinoma;endometrial carcinoma | Paclitaxel (NPC208553) | |
| NCT00014118 | Chemotherapy and Radiation Therapy in Treating Patients With Stage III or Stage IV Larynx or Oropharynx Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04594772 | Adaptive Approach to Neoadjuvant Therapy to Maximize Resection Rates for Pancreatic Adenocarcinoma | pancreatic carcinoma | Fluorouracil (NPC75844) |
❱❱❱ Associated Human Diseases and Detailed Association Evidence
How do we define the Plant-Targeted Human Disease Association?
Associated human diseases of an individual plant are summurized based on FOUR types of association evidence, these include:
❶ Association by Therapeutic Target: Bioactive protein targets of the plant were defined in "Molecular Targets" section, target-disease associations collected from TTD database were subsequently used to build the associations between the plant and its targeted human diseases.
❷ Association by Disease Gene Reversion: Plant and a specific disease will be associated when >= 1 plant target gene overlaped with disease's DEGs.
❸ Association by Clinical Trials of Plant: Plant and a specific disease will be associated when >= 1 clinical trial (the plant is the intervetion) can be matched in ClinicalTrials.gov database.
❹ Association by Clinical Trials of Plant Ingredients: Plant and a specific disease will be associated when >= 1 clinical trial (the plant ingredient is the intervetion) can be matched in ClinicalTrials.gov database.
Associated Disease of the Plant |
Association Type & Detailed Evidence |
|---|---|
Head and neck cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D42 |
MTOR
NCT00003251,NCT00003637,NCT00002839,NCT03727061,NCT00004094,NCT00004097,NCT00006248,NCT00460265,NCT01467115,NCT00544414,NCT00203905,NCT00665392,NCT00004089,NCT00646659,NCT00868491,NCT00003888,NCT00392704,NCT00721513,NCT01084083,NCT02353260,NCT00343083,NCT00006051,NCT00971867,NCT01024101,NCT03887442,NCT00002747,NCT00100789,NCT02997332,NCT00002888,NCT01412229,NCT00122460,NCT01142414,NCT00004865,NCT01264328,NCT00003627,NCT01126216,NCT04338399,NCT00113399,NCT00089297,NCT00002999,NCT00270790,NCT04356170,NCT00176241,NCT00022217,NCT00005647,NCT00566540,NCT00002632,NCT00014118,NCT02013453,NCT00003206,NCT00093665,NCT00002922,NCT00095875,NCT03358472,NCT01081041,NCT02358031,NCT00268372,NCT00851877,NCT00498953,NCT00002476,NCT00833261,NCT00301028,NCT00004901,NCT00319839,NCT00959387,NCT04428333,NCT01911598,NCT00337532,NCT00052429,NCT01333085,NCT00005087,NCT00002496,NCT00352105,NCT00002735,NCT03386838,NCT00608205,NCT04883281,NCT00352118,NCT00731380,NCT00011999,NCT00054054,NCT00004164,NCT00003592,NCT00003576,NCT00003327,NCT00003777,NCT00855764,NCT00083057,NCT00997906,NCT00193284,NCT03342352,NCT00025298,NCT00003193,NCT00468169,NCT02741570 |
Diffuse large B-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A81 |
TOP2A
NCT04824092,NCT02228512,NCT01724021,NCT00140595,NCT04546620,NCT04660799,NCT05189197,NCT02054559,NCT04332822,NCT00575406,NCT01009970,NCT01287741,NCT03274492,NCT05179733,NCT01040871,NCT00355199,NCT02531308,NCT00144807,NCT04263584,NCT05200312,NCT00931918,NCT04529772,NCT02890602,NCT03943901,NCT04231448,NCT01659099,NCT02449265,NCT02733380,NCT02889523,NCT03485118,NCT02285062,NCT05406401,NCT02772822,NCT03018626,NCT02617485,NCT00135499,NCT02753647,NCT04139304,NCT00268853,NCT05018520,NCT02867566,NCT04025593,NCT01004991,NCT03650933,NCT03225924,NCT03003520,NCT04661007,NCT02792491,NCT01148446,NCT04790903,NCT03201471,NCT01649856,NCT02596971,NCT02951728,NCT04023916,NCT04517435,NCT01622439,NCT04835870,NCT00379574,NCT05351346,NCT02428751,NCT02855359,NCT03758989,NCT03399747,NCT01459887,NCT05290090,NCT01848132,NCT05428670,NCT04594798,NCT05498259,NCT03129828,NCT01852435,NCT01415765,NCT01285765,NCT00169130,NCT05234684,NCT01925612,NCT00850512,NCT05422066,NCT02734771,NCT05389423,NCT01087424,NCT04974996,NCT01804127,NCT00499018,NCT02449278,NCT04002947,NCT03536039,NCT04021992,NCT00582725 |
Mature B-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85 |
TOP2A
NCT00054665,NCT00486759,NCT05201248,NCT03677141,NCT04745949,NCT02787239,NCT03467373,NCT00324467 |
Endometrioid adenocarcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0SD2 |
NCT02713386,NCT00262847,NCT00466960,NCT00989651,NCT00079430,NCT00085358,NCT00108745,NCT00002913,NCT01167712
|
Classical Hodgkin lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B30.1 |
NCT03527628,NCT05008224,NCT02414568,NCT04624984,NCT03712202,NCT03233347,NCT05404945,NCT03004833,NCT02661503
|
Malignant neoplasms of rectum, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B92.Z |
NCT04703101,NCT00308516,NCT00068692,NCT02641691,NCT01383343,NCT01333709,NCT02000050,NCT04749108,NCT01804790
|
Malignant neoplasms of stomach, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.Z |
NCT02163291,NCT03081143,NCT00881816,NCT04714190,NCT00237900,NCT00509964,NCT02048540,NCT01748851,NCT00639522
|
Neuroblastoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH85Z0 |
NCT02771743,NCT00578864,NCT01704716,NCT03786783,NCT00135135,NCT04221035,NCT01857934,NCT00808899,NCT00186849
|
Unspecified malignant neoplasms of unspecified sitesDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D4Z |
NCT01653470,NCT00798252,NCT05229497,NCT02640755,NCT00983541,NCT05081609,NCT00479583,NCT01952847,NCT01113476,NCT05332002,NCT00147225,NCT01046864,NCT04611724,NCT03368859,NCT01688791,NCT00500292,NCT01332604,NCT01235897,NCT01376310,NCT02937116,NCT01351350,NCT01705483,NCT00710736,NCT00881166,NCT04196283,NCT01325441,NCT00954642,NCT05217069,NCT01057264,NCT00455247,NCT02892123,NCT02317419,NCT00508326,NCT01636622,NCT01579578,NCT01110603,NCT00608803,NCT04370418,NCT01633970,NCT01187199,NCT02251951,NCT01204996,NCT03143153,NCT00113438,NCT00358163,NCT01183663,NCT02240212,NCT02383212,NCT01497470,NCT01374620,NCT02193633,NCT05235542,NCT00507962,NCT02366949,NCT00473720,NCT03082209,NCT00581971,NCT00337376,NCT00305084,NCT01604863,NCT01750918,NCT01301716,NCT02024607,NCT00943137,NCT02412722,NCT02327169,NCT01192165,NCT02640365,NCT02138812,NCT01015222,NCT00124956,NCT01931592,NCT00003170,NCT05329766,NCT00941499,NCT00002854,NCT02483247,NCT03498521,NCT03328494,NCT00703170,NCT03507491,NCT02630199,NCT02761694,NCT01983969,NCT04632992,NCT01075464
|
AdenocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D40 |
NCT03970252,NCT04752696,NCT04329949,NCT01253525,NCT04469556,NCT01676259,NCT03915444,NCT00384826,NCT01506973,NCT04481204,NCT02440958,NCT04134468,NCT02715804,NCT02178709,NCT02059967,NCT04821284,NCT00005838,NCT02101021,NCT03410030,NCT05209074,NCT05132504,NCT01893294,NCT04927780,NCT00042835,NCT04390763,NCT01431794,NCT05314998,NCT00368992,NCT04634539,NCT00226746,NCT02795988,NCT04827953,NCT03517176,NCT01218516,NCT02732938,NCT04046887,NCT02754726,NCT02468557,NCT00002852,NCT04292743,NCT02048943,NCT00369551,NCT02926183,NCT02047513,NCT00955305,NCT02487277,NCT04257448,NCT04990037,NCT04793932,NCT03697239,NCT04539808,NCT05042128,NCT03496662,NCT02723331,NCT01578551,NCT02583477,NCT02501902,NCT00548548,NCT04929041,NCT04581343,NCT03361319,NCT00040794,NCT03345810,NCT00054873,NCT03908333,NCT04852367,NCT00307723,NCT03806309,NCT02959879,NCT05257993,NCT02186847,NCT02243007,NCT04935359,NCT02993731,NCT00021060,NCT01526135,NCT02303977,NCT05472259,NCT05254171,NCT03331640,NCT00201734,NCT02426281,NCT03977233
|
Thyroid cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D10 |
TSHR
NCT01701349,NCT03085056,NCT02152137,NCT00507429,NCT01882816,NCT00786552,NCT00603941 |
AngiosarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B56 |
NCT02212015,NCT01303497,NCT04859465,NCT03544567,NCT01042379,NCT04339738,NCT01055028,NCT03512834
|
Malignant neoplasms of oesophagusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B70 |
ACHE,BLM,SLCO1B1,AURKA,TOP2A,SLCO1B3
NCT02969473,NCT02395705 |
Other specified malignant neoplasms of kidney, except renal pelvisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90.Y |
ACHE,OPRD1,BCHE,TOP2A,OPRD1,TSHR,TOP2A
NCT04322318 |
Malignant neoplasms of biliary tract, distal bile ductDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C15 |
LMNA,BLM,TUBB6,ITGAV,PMP22,AURKA,TOP2A,GMNN
|
Squamous cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.2 |
BLM,TSHR,SLCO1B1,AURKA,SLC22A2,TOP2A,SLCO1B3,GMNN
|
Hepatocellular carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH4W48 |
NCT05493332,NCT02527772,NCT00143403,NCT03803254,NCT03937830,NCT00877071,NCT00849264,NCT05092373,NCT03780049,NCT02580253,NCT01840592,NCT02967887,NCT04994236,NCT02755311,NCT02981498,NCT00559455,NCT00083226,NCT03812770,NCT00471965,NCT05135364,NCT05093920,NCT02534337,NCT01966133,NCT00988195,NCT04175912,NCT00108953,NCT05166772,NCT03192618,NCT03563170,NCT01381211,NCT01327521,NCT05171166,NCT02452853,NCT05263219,NCT01281943,NCT03468231,NCT02767375,NCT01116635,NCT05476432,NCT02987699,NCT01655693,NCT03048123,NCT03008512,NCT01953406,NCT03869034,NCT01004978,NCT02774187,NCT01055743,NCT00990860,NCT02069041,NCT03605706,NCT01171482,NCT00933816,NCT03812783,NCT03145558,NCT01858207,NCT05033522,NCT04962958,NCT03780634,NCT03469479,NCT03722498,NCT02426450,NCT00619541,NCT05214339,NCT00921531,NCT02973685,NCT02958163,NCT03192644,NCT01775501,NCT05029973,NCT00057382
|
Solid tumour/cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00-2F9Z |
EPAS1,HIF1A,MTOR,APP,TOP2A,ITGAV,AURKA
|
Renal cell carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90 |
MTOR,EPAS1
NCT02419495,NCT05092373,NCT00083109,NCT00001383,NCT00068393 |
LeiomyosarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B58 |
NCT02131480,NCT04996004,NCT02997358,NCT05099666,NCT03420014,NCT00815945,NCT03437070
|
Malignant neoplasms of cervix uteri, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C77.Z |
NCT05290935,NCT02312804,NCT02595554,NCT03556839,NCT04652076,NCT04188860,NCT02492503
|
Malignant neoplasms of kidney, except renal pelvisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90 |
NCT00024388,NCT00053807,NCT03678883,NCT00003585,NCT00003664,NCT00077129,NCT00053820
|
Herpes simplex infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1F00 |
NCT01994993,NCT00600925,NCT00600483,NCT04789928,NCT03632109,NCT03213249,NCT04967859
|
Transitional cell carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH8EH1 |
NCT03197571,NCT04603846,NCT02412670,NCT03871036,NCT04211012,NCT03464734,NCT03240016
|
Carcinosarcoma of uterusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.43 |
TSHR,DHCR7,OPRD1,TOP2A,GMNN
NCT00954174,NCT00687687 |
Serous cystadenoma,borderline malignancy of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.4 |
TSHR,OPRD1,THRB,AURKA,TUBB3,SLCO1B3,GMNN
|
Hepatocellular carcinoma of liverDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.02 |
BLM,AURKA,TOP2A,GMNN,AURKA,TOP2A,GMNN
|
Glioblastoma of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.00 |
LMNA,BLM,TUBB6,TSHR,ITGAV,RAB9A,GMNN
|
Malignant neoplasms of adrenal glandDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D11 |
BLM,DHCR7,AURKA,BCHE,TOP2A,TUBB3,GMNN
|
Fallopian tube cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C74 |
NCT00085358,NCT00373217,NCT00189566,NCT01493505,NCT00003944,NCT00517621,NCT01666444,NCT01204749,NCT03029611,NCT01253681,NCT00672295,NCT01219777,NCT02606305,NCT02050009,NCT00091377,NCT00838656,NCT03126812,NCT00466986,NCT02834975,NCT02012192,NCT00993655,NCT03393884,NCT02520154,NCT00079430,NCT00031954,NCT01100372,NCT00466960,NCT01402271,NCT00520013,NCT01447706,NCT00003896,NCT01570582,NCT01196741,NCT01649336,NCT00652119,NCT02713386,NCT00483782,NCT00226915,NCT00331422,NCT00003120,NCT00052468,NCT00479817,NCT00003064,NCT01091428,NCT00189553,NCT00075712,NCT00006454,NCT03038100,NCT00004934,NCT03740165,NCT00903630,NCT01220154,NCT00028743,NCT00702299,NCT00008138,NCT00003385,NCT00003160,NCT01083537,NCT00550784,NCT00003624,NCT03056833,NCT03462212,NCT02121990,NCT03635489,NCT00408070,NCT00262990,NCT02865811,NCT01146795
|
Neoplasms of unknown behaviour of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F95 |
NCT04895358,NCT00373256,NCT00524303,NCT00121992,NCT00075270,NCT00111787,NCT02051751,NCT00367471,NCT03396445,NCT01026116,NCT00388076,NCT01220128,NCT00353717,NCT00356811,NCT00849472,NCT00709761,NCT00048893,NCT00550771,NCT01205217,NCT00553358,NCT00448305,NCT00021255,NCT04251533,NCT02455141,NCT00174707,NCT00246571,NCT00194753,NCT00251095,NCT00272987,NCT01779050,NCT03678883,NCT02162719,NCT01953536,NCT03742102,NCT05067530,NCT00429299,NCT05244993,NCT00281658,NCT01953445,NCT00779129,NCT02776917,NCT01137994,NCT00201435,NCT00174434,NCT00687440,NCT03272477,NCT00525642,NCT03036488,NCT03273595,NCT01572038,NCT00722293,NCT00174655,NCT00046514,NCT02307227,NCT01138046,NCT00046527,NCT03997123,NCT02622074,NCT00191672,NCT01271725,NCT03705429,NCT00446030,NCT00543829,NCT00110695
|
Pain, unspecifiedDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG3Z |
OPRD1,ACHE,BCHE,MTOR
NCT02479971,NCT02311907 |
Kaposi sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B57 |
TOP2A
NCT02659930,NCT00003350,NCT00002318,NCT00923936,NCT00002105 |
Urogenital cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C95-2C9Z |
NCT02033993,NCT02256436,NCT04527991,NCT00034177,NCT02756013,NCT00151034
|
SepsisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1G40-1G41 |
NCT00189384,NCT01263041,NCT00133978,NCT00005775,NCT01551394,NCT00395161
|
Sickle-cell disorderDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3A51 |
NCT00125788,NCT00586209,NCT00131508,NCT04684381,NCT05371184,NCT01179217
|
Adenocarcinoma, endocervical typeDisease Category: X.Extension CodesDisease ICD-11 Code: XH0GS9 |
NCT00064077,NCT02020707,NCT01755897,NCT04723875,NCT00295789,NCT04516616
|
Uterine ligament/parametrium/uterine adnexa neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C72 |
NCT00147680,NCT01650376,NCT00584909,NCT00231868,NCT00506779,NCT00584857
|
Malignant neoplasms of corpus uteriDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76 |
NCT01970722,NCT00505492,NCT02112552,NCT00782041,NCT02684227,NCT00063999
|
GerminomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH1E13 |
NCT01873326,NCT02414685,NCT00864318,NCT05455918,NCT00611962,NCT04581265
|
Polyneuropathy, unspecifiedDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C0Z |
NCT03492047,NCT00195013,NCT02590367,NCT00979082,NCT02024191,NCT02215083
|
PheochromocytomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH3854 |
ACHE,BLM,APP,OPRD1,EPAS1,BCHE
|
Superficial ovarian endometriosisDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA10.B4 |
ACHE,LMNA,TUBB6,PMP22,TUBB3,RAB9A
|
Urothelial carcinoma of bladderDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94.2 |
BLM,DHCR7,SLCO1B1,AURKA,TOP2A,SLCO1B3
|
Malignant neoplasms of retroperitoneumDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C50 |
NCT00003896,NCT01083537,NCT00005026,NCT02865811,NCT00002894,NCT01447706,NCT00008138,NCT02272790,NCT00652119,NCT00023907,NCT01100372,NCT00003385,NCT00003160,NCT00084448,NCT00331422,NCT00005046,NCT00028743,NCT00408070,NCT00017303,NCT00993655,NCT00031954,NCT00550784,NCT00672295,NCT01493505,NCT00003624,NCT00003322,NCT00085358,NCT00091377,NCT00003998,NCT00903630,NCT00483782,NCT00004934,NCT00625092,NCT01402271,NCT02050009,NCT00825201,NCT03126812,NCT00075712,NCT00373217,NCT00003944,NCT01821859,NCT00003880,NCT00702299,NCT00079430,NCT00002734,NCT00491855,NCT00002717,NCT02020707,NCT00003120,NCT00003378,NCT00003064,NCT02834975,NCT01649336,NCT01091428,NCT00466960,NCT00838656
|
Cervical cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C77 |
NCT01566240,NCT05367206,NCT04799639,NCT00980954,NCT04884906,NCT02446652,NCT01594099,NCT02036164,NCT00626561,NCT03340376,NCT04974944,NCT03534713,NCT04731038,NCT04973904,NCT03852979,NCT03367871,NCT03912415,NCT04551950,NCT00002949,NCT04341883,NCT05179239,NCT04864782,NCT00462397,NCT00276796,NCT01756170,NCT03635567,NCT05247619,NCT02467907,NCT04806945,NCT04016142,NCT00003209,NCT04150575,NCT00023660,NCT00003065,NCT04868708,NCT01667211,NCT02432365,NCT03912402,NCT00340184,NCT01487226,NCT00138151,NCT00003379,NCT00003624,NCT04238988,NCT00057928,NCT01229930,NCT00823186,NCT00997009,NCT02422563,NCT01405235,NCT01755845,NCT00003377,NCT00003078,NCT02039791,NCT04982237
|
Pulmonary hypertensionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BB01 |
HIF1A,MTOR
NCT01340131,NCT01352689,NCT01246193 |
Alzheimer diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A20 |
TOP2A,BACE1,BCHE,APP,ACHE
|
Pleural mesotheliomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C26 |
MTOR
NCT00634205,NCT00859495,NCT00886028,NCT02798536 |
OsteosarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B51 |
NCT00180908,NCT00691236,NCT01258634,NCT00586846,NCT00145639
|
Testicular cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C80 |
NCT00072215,NCT00531687,NCT00231582,NCT00004077,NCT00104676
|
Intrahepatic cholangiocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.10 |
NCT02392637,NCT03579771,NCT04961970,NCT04175912,NCT03771846
|
ovarian cystadenocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.Y |
NCT00466960,NCT00079430,NCT00085358,NCT00002913,NCT02050009
|
Squamous cell carcinoma of oropharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6A.0 |
NCT03829722,NCT04572100,NCT03323463,NCT05108870,NCT04718415
|
LeishmaniasisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1F54 |
TSHR,BLM
NCT01083576,NCT00657917,NCT01032382 |
Adenocarcinoma of prostateDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C82.0 |
SLCO1B3,SLCO1B1
NCT04221828,NCT02560051,NCT00521781 |
Duchenne muscular dystrophyDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C70.1 |
NCT00018109,NCT00296621,NCT00016653,NCT00005574,NCT00451074
|
Invasive carcinoma of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C61 |
BLM,OPRD1,AURKA,THPO,TOP2A
|
Multiple myelomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A83 |
MTOR
NCT04877275,NCT00925821,NCT00734877,NCT00083538,NCT01548573,NCT01492881,NCT01177683,NCT00750815,NCT01246063,NCT01889420,NCT00516191,NCT02075021,NCT00366106,NCT00003401,NCT01646762,NCT02114502,NCT01328236,NCT01078441,NCT02985333,NCT00706953,NCT00572169,NCT01394354,NCT00871013,NCT00003399,NCT01101594,NCT01541332,NCT00617591,NCT00083915,NCT00441168,NCT02471820,NCT01481194,NCT00083551,NCT00148317,NCT00003853,NCT01215344,NCT00215943,NCT03004287,NCT00319865,NCT00003331,NCT01070862,NCT02362165,NCT00574080,NCT00814541,NCT02186834,NCT00869232 |
Malignant neoplasm metastasis in lymph nodes of head, face or neckDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D60.0 |
SLCO1B1,AURKA,BCHE,SLCO1B3
NCT05189184,NCT04278092,NCT01154920,NCT03169764,NCT00274937,NCT00513383,NCT05446467,NCT04882462,NCT01086826,NCT05294900,NCT01732640,NCT03723967,NCT02268695,NCT01612351,NCT02124707,NCT05283226,NCT01233843,NCT04428151,NCT02270814,NCT00736944,NCT01177956,NCT05272696,NCT00971932,NCT04854499,NCT04831320,NCT04164238,NCT05459129,NCT03340896,NCT04826679,NCT04807140,NCT00139269,NCT03830385,NCT00002774,NCT02573493,NCT00999700,NCT03855384,NCT01852292,NCT02047201,NCT02551159,NCT04843098,NCT04282109,NCT05054439 |
Breast cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C60-2C6Y |
MTOR,TOP2A,AURKA
NCT00336791,NCT00006459,NCT00140140,NCT00479674,NCT00006110,NCT00041470,NCT03409198,NCT02790580,NCT05207514,NCT00872625,NCT01204801,NCT00015886,NCT00129389,NCT00525759,NCT00033683,NCT00096343,NCT02506777,NCT01959490,NCT00618826,NCT04177108,NCT01036087,NCT01172223,NCT00004013,NCT00793377,NCT00136539,NCT03805399,NCT02132949,NCT03872791,NCT00002784,NCT00820547,NCT01937507,NCT00915018,NCT00002627,NCT01998906,NCT03497702,NCT04129996,NCT00723125,NCT01206881,NCT00009997,NCT05113251,NCT00003012,NCT05192798,NCT05498896,NCT00002580,NCT00082095,NCT00404404,NCT02897050,NCT00093795,NCT02897700,NCT00003352,NCT00024102,NCT00003680,NCT00589238,NCT01358877,NCT03595592,NCT03498716,NCT01131364,NCT00002582,NCT01270373,NCT03175666,NCT01042379,NCT00054587,NCT00002498,NCT00707707,NCT00003035,NCT00789581,NCT02681003,NCT00295893,NCT05159193,NCT01796197,NCT00334802,NCT00574587,NCT00201708,NCT00289263,NCT01176799,NCT02413320,NCT00971945,NCT00776724,NCT00944047,NCT00667251,NCT00312208,NCT00005581,NCT00645177,NCT05346107,NCT04031703,NCT01008150,NCT00542191,NCT00591851,NCT00486668,NCT00820170,NCT01966471,NCT01354522,NCT02742051,NCT00331097,NCT05097248,NCT00002772,NCT00533936,NCT00768859,NCT00559845,NCT00370552,NCT00236899,NCT03726879,NCT00016406,NCT01090128,NCT00128856,NCT00194792,NCT01855828,NCT01593020,NCT00563953,NCT00004125,NCT00003440,NCT03575520,NCT02995772,NCT00346229,NCT00574236,NCT02299999,NCT04296175,NCT00434356,NCT00194779,NCT00274456,NCT00146549,NCT00038402,NCT00455533,NCT00301730,NCT00887575,NCT00846027,NCT00196872,NCT00654836,NCT00616967,NCT00311636,NCT00675259,NCT04584112,NCT04138719,NCT00356681,NCT00316199,NCT02562378,NCT00070564,NCT00039546,NCT00002628,NCT01705691,NCT00001383,NCT00206518,NCT00951665,NCT00622466,NCT00146562,NCT02225652,NCT00445458,NCT03285607,NCT00314977,NCT00499122,NCT00433420,NCT04540692,NCT04038489,NCT00309478,NCT03742986,NCT00856492,NCT00691912,NCT00679029,NCT00609791,NCT01818063,NCT00193206,NCT02846428,NCT00148681,NCT00407888,NCT00002953,NCT01990352,NCT00408408,NCT00721747,NCT02499367,NCT01329627,NCT00788931,NCT05020860,NCT00950300,NCT00003782,NCT02838225,NCT00392392,NCT00068757,NCT00004174,NCT02115204,NCT04762901,NCT00102219,NCT00930930,NCT00669773,NCT00629499,NCT05386524,NCT00001384,NCT04443348,NCT00001498,NCT00070278,NCT00072319,NCT00404066,NCT00003050,NCT00524810,NCT00006108,NCT00129922,NCT00688740,NCT00532285,NCT02393833,NCT00055679,NCT00124111,NCT00005800,NCT02365805,NCT00003088,NCT00756470,NCT00002662,NCT00900627,NCT00014222,NCT04083963,NCT00365417,NCT00258960,NCT00668616,NCT00499603,NCT01547741,NCT01333423,NCT01216111,NCT00331630,NCT00129376,NCT00152178,NCT01940497,NCT00002707,NCT03933319,NCT04085276,NCT05491694,NCT00476827,NCT00251472,NCT01670500,NCT01847001,NCT00320541,NCT00191854,NCT00256243,NCT00007904,NCT00003042,NCT00254592,NCT03248427,NCT00777673,NCT02314806,NCT01969032,NCT00003577,NCT04172259,NCT00003927,NCT00003877,NCT00003539,NCT00615602,NCT03412643,NCT03387085,NCT00919880,NCT04770272,NCT00724386,NCT04159818,NCT03197935,NCT00625898,NCT02053597,NCT00028990,NCT00004067,NCT00003013,NCT00629278,NCT00963729,NCT01784120,NCT03609047,NCT01120171,NCT04301739,NCT00309569,NCT02379585,NCT04498793,NCT00107094,NCT00010075,NCT00903656,NCT00934895,NCT05275777,NCT00915603,NCT00795899,NCT00431795,NCT00807859,NCT00394251,NCT00436566,NCT00322400,NCT03071926,NCT00378313,NCT00050167,NCT03554109,NCT01622361,NCT00002542,NCT02400567,NCT03425656,NCT01669239,NCT00087178,NCT05112536,NCT03301350,NCT01415336,NCT00503750,NCT00448266,NCT00442260,NCT00773695,NCT00154882,NCT00005649,NCT00193011,NCT01396655,NCT00915369,NCT01222052,NCT03888677,NCT00203372,NCT01985841,NCT00003086,NCT02032277,NCT00475670,NCT00482391,NCT03725059,NCT00278109,NCT00031876,NCT00003893,NCT00028873,NCT02488564,NCT02641847,NCT00206466,NCT00530101,NCT03949634,NCT00580333,NCT04148911,NCT00212082,NCT00479856,NCT00493870,NCT02450058,NCT00002679,NCT04243616,NCT00680758,NCT00748553,NCT04958785,NCT00266799,NCT00467012,NCT00009763,NCT00004092,NCT00002755,NCT00290732,NCT01964391,NCT00960960,NCT00193037,NCT00803556,NCT00876395,NCT00434031,NCT00193115,NCT02980965,NCT02012608,NCT03057600,NCT00250874,NCT00012311,NCT00403130,NCT00054028,NCT00003392,NCT00152191,NCT00618657,NCT00513292,NCT00397761,NCT04127019,NCT00110084,NCT00003612,NCT01985724,NCT01204437,NCT00691379,NCT00544232,NCT00546156,NCT00499525,NCT03329378,NCT00637897,NCT02472353,NCT00536939,NCT00912639,NCT00052351,NCT00123929,NCT00036868,NCT00456846,NCT03994107,NCT01642771,NCT01672671,NCT05088057,NCT02315196,NCT02115152,NCT00002826,NCT00002870,NCT00365365,NCT00002837,NCT00912444,NCT00499083,NCT04213898,NCT00561119,NCT00426556,NCT00717340,NCT01314833,NCT00191789,NCT00047099,NCT00022230,NCT00003972,NCT00003165,NCT00636441,NCT02938442,NCT00003992,NCT01009983,NCT00662129,NCT00733408,NCT00567554,NCT00394082,NCT02685657,NCT00006120,NCT00002696,NCT00053911,NCT00590785,NCT00630032,NCT03002103,NCT02605915,NCT02850419,NCT00025688,NCT00465673,NCT00983424,NCT00301925,NCT03493854,NCT05390710,NCT00431080,NCT01646034,NCT04024462,NCT01227408,NCT00149214,NCT00303108,NCT02215876,NCT00002581,NCT00511459,NCT00041119,NCT00019812,NCT01057069,NCT00006256,NCT00503906,NCT04293393,NCT01881230,NCT04664972,NCT01231802,NCT00448591,NCT00887536,NCT00929591,NCT01031030,NCT01210768,NCT01003158,NCT00532857,NCT00002937,NCT00607438,NCT00600340,NCT05227664,NCT00005635,NCT00093145,NCT00096668,NCT03125902,NCT02096588,NCT00002579,NCT00635050,NCT00017095 |
Breast in situ carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E65 |
NCT00542451,NCT02474173,NCT04001829,NCT05092373,NCT03201861,NCT02187991,NCT02672475,NCT03971409,NCT05177796,NCT03164993,NCT02632071,NCT04081389,NCT01498588,NCT02419495,NCT01750073,NCT01935492,NCT03725436,NCT03101748,NCT05233696,NCT04216472,NCT03179904,NCT01238133,NCT02654119,NCT04862585,NCT05422794,NCT03606967,NCT02883062,NCT04266249,NCT00785291,NCT00520975,NCT01263145,NCT03853707,NCT01885013,NCT02593175,NCT02436993,NCT01275677,NCT02603679,NCT02689427,NCT01463072,NCT04249167,NCT01091428,NCT03554044
|
Malignant neoplasms of nasopharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6B |
NCT03175939,NCT01534585,NCT04437329,NCT00188877,NCT04850235,NCT00484601,NCT00592501,NCT04834206,NCT00817583,NCT02016417,NCT05433597,NCT00697905,NCT03020329,NCT01712919,NCT03668730,NCT04004871,NCT03047265,NCT03574324,NCT01528618,NCT02250599,NCT03604965,NCT01536223,NCT02434614,NCT00201396,NCT02500940,NCT04223024,NCT03306121,NCT01245959,NCT04282070,NCT00816816,NCT03503136,NCT00677118,NCT04015661,NCT02590133,NCT00816855,NCT01694576,NCT02012062,NCT00379262,NCT04766359,NCT00747799,NCT01797900
|
Soft tissue disorderDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FB56 |
NCT04910126,NCT03283696,NCT01514188,NCT00017160,NCT00755261,NCT00718484,NCT01440088,NCT04650984,NCT00502411,NCT04032964,NCT03042819,NCT02784015,NCT03056001,NCT00102609,NCT02049905,NCT01168791,NCT05189483,NCT03317457,NCT02783599,NCT00484341,NCT04012827,NCT04765228,NCT05448820,NCT03698227,NCT02255110,NCT02451943,NCT03805022,NCT04757337,NCT04780464,NCT03719430,NCT00790244,NCT01861951,NCT05100628,NCT00626704,NCT00878800,NCT04656262,NCT03524898,NCT03108300,NCT04776525,NCT04874311
|
Brain cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00 |
HIF1A,EPAS1,TOP2A
NCT01014767 |
Acute myeloid leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60 |
TOP2A,AURKA
NCT03860844,NCT03059615 |
Malignant haematopoietic neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B33 |
TOP2A,AURKA,MTOR
NCT00051311 |
Metastatic digestive system neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D8Y |
NCT01434069,NCT03800693,NCT04755543,NCT02319018
|
Glioblastoma, primary, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0MB1 |
NCT00944801,NCT01851733,NCT02372409,NCT00479765
|
Burkitt lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH4KA9 |
NCT00669877,NCT00126191,NCT05389423,NCT05270057
|
Large cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.3 |
NCT03345810,NCT00596830,NCT00955305,NCT02186847
|
Central nervous system diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A04-8D87 |
NCT00031577,NCT01498783,NCT00014573,NCT00002814
|
Diabetic foot ulcerDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD54 |
NCT00593567,NCT00658957,NCT00659646,NCT02036528
|
Adult T-cell lymphoma or leukaemia, human T-cell lymphotropic virus type 1-associatedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90.5 |
NCT01788137,NCT02228772,NCT01000285,NCT03007147
|
Ewing sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B52 |
NCT02063022,NCT00568464,NCT00038142,NCT01864109
|
Angioimmunoblastic T-cell lymphomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH1J86 |
NCT01719835,NCT03853044,NCT00725231,NCT00169156
|
Hypopharyngeal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6D |
NCT00508664,NCT01249443,NCT01312350,NCT04502641
|
Thymic carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH6AK2 |
NCT01100944,NCT00010257,NCT03694002,NCT00818090
|
Other specified malignant neoplasms of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.Y |
AURKA,TOP2A,TUBB3,SLCO1B3
|
Cytomegaloviral diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D82 |
ACHE,OPRD1,THPO,THRB
|
Renal cell carcinoma, chromophobe typeDisease Category: X.Extension CodesDisease ICD-11 Code: XH6153 |
DHCR7,AURKA,TOP2A,NPC1
|
Endometrial cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76 |
MTOR
NCT00003691,NCT02630823,NCT01767155,NCT00373620,NCT03603184,NCT02476955,NCT00006027,NCT00052312,NCT03932409,NCT04527900,NCT01100359,NCT02730416,NCT00515073,NCT02744898,NCT00003624,NCT00513786,NCT00334321,NCT01770171,NCT05201547,NCT00003127,NCT00022620,NCT05481645,NCT03503786,NCT05092373,NCT00006377,NCT03189446,NCT00002949,NCT05489848,NCT00016341,NCT01367002,NCT00879359,NCT00883116,NCT04159155,NCT01244789,NCT00401635,NCT03935256,NCT04652076,NCT00411138 |
Hodgkin lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B30 |
NCT01555853,NCT01652261,NCT00816959,NCT00026208,NCT03033914,NCT01468740,NCT00512980,NCT01056679,NCT00797472,NCT02247869,NCT02505269,NCT02979522,NCT01060904,NCT01929941,NCT01712490,NCT03755804,NCT03407144,NCT01356680,NCT00795613,NCT00038558,NCT00736320,NCT00784537,NCT01251107,NCT03646123,NCT04638790,NCT00225173,NCT03159897,NCT04685616,NCT02292979,NCT02398240,NCT02298283,NCT01920932,NCT00443677,NCT01569204,NCT01358747,NCT03618550,NCT02181738,NCT02275598,NCT03517137
|
Malignant neoplasms of colonDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B90 |
NCT01383343,NCT03883802,NCT00101894,NCT00337389,NCT02138617,NCT05174169,NCT03641976,NCT00070122,NCT00646607,NCT05062889,NCT04259944,NCT00003835,NCT00235898,NCT02510118,NCT01198548,NCT04120701,NCT05179889,NCT04832776,NCT04003792,NCT03426904,NCT00193219,NCT04303429,NCT01238965,NCT00004931,NCT00025337,NCT00079274,NCT00499369,NCT00003873,NCT00309530,NCT02041481,NCT01205022,NCT00942266,NCT01270438,NCT02967289,NCT03475680,NCT01111604,NCT04488159,NCT01347645,NCT00309543
|
Precursor cell lymphoblastic leukaemia, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5J37 |
NCT03515200,NCT02420717,NCT00549848,NCT03991884,NCT01186328,NCT00187161,NCT00600977,NCT05303792,NCT00439296,NCT03349281,NCT03384654,NCT01324180,NCT01887587,NCT02419755,NCT02881086,NCT00165178,NCT03553238,NCT00671658,NCT03117751,NCT04996160,NCT00165087,NCT00973752,NCT04307576,NCT00968253,NCT03150693,NCT03817320,NCT03571321,NCT00136435,NCT00440726,NCT00131027,NCT00137111,NCT03023046,NCT05453500,NCT01523977,NCT03860844,NCT00476190,NCT00890656,NCT03020030,NCT03643276
|
Malignant neoplasms of bladder, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94.Z |
NCT00006118,NCT00003701,NCT00136175,NCT01812369,NCT00003133,NCT00585689,NCT02302807,NCT00055601,NCT02177695,NCT00028860,NCT04060459,NCT00003105,NCT04602117,NCT00268450,NCT00583349,NCT05328336,NCT00808639,NCT00003642,NCT00005644,NCT00003376,NCT00022191,NCT00024349,NCT00635726,NCT00981656,NCT00006111,NCT00506155,NCT00002917,NCT00002949,NCT00022633,NCT00933374,NCT04101812,NCT00003930,NCT00045630,NCT00003175,NCT00003342,NCT00310011
|
Neuroendocrine carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C34 |
NCT00873119,NCT03168607,NCT00183742,NCT01593306,NCT00003558,NCT00001569,NCT00731861,NCT02246127,NCT00022178,NCT02215447,NCT00193531,NCT03837977,NCT02172976,NCT04705519,NCT00001339,NCT00953394,NCT00894569,NCT03387592,NCT01489566,NCT04919226,NCT01253681,NCT01678664,NCT00003943,NCT05015621,NCT03164382,NCT00094497,NCT03092895,NCT01858025,NCT04325425,NCT02820857,NCT00737243,NCT00936702,NCT03136055,NCT01524094
|
Prostate cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C82 |
MTOR,AURKA
NCT04148885,NCT01558492,NCT00005028,NCT00003614,NCT00049257,NCT00005847,NCT00176293,NCT00084565,NCT01607879,NCT00030654,NCT00003717,NCT00933426,NCT00003622,NCT02494713,NCT00193193,NCT00009750,NCT02911350,NCT00038168,NCT01240629,NCT00004054,NCT00284752,NCT00038246,NCT00024414,NCT00003394,NCT00151060,NCT00003084,NCT00016913,NCT00005048,NCT00028769 |
Pleomorphic sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B54 |
MTOR
NCT00003624,NCT00204646,NCT01104298,NCT00189137,NCT00003008,NCT03678883,NCT00288431,NCT00003054,NCT02326025,NCT04199026,NCT00796120,NCT04535713,NCT02448537,NCT02732015,NCT01185964,NCT00217607,NCT03880695,NCT01027910,NCT02812654,NCT01605526,NCT03802071,NCT00949325,NCT00204568,NCT01746238,NCT04968106,NCT00020449,NCT00112489,NCT00001300,NCT05210374,NCT00003128 |
Adenocarcinoma of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.0 |
BLM,SLCO1B1,AURKA,TOP2A,SLCO1B3
NCT03281369,NCT00183911,NCT01567618,NCT03193918,NCT04523818,NCT02449655,NCT01336062,NCT04604132,NCT02494583,NCT01641783,NCT03959293,NCT04572542,NCT05268510,NCT02625610,NCT02545504,NCT04908566,NCT04510064,NCT03990103,NCT04047004,NCT02615730,NCT04209686,NCT02448329,NCT04077255,NCT04675983,NCT02625623,NCT04190745 |
Other specified malignant neoplasms of colonDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B90.Y |
NCT00387387,NCT01383343,NCT02413853,NCT00079274,NCT03050814,NCT04068103,NCT03069950,NCT00166465,NCT00942266,NCT00003873,NCT03300609,NCT00096278,NCT00707889,NCT04094688,NCT01862003,NCT00851045,NCT01704703,NCT02355379,NCT01605344,NCT00034190,NCT02508077,NCT00967616,NCT00003835,NCT00499850,NCT00507091,NCT00025337,NCT01277406,NCT02071069,NCT01270438,NCT00070122
|
GlaucomaDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9C61 |
ACHE
NCT00000122,NCT02767219 |
Dystrophic epidermolysis bullosaDisease Category: 14.Diseases of the skinDisease ICD-11 Code: EC32 |
APP
NCT02698735,NCT03392909 |
ThymomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C27 |
NCT01100944,NCT00818090,NCT00010257
|
Gastrointestinal stromal tumourDisease Category: X.Extension CodesDisease ICD-11 Code: XH9HQ1 |
NCT02607332,NCT02574663,NCT03944304
|
Malignant mixed epithelial mesenchymal tumour of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B5D.0 |
NCT01249443,NCT02822157,NCT02107937
|
Hereditary breast and ovarian cancer syndromeDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C65 |
NCT05485766,NCT00535119,NCT02978495
|
Atopic eczemaDisease Category: 14.Diseases of the skinDisease ICD-11 Code: EA80 |
NCT04019769,NCT04544943,NCT01429701
|
Rhabdomyosarcoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0GA1 |
NCT04213794,NCT04625907,NCT01871766
|
Skin cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30-2C37 |
NCT03167164,NCT02054884,NCT00003549
|
Mixed tumour, malignant, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0V86 |
NCT04825938,NCT05000892,NCT01969578
|
Other specified malignant neoplasms ofcolonDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B90.Y |
OPRD1,SLCO1B1,SLCO1B3
|
Systemic lupus erythematosusDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4A40.0 |
TUBB3,ACHE,EPAS1
|
MelanomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30 |
NCT01676649,NCT00255762,NCT03167177,NCT00434252,NCT02023710,NCT02617849,NCT01009515,NCT01174238,NCT00111007,NCT01006252,NCT01039844,NCT04979585,NCT00462423,NCT00329641,NCT00864253,NCT02300935,NCT01666418,NCT01107665,NCT00093119,NCT00288041,NCT00084214,NCT00970996,NCT00483301,NCT01827111,NCT01704287,NCT00796991,NCT00522834,NCT01200342,NCT00779714
|
Malignant lymphoma, not elsewhere classifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B33.5 |
NCT03571308,NCT02055820,NCT00038545,NCT03169790,NCT01332968,NCT00361621,NCT00825149,NCT05238064,NCT00133302,NCT01200758,NCT01777152,NCT03467373,NCT02406092,NCT03013218,NCT00088530,NCT00323323,NCT01555853,NCT02626455,NCT00324467,NCT01746173,NCT00455897,NCT00201318,NCT00809341,NCT01516580,NCT00719472,NCT00184002,NCT00193440
|
Colorectal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B91 |
TOP2A,ITGAV
NCT00003446,NCT00005979,NCT00265811,NCT00628810,NCT00612586,NCT00002523,NCT00077233,NCT01529164,NCT00006366,NCT01047293,NCT01374425,NCT00126256,NCT00786643,NCT05193292,NCT00008060,NCT00182715,NCT00098787,NCT01383707,NCT00006037,NCT00501410,NCT02826837,NCT01286818,NCT00839111,NCT00006786,NCT00091312,NCT00069121,NCT01276379,NCT03487939,NCT00425152,NCT00537823,NCT01802645,NCT04337879,NCT00625573,NCT00212589,NCT01289821,NCT01736904,NCT00336960,NCT00873275,NCT00023751,NCT01263171,NCT00640081,NCT00006479,NCT00062426,NCT00006468,NCT00061815,NCT01836653,NCT01338558,NCT01418222,NCT00070213,NCT01087658,NCT01478594,NCT00349336,NCT00004885,NCT03169777,NCT02415699,NCT00111761,NCT02291289,NCT00454116,NCT00721916,NCT00467142,NCT00286000,NCT00671372,NCT01417494,NCT00268333,NCT01077739,NCT01765582,NCT00647530,NCT02244632,NCT00046995,NCT00016952,NCT00280176,NCT00004150,NCT00016978,NCT05081687,NCT00551213,NCT00378716,NCT00025142,NCT00009737,NCT00001428,NCT00427570,NCT00384176,NCT00719199,NCT00075595,NCT00291785,NCT00335816,NCT00098982,NCT02494973,NCT01622543,NCT00006115,NCT00002692,NCT01465451,NCT00321828,NCT00066274,NCT00002551,NCT02376452,NCT00642577,NCT00058474,NCT00813137,NCT00265850,NCT02885753,NCT01490866,NCT00268398,NCT01279681,NCT01167725,NCT00085163,NCT05239741,NCT00720512,NCT00002793,NCT00202787,NCT00002593,NCT01227707,NCT01226719,NCT00862784,NCT00069095,NCT01479465,NCT02124148,NCT00559741,NCT02384850,NCT00889343,NCT05253651,NCT02141295,NCT00296608,NCT00080951,NCT00423696,NCT00027833,NCT00066846,NCT00005586,NCT00003543,NCT00003287,NCT00006015,NCT00003063,NCT00003594,NCT00831181,NCT00940303,NCT00571740,NCT00209703,NCT02842580,NCT00469443,NCT00055822,NCT00199797,NCT00385021,NCT00952029,NCT00478946,NCT01515787,NCT00541125,NCT00064181,NCT00143403,NCT00021398,NCT02103062,NCT00016198,NCT01193452,NCT00388700,NCT00065117,NCT01183780,NCT00613080,NCT00278889,NCT00003422,NCT00427310,NCT02830139,NCT00449137,NCT00002896,NCT01679327,NCT01326000,NCT00884767,NCT00797485,NCT00577109,NCT02515734,NCT00002893,NCT00303771,NCT00866944,NCT00176774,NCT00112918,NCT00020501,NCT00023868,NCT00541112,NCT01133990,NCT01375816,NCT00544011,NCT00026364,NCT00755118,NCT01628211,NCT00026273,NCT00354978,NCT01205711,NCT00002570,NCT00081289,NCT00006103,NCT00069108,NCT00005050,NCT01652482,NCT00003260,NCT05035381,NCT00002525,NCT00024401,NCT00323011,NCT01442649,NCT00004005,NCT00049101,NCT00008281,NCT00274872,NCT01150045,NCT00988897,NCT00719797,NCT00003001,NCT01442935,NCT00749450,NCT02246049,NCT00262808,NCT01730586,NCT02375672,NCT00002828,NCT00284258,NCT01068132,NCT00118261,NCT00497497,NCT01718873,NCT00072553,NCT00615056,NCT00020917,NCT00973609,NCT00609622,NCT00110721,NCT00209625,NCT01271166,NCT02574663,NCT00202774,NCT00016133,NCT00070434,NCT00003254,NCT00004252,NCT00002716,NCT00004102,NCT00021281,NCT00556413,NCT00958737,NCT00039208,NCT00778102 |
Malignant neoplasm of pancreas, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.Z |
NCT00398086,NCT00026130,NCT02717091,NCT02975141,NCT00786006,NCT04431258,NCT01888978,NCT01488552,NCT04498689,NCT00002955,NCT00089024,NCT00323583,NCT02559674,NCT05047991,NCT03586869,NCT03600623,NCT00463840,NCT01992705,NCT01834235,NCT01693276,NCT00045747,NCT02241551,NCT03899636,NCT03563144,NCT03344172,NCT01688336,NCT02919787,NCT00005870,NCT02873598,NCT02651727,NCT05251038,NCT01470417,NCT04594772,NCT03502343,NCT01851174,NCT03387098,NCT02340117,NCT03316599,NCT02135822,NCT03487016,NCT03649321,NCT01621243,NCT00003049,NCT02351219,NCT03329248,NCT01934634,NCT01063192,NCT05241249,NCT02672917,NCT00262951,NCT00417976,NCT02028806,NCT02005315,NCT01867892,NCT03777462,NCT02506842,NCT00691054,NCT01647828,NCT02207465,NCT03278015,NCT00426127,NCT03443492,NCT02124317,NCT04054362,NCT00003029,NCT04707118,NCT03797443,NCT04617067,NCT02506803,NCT03941093,NCT02574663,NCT04897854,NCT02109445,NCT02047500,NCT03991962,NCT04858009,NCT02827201,NCT03257033,NCT02382263,NCT02581501,NCT02608229,NCT02050178,NCT05065801,NCT01473303,NCT02021422,NCT04753879,NCT03585062,NCT02391662,NCT01730222,NCT01632306,NCT03693677,NCT03760614,NCT05168527,NCT00609765,NCT01858883,NCT00882973,NCT00049348,NCT03721744,NCT04581876,NCT00002689,NCT05462496,NCT03714555,NCT00416507,NCT03850769,NCT03136406,NCT02588443,NCT04371224,NCT01836432,NCT00111904,NCT00024375,NCT04161755,NCT03699319,NCT00251355,NCT03989310,NCT03641183,NCT01088815,NCT01893801,NCT03885219,NCT04203641,NCT02930902,NCT02481635,NCT00112697,NCT01461915,NCT03336216,NCT05360732,NCT01661088,NCT00004003,NCT02676349,NCT04589234,NCT03412799,NCT02767557,NCT00003018,NCT00602602,NCT02355119,NCT04592861,NCT03520790,NCT01822756,NCT04672005,NCT02155088,NCT00010062,NCT01754623,NCT01652976,NCT03815461,NCT04158635,NCT02138383,NCT02283372,NCT02539537,NCT01591733,NCT04089150,NCT00068575,NCT03563248,NCT00059826,NCT04723030,NCT02570711,NCT02358161,NCT02352337,NCT02106884,NCT04324307,NCT04683939,NCT03435289,NCT03825328,NCT02210559,NCT02923921,NCT02514031,NCT00003216,NCT01821729,NCT05218889,NCT04683315,NCT05298020,NCT01560949,NCT02043730,NCT03415802,NCT01489865,NCT01905150,NCT04390399,NCT03042780,NCT05193604,NCT04814485,NCT00058201,NCT01839487,NCT04643405,NCT04241276,NCT03417921,NCT03167112,NCT02272738,NCT02399137,NCT04247126,NCT05466799,NCT02128100,NCT00005871,NCT00960284,NCT01964534,NCT01523457,NCT04674956,NCT01446458,NCT05346146,NCT00112658,NCT04902261,NCT04104672,NCT01978184,NCT04228601,NCT01827553,NCT01494506,NCT04084496,NCT03861702,NCT03519308,NCT04447092,NCT03252808,NCT05185869,NCT00844649,NCT00436267,NCT02705196,NCT04807972,NCT02109341,NCT03636308,NCT03504423,NCT03086369,NCT01485744,NCT00275119,NCT00601627,NCT00303758,NCT00024427,NCT02405585,NCT05497778,NCT02581215,NCT00003591,NCT02311439,NCT00660270,NCT00033735,NCT00424827,NCT01454180,NCT03484299,NCT01010945,NCT03883919,NCT04769414,NCT02551991
|
Ovarian cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73 |
TOP2A
NCT00102622,NCT00750386,NCT02606305,NCT01802749,NCT01379989,NCT02389985,NCT00722592,NCT00652119,NCT01649336,NCT00886717,NCT00228319,NCT00003160,NCT01219777,NCT00390611,NCT03025477,NCT03029611,NCT03804866,NCT00657878,NCT00840450,NCT00326456,NCT00003322,NCT02766582,NCT03467178,NCT01016054,NCT04679064,NCT01083537,NCT03393884,NCT03393507,NCT02470585,NCT00550784,NCT00825201,NCT00585052,NCT05467670,NCT00003378,NCT00002568,NCT01357161,NCT03639246,NCT03246074,NCT00861120,NCT02475772,NCT00945191,NCT00005026,NCT00610714,NCT00003644,NCT00408070,NCT02312661,NCT03126812,NCT05145569,NCT03989336,NCT00189553,NCT00005612,NCT00006454,NCT01485848,NCT00008138,NCT00937560,NCT00001383,NCT00003064,NCT02718417,NCT04000295,NCT03539328,NCT01146795,NCT02440425,NCT00028743,NCT00004934,NCT03763123,NCT00129727,NCT02004093,NCT01204749,NCT01447706,NCT00003449,NCT00004921,NCT00189566,NCT02431559,NCT02012192,NCT00660842,NCT00331422,NCT01388621,NCT03117933,NCT03287271,NCT00193297,NCT01493505,NCT00052468,NCT00002734,NCT04261465,NCT03818282,NCT00635193,NCT00003944,NCT00003732,NCT00017303,NCT03410784,NCT01954355,NCT00520013,NCT00003624,NCT00002717,NCT00023907,NCT04729608,NCT05255471,NCT01113957,NCT03038100,NCT01809379,NCT00075712,NCT00003880,NCT02092363,NCT01637532,NCT01239732,NCT01081951,NCT00003896,NCT00005046,NCT03699449,NCT04661696,NCT01100372,NCT05145218,NCT03737643,NCT03398655,NCT04931342,NCT03635489,NCT01778803,NCT00738699,NCT00226915,NCT04908787,NCT01570582,NCT01711970,NCT00889382,NCT00976911,NCT03596281,NCT00002928,NCT01705158,NCT04729387,NCT00903630,NCT00084448,NCT00003385,NCT03161132,NCT04815408,NCT03394885,NCT02125513,NCT02834975,NCT00610792,NCT04348032,NCT00034151,NCT04938583,NCT02948075,NCT00391118,NCT00003998,NCT04887961,NCT05044871,NCT02520154,NCT00702299,NCT01402271,NCT00091377,NCT00011986,NCT00031954,NCT03740165,NCT01644825,NCT00003413,NCT00003120,NCT02421588,NCT04590625,NCT04921527,NCT03942068,NCT01654146,NCT01253681,NCT00231075,NCT00003386,NCT00484432,NCT00002894,NCT01650376,NCT00838656,NCT00479817,NCT00005051,NCT03776812,NCT05316376,NCT01653912,NCT00964626,NCT02098343,NCT00170664,NCT00672295,NCT04374630,NCT01196741,NCT02865811,NCT00314678,NCT00003733,NCT02483104,NCT02121990,NCT03901118,NCT05371301,NCT00772798,NCT00993655,NCT04072263,NCT03335241,NCT01696032,NCT01821859,NCT02383251,NCT01666444,NCT01220154,NCT00373217,NCT03197584,NCT01227941,NCT02157870,NCT00002819,NCT00179725,NCT00262990,NCT01332656,NCT01358071,NCT01684878,NCT00929162,NCT00158379,NCT00523380,NCT01739218,NCT02001272,NCT00189371,NCT02545010,NCT02159820,NCT00466986,NCT04337632,NCT00063401,NCT00758732,NCT03056833,NCT02476955,NCT00004206,NCT01838538,NCT00517621,NCT00483782,NCT03940196,NCT04608409,NCT00401674,NCT00490711,NCT00003214,NCT03806049 |
Malignant neoplasms of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73 |
NCT04711161,NCT02903004,NCT04561817,NCT00628251,NCT03314740,NCT01121406,NCT00248248,NCT00001426,NCT00350948,NCT00919984,NCT01608009,NCT00913835,NCT00659178,NCT00191646,NCT01279291,NCT00001272,NCT00653952,NCT01616303,NCT02751918,NCT00780039,NCT03574779,NCT00069901
|
Small cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.1 |
NCT04314895,NCT05420636,NCT00317200,NCT04210037,NCT02551432,NCT04056949,NCT02262897,NCT00527735,NCT02261805,NCT04672928,NCT03219762,NCT02769832,NCT00483509,NCT00617409,NCT02038647,NCT00454324,NCT04213937,NCT01441349,NCT03810872,NCT04247126,NCT04698941,NCT02566993
|
NeoplasmsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 02 |
NCT02630264,NCT04778839,NCT01047007,NCT01566435,NCT00287937,NCT04035473,NCT04742036,NCT01930552,NCT00004863,NCT02341456,NCT00496028,NCT05322720,NCT04143711,NCT01702129,NCT00005819,NCT01868022,NCT02817113,NCT01930292,NCT01617928,NCT01522989,NCT02597036,NCT04176952,NCT01099358,NCT00021073,NCT01362374,NCT02715531,NCT00003130,NCT04675528,NCT01281176,NCT01494688,NCT04180384,NCT00005068,NCT03326947,NCT03421353,NCT01525602,NCT01992341,NCT00080418,NCT00024141,NCT05381909,NCT00838370,NCT03154294,NCT00054548,NCT00793897,NCT01472016,NCT03085914,NCT00020488,NCT01297452,NCT00653939,NCT04047862,NCT04999969,NCT00006018,NCT00261703,NCT01491204,NCT00413322,NCT02051751,NCT02057380,NCT02723955,NCT00003555,NCT03981796,NCT00001442,NCT00193596,NCT00024310,NCT00003004,NCT02648711,NCT00095914,NCT00458315,NCT00044785,NCT03588039,NCT01946074,NCT01994031,NCT00819221,NCT03330106,NCT02122770,NCT03928314,NCT00556088,NCT00004979,NCT01206465,NCT00004059,NCT02317874,NCT01967043,NCT03503604,NCT00422682,NCT00009802,NCT00002939,NCT00042861,NCT03309150,NCT02430311,NCT00768469,NCT00009828,NCT01455532,NCT03057366,NCT00215501,NCT02711137,NCT01240655,NCT02588105,NCT00100139,NCT00042874,NCT04607421,NCT04151277,NCT01411410,NCT02794571,NCT00891605,NCT00454649,NCT00003899,NCT02283489,NCT05344742,NCT04794699,NCT03595059,NCT02593708,NCT00307255,NCT04336098,NCT02134067,NCT02762981,NCT01419548,NCT00289445,NCT00003242,NCT03617432,NCT00402545,NCT04481009,NCT03564691,NCT01159418,NCT02720068,NCT05262335,NCT03894540,NCT01285466,NCT00954512,NCT03600090,NCT03486314,NCT00003742,NCT03537690,NCT00811993,NCT04541108,NCT00360360,NCT00003898,NCT03693612,NCT04393298,NCT02482441,NCT00007878,NCT02379416,NCT01938846,NCT01548924,NCT01031212,NCT01593228,NCT00350025,NCT02377752,NCT02884128,NCT03430882,NCT03892018,NCT00881101,NCT00357149,NCT00042809,NCT00003092,NCT02607202,NCT00610948,NCT03250832,NCT01515306,NCT00399126,NCT00080990,NCT03834948,NCT03907475,NCT03577704,NCT00511849,NCT00030368,NCT00520000,NCT00002587,NCT01214668,NCT04046016,NCT02730481,NCT02937272,NCT03918278,NCT00088114,NCT00016874,NCT03023124,NCT00004173,NCT00848718,NCT05017012,NCT00995293,NCT04185883,NCT00087217,NCT01968915,NCT04594005,NCT00046423,NCT00006257,NCT00028587,NCT01293630,NCT00607048,NCT00009932,NCT04420884,NCT00005078,NCT02648425,NCT00448136,NCT00005791,NCT00043121,NCT00246103,NCT00809133,NCT05214976,NCT04802980,NCT02979392,NCT01148628,NCT00486954,NCT00004913,NCT04449549,NCT00004242,NCT01634555,NCT01248949,NCT00001387,NCT03601897,NCT04761601,NCT00502567,NCT01862328
|
Non-small cell lung cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25 |
NCT03808480,NCT02412371,NCT02366143,NCT01993810,NCT02204345,NCT00966914,NCT01966003,NCT00603538,NCT01171170,NCT00976677,NCT00861627,NCT00161278,NCT00686322,NCT00168883,NCT00097227,NCT00806286,NCT00198354,NCT00085839,NCT01702844,NCT04224337,NCT01872403,NCT01560104,NCT00558636,NCT00578149,NCT00147537,NCT02477826,NCT02016209,NCT02367794,NCT00198432,NCT01100931,NCT01236716,NCT00850577,NCT01090830,NCT00903942,NCT01702714,NCT01165216,NCT00974584,NCT00480831,NCT01249443,NCT00273507,NCT00191256,NCT02319889,NCT00787852,NCT00723957,NCT00034541,NCT00199758,NCT00088088,NCT00093756,NCT03169738,NCT02276560,NCT00662311,NCT00313768,NCT00300885,NCT02064491,NCT01820325,NCT00828841,NCT00702572,NCT00540514,NCT00291850,NCT00762034,NCT00050960,NCT02264990,NCT00073723,NCT02405910,NCT01310244,NCT01383148,NCT01454102,NCT00866528,NCT00449657,NCT00269828,NCT01763645,NCT01620190,NCT00087802,NCT00035152,NCT01783197,NCT01496742,NCT00006929,NCT00666692,NCT00298415,NCT00481078,NCT00088556,NCT01711697,NCT00735696,NCT00752115,NCT00508625,NCT00369070,NCT02250326,NCT02419495,NCT00800202,NCT01199055,NCT03574649,NCT00583830,NCT00702975,NCT02083679,NCT01366131,NCT01507428,NCT01011075,NCT00680940,NCT00322452,NCT01836679,NCT00948675,NCT00576225,NCT00662597,NCT00112294,NCT01493843,NCT00991796,NCT01069328,NCT00716534,NCT00178256,NCT00005065,NCT00113516,NCT00061646,NCT02172846,NCT01413750,NCT00986674,NCT00768131,NCT00654758,NCT02392507,NCT02322281,NCT01763671,NCT00252798,NCT00001499,NCT01160601,NCT01527864,NCT00708812,NCT00254891,NCT00437749,NCT00473889,NCT00268970,NCT01364012,NCT00614484,NCT00832494,NCT00294762,NCT01158144,NCT01024062,NCT00563784,NCT00539331,NCT00434135,NCT01179269,NCT01326767,NCT02453282,NCT02289456,NCT02182232,NCT01822496,NCT02142738,NCT00874107,NCT00347412,NCT00259675,NCT01303926,NCT01649947,NCT00527735,NCT00642759,NCT00049790,NCT02420314,NCT02220894,NCT01769391,NCT00933803,NCT00687817,NCT00343291,NCT01519804,NCT00280787,NCT00915005,NCT00318136,NCT00434226,NCT00970580,NCT00094835,NCT00460317,NCT01234038,NCT01757288,NCT00042302,NCT01820754,NCT01225302,NCT01196234,NCT02117024,NCT00874848,NCT00602797,NCT02106546,NCT02364999,NCT01980472,NCT02259621,NCT00071188,NCT02403895,NCT00655850,NCT02073968,NCT01912625,NCT00918203,NCT00034164,NCT00753909,NCT00832819,NCT00152477,NCT02252796,NCT00730925,NCT00686959,NCT00807612,NCT02039674,NCT02041533,NCT00001450,NCT01268059,NCT02382406,NCT00960297,NCT00070629,NCT00174356,NCT02226757,NCT00198367
|
Malignant neoplasm metastasis, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E2Z |
NCT00126256,NCT02677116,NCT00046423,NCT00374036,NCT01014351,NCT01933061,NCT02158520,NCT00046527,NCT00048893,NCT02419495,NCT00731861,NCT00046514,NCT03678883,NCT01721746,NCT00289445,NCT02124148,NCT00984464,NCT00781612,NCT00065117,NCT00149201
|
Biliary tract cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C17 |
NCT03110510,NCT03291899,NCT03778593,NCT04492033,NCT03830606,NCT04027764,NCT01127555,NCT04692051,NCT05170438,NCT03785873,NCT00090025,NCT03524508,NCT05052099,NCT02632305,NCT04005339,NCT04217954,NCT01572324,NCT01494363,NCT04004234,NCT04722133
|
Carcinomas of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.0 |
NCT03598270,NCT00312650,NCT02107378,NCT01847677,NCT03030287,NCT02419495,NCT05158062,NCT05446870,NCT03542669,NCT00181701,NCT01091428,NCT04602117,NCT03400306,NCT03756818,NCT02244502,NCT02437812,NCT01970722,NCT03690739,NCT02272790,NCT05092373
|
Mild neurocognitive disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6D71 |
ACHE,BCHE
|
Bacterial infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A00-1C4Z |
TOP2A
NCT00189384 |
Pancreatic cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10 |
MTOR,TOP2A
|
Hypo-thyroidismDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A00 |
THRB,TSHR
|
Multiple sclerosisDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A40 |
TOP2A,ITGAV
|
Irritable bowel syndromeDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DD91 |
OPRD1
NCT01414244 |
Bladder cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94 |
MTOR,TOP2A
|
Adrenomedullary hyperfunctionDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A75 |
EPAS1,HIF1A
|
PsoriasisDisease Category: 14.Diseases of the skinDisease ICD-11 Code: EA90 |
TOP2A
NCT00006276 |
Rheumatoid arthritisDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FA20 |
OPRD1
NCT00055133 |
Muscular atrophyDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B61 |
SMN2,SMN1
|
RetinoblastomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D02.2 |
NCT01783535,NCT00186888
|
Oral mucositisDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DA01 |
NCT00006994,NCT02282839
|
Lymphoid leukaemia, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH7Q12 |
NCT00866749,NCT00801580
|
Ductal carcinoma in situ of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E65.2 |
NCT02779855,NCT00461344
|
Neoplasms of unknown behaviour of larynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F91.0 |
NCT00599131,NCT00117572
|
Diseases of the digestive system, unspecifiedDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DE2Z |
NCT00526110,NCT00208936
|
Glioma, malignantDisease Category: X.Extension CodesDisease ICD-11 Code: XH4RQ3 |
NCT02861222,NCT03678883
|
Primary haemophagocytic lymphohistiocytosisDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4A01.23 |
NCT04077905,NCT02631109
|
Neoplasms of unknown behaviour of colonDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F90.0 |
NCT03484195,NCT02777437
|
Neuroendocrine neoplasms of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.1 |
NCT03736720,NCT03042780
|
Cystic fibrosisDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA25 |
NCT01051999,NCT00376428
|
strictureDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BB80.Z-LB15.1 |
NCT02040454,NCT01868984
|
Penile cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C81 |
NCT00512096,NCT04475016
|
Chronic arterial occlusive disease, unspecifiedDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD4Z |
NCT00518284,NCT03064126
|
Chronic lymphocytic leukaemia or small lymphocytic lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A82.0 |
NCT03003546,NCT00801281
|
Malignant neoplasms of other or unspecified parts of mouthDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B66 |
NCT00933387,NCT05456022
|
Myelodysplastic syndromeDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A37 |
NCT00003331,NCT03059615
|
Lip/oral cavity/pharynx neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6E |
NCT00117572,NCT00117572
|
Anaplastic large cell lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH1LC0 |
NCT01719835,NCT01309789
|
Unspecified undernutritionDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5B7Z |
NCT00338221,NCT00133562
|
Crohn diseaseDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DD70 |
SLCO1B3
NCT00838149 |
Carcinoma, undifferentiated, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH1YY4 |
NCT00989651,NCT00108745
|
ThalassaemiaDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3A50 |
NCT00586209,NCT00125788
|
Type 2 diabetes mellitusDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A11 |
NCT03947879,NCT00673894
|
Malignant lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5FJ5 |
NCT00002524,NCT00126243
|
NeutropeniaDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4B00 |
NCT04227990,NCT03294577
|
Myotonic dystrophyDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C71.0 |
CHRNE,TOP2A
|
Chikungunya virus diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D40 |
SLC22A2,LMNA
|
Malignant neoplasms of thymusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C27 |
ACHE,GMNN
|
Carpal tunnel syndromeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C10.0 |
TUBB6,THPO
|
Malignant neoplasms of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72 |
NCT00313872,NCT03015675,NCT05319639,NCT03939962,NCT01285557,NCT05400915,NCT05441254,NCT01198392,NCT00003172,NCT01224652,NCT00020787,NCT00343239,NCT00154726,NCT02486601,NCT04358354,NCT01769508,NCT04592211,NCT03579784,NCT01216644,NCT00003298,NCT01590719,NCT00595972,NCT04694183,NCT01471132,NCT03977220,NCT01924819,NCT03766607,NCT03675737,NCT01896531,NCT04149015,NCT02855788,NCT00941655,NCT01249443,NCT05410847,NCT04888663,NCT01924533,NCT04358341,NCT01739894,NCT02934464,NCT00384878,NCT02072317,NCT00555672,NCT00857246,NCT03825861,NCT02114359,NCT01746771,NCT05095467,NCT03718624,NCT00992199,NCT00002783,NCT01041404,NCT03475615,NCT02845908,NCT03026881,NCT00210184,NCT00002722,NCT02442362,NCT01286766,NCT00054873,NCT00811447,NCT00149201,NCT00611507,NCT00103103,NCT00400179,NCT04182724,NCT04526470,NCT04308837,NCT04563975,NCT02142322,NCT00004233,NCT05204173,NCT01854255,NCT05002686,NCT04781413,NCT04943653,NCT01491217,NCT00003862,NCT01283204,NCT02038621,NCT01183559,NCT00188266,NCT00202969,NCT03704077,NCT01063517,NCT00004099,NCT01662869,NCT00661167,NCT02451956,NCT00374036,NCT04155671,NCT04001569,NCT01457846,NCT04483076,NCT03322969,NCT04342910,NCT04195828,NCT00052910,NCT01889303,NCT04757363,NCT04835896,NCT02697838,NCT01839773,NCT01170663,NCT04258657,NCT00123318,NCT01851941,NCT01503372,NCT00142350,NCT01980810,NCT01136031,NCT03607656,NCT00447967,NCT04258644,NCT05190445,NCT04602117,NCT00002883,NCT04286711,NCT03801668,NCT02574663,NCT02931890,NCT03751761,NCT02229058,NCT02563054,NCT05052931,NCT03286244,NCT01015339,NCT04135781,NCT00711243,NCT00525785,NCT04937738,NCT00155883,NCT01641939,NCT05318794,NCT00004873,NCT00215514,NCT03263741,NCT02890511,NCT04267549,NCT04859582,NCT03130790,NCT04683939,NCT00976768,NCT00270543,NCT01248403,NCT04393584,NCT03606928,NCT00005060,NCT04354662,NCT05025033,NCT01774786,NCT02578368,NCT00209612,NCT02296671,NCT00514020,NCT03067792,NCT03428425,NCT05427383,NCT00002615,NCT03694522,NCT05070598,NCT05171530,NCT04997837,NCT04379596
|
Follicular lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A80 |
TOP2A
NCT00634179,NCT05371093,NCT00801281,NCT02889523,NCT02449252,NCT01852435,NCT01650701,NCT00494780,NCT00907348,NCT05058404,NCT02855359,NCT00715208,NCT01476787,NCT04745832,NCT00774826 |
Peritoneal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C51 |
NCT03056833,NCT00517621,NCT00189566,NCT00001569,NCT00063401,NCT00391118,NCT03030287,NCT03740165,NCT00666991,NCT01279291,NCT00189371,NCT00262990,NCT02125513,NCT00191646,NCT03038100,NCT00352755
|
Peripheral T-cell lymphoma, not otherwise specifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90.C |
NCT00725231,NCT04480099,NCT00930605,NCT00791947,NCT01839097,NCT01719835,NCT01796002,NCT03553537,NCT00136565,NCT03542266,NCT02445404,NCT04922567,NCT03023358,NCT03952572,NCT04569032,NCT00970385
|
Liver cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12 |
AURKA,TOP2A,MTOR
NCT00507585,NCT00471484,NCT01009801,NCT00956930,NCT03164382,NCT03092895,NCT02753881,NCT01324076,NCT00732836,NCT00238160,NCT00003907,NCT00093444 |
Mantle cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85.5 |
NCT02427620,NCT00209222,NCT00878254,NCT01865110,NCT02633137,NCT05051891,NCT04566887,NCT00877006,NCT00401817,NCT00477412,NCT00022945,NCT00722137,NCT02858258,NCT00209209,NCT00581776
|
Inflammatory carcinoma of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C62 |
NCT01583426,NCT02879513,NCT02125344,NCT02221999,NCT01880385,NCT02682693,NCT02199418,NCT02623972,NCT01938833,NCT00513695,NCT03515798,NCT05383196,NCT01730833,NCT02876302,NCT00016276
|
Malignant neoplasms of anus or anal canalDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C00 |
NCT00025090,NCT00316888,NCT01249443,NCT00003652,NCT02059499,NCT00324415,NCT01621217,NCT00003596,NCT00955240,NCT01324141,NCT00423293,NCT02369939,NCT02402842,NCT00068744
|
Malignant neoplasm of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10 |
NCT02301143,NCT05083247,NCT04808687,NCT01121848,NCT04289792,NCT02017015,NCT02810418,NCT05222204,NCT00417209,NCT05493995,NCT02024009,NCT00220649,NCT01413022,NCT03807999
|
Malignant neoplasms of corpus uteri, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.Z |
BLM,AURKA,TOP2A,SLCO1B3
NCT03005015,NCT03517449,NCT02725268,NCT04865289,NCT03884101,NCT04602117,NCT05173987,NCT04269200,NCT01820858,NCT04634877 |
Diffuse large B-cell lymphomasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A81 |
ACHE,LMNA,BLM,SMN1,TUBB6,TSHR,ITGAV,PMP22,AURKA,EPAS1,THPO,TOP2A,THRB,GMNN
|
Malignant neoplasms of fallopian tube, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C74.Z |
NCT00191646,NCT05092373,NCT01279291,NCT01167712,NCT01970722,NCT00954174,NCT02419495,NCT00063401,NCT02125513,NCT00391118,NCT02272790,NCT03030287,NCT03776812
|
Carcinoma of male breastDisease Category: X.Extension CodesDisease ICD-11 Code: XH5KW8 |
NCT01281150,NCT00470301,NCT00513695,NCT01276496,NCT00770809,NCT00119262,NCT00368875,NCT01493310,NCT01938833,NCT02060253,NCT00861705,NCT03018080,NCT01288261
|
Esophageal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B70 |
NCT01843049,NCT00016900,NCT01348217,NCT03281369,NCT00069953,NCT01704690,NCT00008047,NCT00209716,NCT00006245,NCT00632333,NCT04278287,NCT00002631,NCT04005170,NCT03691090,NCT00258323,NCT00525915,NCT00453323,NCT00107341,NCT03110926,NCT01998347,NCT02861690,NCT02735239,NCT00054873,NCT00002884,NCT03328234,NCT01372202,NCT00075738,NCT00393068,NCT02389751,NCT00539617,NCT05013697,NCT02607982,NCT03279237,NCT00003087,NCT00003118,NCT02741856,NCT00281788,NCT00004257,NCT00021320,NCT03126708,NCT00493025,NCT01608464,NCT02133612,NCT00730353,NCT03002064,NCT05174156,NCT03731442,NCT00655876,NCT00759226,NCT00551759,NCT02762474,NCT03881111,NCT02574663,NCT02317991,NCT00815308,NCT01444547,NCT00544362,NCT04540211,NCT00193141,NCT00086996,NCT01611857,NCT00154700,NCT02858206,NCT02023593,NCT00209690,NCT01249443,NCT02359968,NCT00861094,NCT03910634,NCT02461043,NCT00733889,NCT00344552,NCT00193882,NCT01183559,NCT00004233,NCT01787006,NCT00230451,NCT00002984,NCT02644863,NCT00002883,NCT00020787,NCT00735345,NCT00416858,NCT04879368,NCT00066716,NCT00139633,NCT00052910,NCT04046575,NCT00047112,NCT02891083,NCT00259402,NCT00041262,NCT00006472,NCT02297217,NCT00002897,NCT00425425,NCT00160030,NCT02924909,NCT00381706,NCT00004897,NCT03165994,NCT03764553,NCT02570893,NCT02429622,NCT00971841,NCT01034189,NCT00154804,NCT00570531,NCT00915850,NCT04426955,NCT01217060,NCT01843829,NCT00002711,NCT00686114,NCT04460066,NCT04821765,NCT02569242,NCT03308552,NCT00003326,NCT04006041,NCT02279134,NCT03189719,NCT02273713,NCT02446574,NCT03490292,NCT05357846,NCT03087864,NCT02598687
|
Lung cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25 |
MTOR,AURKA,TOP2A
NCT00004055,NCT02279732,NCT00807573,NCT00003317,NCT00278148,NCT00290537,NCT00547443,NCT00003111,NCT00006484,NCT00043108,NCT00003589,NCT02525653,NCT00544648,NCT02044601,NCT00081302,NCT01486602,NCT00005032,NCT00828009,NCT00025389,NCT00004137,NCT00006049,NCT00006374,NCT00245154,NCT00004202,NCT00062010,NCT00550537,NCT00003159,NCT01057342,NCT00033410,NCT00062062,NCT00729612,NCT00979212,NCT02513563,NCT00551733,NCT00193362,NCT00004887,NCT00280150,NCT00144053,NCT00006469,NCT00003089,NCT03432598,NCT00077220,NCT00006012,NCT00003235,NCT01107626,NCT01862081,NCT00452803,NCT00077246,NCT00005059,NCT00039039,NCT00003587,NCT00748163,NCT01024712,NCT00020007,NCT00459121,NCT00533949,NCT00062179,NCT00003881,NCT00017407,NCT00005849,NCT03322566,NCT00226590,NCT00004126,NCT00661193,NCT00079287,NCT00276588,NCT00006378,NCT00005806,NCT00033696,NCT04647344,NCT00004859,NCT00002972,NCT00795340,NCT00453167,NCT00006004,NCT00025480,NCT00003117,NCT00004093,NCT00004265,NCT00032032,NCT00011921,NCT00002969,NCT00616031,NCT00016315,NCT00054392,NCT00003387,NCT00495170,NCT00033553,NCT00096226,NCT00003943,NCT03348904,NCT00054210,NCT00055887,NCT00003812,NCT00003158,NCT00003284,NCT02328105,NCT00085501,NCT00005646,NCT00028938,NCT00004011,NCT00004160,NCT00193310,NCT00004159,NCT02733250,NCT00533585,NCT00023673,NCT00006229,NCT00287989,NCT00801736,NCT00728845,NCT00553462,NCT00526890,NCT00465907,NCT00003299,NCT00334763,NCT00003696,NCT00063258,NCT00003281,NCT00002519,NCT00003313,NCT00004924,NCT01285609,NCT00004253 |
Actinic keratosisDisease Category: X.Extension CodesDisease ICD-11 Code: XH36H6 |
NCT05078827,NCT00377273,NCT02281682,NCT01354717,NCT02616601,NCT03037541,NCT01525329,NCT00696488,NCT03083470,NCT04875026,NCT02019355,NCT02289768
|
Precursor B-lymphoblastic neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A70 |
NCT02881086,NCT04043494,NCT05303792,NCT02228772,NCT00866749,NCT03023046,NCT01451515,NCT03564704,NCT03991884,NCT01887587,NCT02845882,NCT03817320
|
Adenocarcinoma of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.0 |
ACHE,LMNA,BLM,APP,DHCR7,TUBB6,ITGAV,PMP22,AURKA,BCHE,SLCO1B3,NPC1
|
CholangiocarcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH7M15 |
NCT02181634,NCT04683939,NCT05024513,NCT05348811,NCT03943043,NCT04077983,NCT01963325,NCT02456714,NCT04480190,NCT02994251,NCT03603834
|
LeukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60-2B33 |
NCT00072007,NCT01516580,NCT02723994,NCT00003402,NCT03975205,NCT00003230,NCT00003294,NCT00038610,NCT01319981,NCT00613457,NCT00109837
|
Neoplasms of unknown behaviour of urinary organsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F98 |
NCT04506554,NCT00005831,NCT00478361,NCT04241185,NCT02560038,NCT03669783,NCT05024773,NCT04876313,NCT02710734,NCT04383743,NCT05203913
|
HepatoblastomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.01 |
BLM,OPRD1,PMP22,AURKA,TOP2A,TUBB3,GMNN
NCT03017326,NCT04478292,NCT00980460,NCT00003994 |
Melanoma of skin, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30.Z |
NCT02419495,NCT02020707,NCT00081042,NCT00278122,NCT00110019,NCT02308553,NCT00976573,NCT00568451,NCT00626405,NCT02158520,NCT00404235
|
Rectum cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B92 |
NCT04838496,NCT00278694,NCT00527111,NCT01740648,NCT00307736,NCT03085992,NCT00025337,NCT00807911,NCT01481545,NCT05111860,NCT02217020,NCT01111604,NCT00070122,NCT03879109,NCT00006094,NCT01871571,NCT05253846,NCT02423226,NCT03043729,NCT01941641,NCT01238965,NCT03391843,NCT04165772,NCT00235898,NCT02280070,NCT01270438,NCT05378919,NCT02574663,NCT00321685,NCT02041481,NCT03781323,NCT03127007,NCT00611858,NCT00003873,NCT01060007,NCT05358704,NCT03428529,NCT04215731,NCT01269229,NCT05108428,NCT01716949,NCT00337389,NCT03259035,NCT02008656,NCT01205022,NCT05024097,NCT04130854,NCT02887313,NCT03152370,NCT01211210,NCT02465593,NCT03484221,NCT04170530,NCT02941562,NCT01347645,NCT01013805,NCT02942563,NCT03300544,NCT03161574,NCT00580073,NCT03975049,NCT05412082,NCT00101894,NCT05201430,NCT02514278,NCT00052559,NCT01500993,NCT01274962,NCT03299660,NCT01554059,NCT04423965,NCT01650428,NCT03921684,NCT01198548,NCT04495088,NCT04621370,NCT01376453,NCT05074966,NCT04808323,NCT00422864,NCT00942266,NCT04009876,NCT01197664,NCT04380337,NCT00145769,NCT02921256,NCT02340949,NCT03000374,NCT01302613,NCT00832299,NCT02319304,NCT02688712,NCT03443661,NCT00003799,NCT00707889,NCT00499369,NCT05022030,NCT00349076,NCT04418895,NCT03548961,NCT02270606,NCT00303628,NCT02167321,NCT01269216,NCT02704520,NCT03671252,NCT05009069,NCT00865189,NCT03491540
|
Mature T-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90 |
TOP2A,HIF1A,MTOR
NCT03188198,NCT02961816,NCT00008021,NCT05006664,NCT00003294,NCT01321008,NCT00005021,NCT00822120,NCT02701673,NCT00265018,NCT02670317,NCT01855750,NCT00554164,NCT00945724,NCT00003595,NCT00003397,NCT04856137,NCT00911183,NCT01490047,NCT01527422,NCT00002557,NCT03884556,NCT00118209,NCT00787527,NCT03975205,NCT01414855,NCT00003957,NCT00006721,NCT00264953,NCT00003784,NCT00451178,NCT00854568,NCT00004112,NCT02359162,NCT00299182,NCT03435250,NCT04884035,NCT00049595,NCT00003541,NCT00536393,NCT00265031,NCT00003956,NCT00217425,NCT00041132,NCT00841945,NCT01992653,NCT00004057,NCT01516567,NCT01831505,NCT01139359,NCT00005584,NCT00433433,NCT02918747,NCT05075460,NCT00920153,NCT03647072,NCT00504504,NCT00004031,NCT00003421,NCT01390584,NCT00003150,NCT04164368,NCT00003402,NCT00003215,NCT00003578,NCT00060385,NCT01118026,NCT00004179,NCT00577629,NCT01889069,NCT00210379,NCT00041210,NCT00577993,NCT00369681,NCT00974324,NCT01983969,NCT00450385,NCT02589145,NCT02529852,NCT01404936,NCT01046825,NCT02605694,NCT00002565,NCT00101101,NCT00001579,NCT00003389,NCT00893516,NCT02012088,NCT00021372,NCT00064116,NCT01974440,NCT00004010,NCT00004916,NCT00770224,NCT00027937,NCT00005867,NCT00290498,NCT00052936,NCT00450801,NCT00060346,NCT00001383,NCT00211185,NCT04980222,NCT00009776 |
Squamous cell carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B60-2D01 |
NCT04460352,NCT01822613,NCT04390958,NCT02027428,NCT02677597,NCT05319730,NCT03946969,NCT03116152,NCT01752205,NCT01248299,NCT03603756,NCT04718415,NCT01225523,NCT00596830,NCT04354961,NCT03278626,NCT04926753,NCT03866993,NCT02272699,NCT02916511,NCT04804696,NCT03748134,NCT02218164,NCT02459457,NCT04506138,NCT04625543,NCT02017600,NCT04767295,NCT04929041,NCT03940001,NCT04888403,NCT03964753,NCT04844385,NCT05221658,NCT04649476,NCT04949256,NCT03732508,NCT01391572,NCT00001442,NCT01166542,NCT02350517,NCT01181401,NCT05342636,NCT03762564,NCT05125055,NCT01386385,NCT02611700,NCT00176267,NCT04548440,NCT04937673,NCT00816634,NCT01542931,NCT01969955,NCT05449483,NCT03292822,NCT01627379,NCT05007145,NCT03387111,NCT04225364,NCT03109158,NCT04644250,NCT00042835,NCT03719924,NCT01591135,NCT04550260,NCT03783442,NCT04568200,NCT03975270,NCT00176254,NCT04138212,NCT04063683,NCT00753038,NCT05312372,NCT01914900,NCT02383966,NCT04229459,NCT05394415,NCT00998192,NCT02059967,NCT01688700,NCT04472429,NCT05189730,NCT04084158,NCT01249443,NCT04764227,NCT02976909,NCT00705016,NCT03370406,NCT04943445,NCT02485548,NCT03430843,NCT04807673,NCT02784288,NCT05213312,NCT04839731,NCT02325986,NCT05281003,NCT05469061,NCT05322499,NCT01836029,NCT01258192,NCT02033538,NCT03957590,NCT03224221
|
Urethral cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C93 |
NCT00022191,NCT00006111,NCT00003376,NCT00022633,NCT00003175,NCT00478361,NCT00479128,NCT00045630,NCT00005644,NCT04579224
|
Mesothelioma of pleuraDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C26.0 |
LMNA,SMN1,DHCR7,OPRD1,AURKA,BCHE,TOP2A,NPC1,BACE1,GMNN
|
Adenocarcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.0 |
ACHE,BLM,TSHR,OPRD1,SLCO1B1,AURKA,THPO,TOP2A,SLCO1B3,GMNN
|
Germ cell tumour of testisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C80.2 |
ACHE,BLM,SMN1,APP,OPRD1,THRB,SLCO1B1,BCHE,RAB9A,GMNN
|
Bowel habit changeDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: ME05 |
OPRD1
|
ParkinsonismDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A00 |
ACHE
|
Coronary vasospastic diseaseDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA85 |
OPRD1
|
Metastatic lymph node neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D60 |
MTOR
|
LymphangioleiomyomatosisDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB07 |
MTOR
|
Arteries/arterioles disorderDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD52 |
MTOR
|
Otitis externaDisease Category: 10.Diseases of the ear or mastoid processDisease ICD-11 Code: AA00-AA13 |
TOP2A
|
Oesophageal/gastroduodenal disorderDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DD90 |
ACHE
|
Transplant rejectionDisease Category: 22.Injury, poisoning or certain other consequences of external causesDisease ICD-11 Code: NE84 |
MTOR
|
AnxietyDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MB24.3 |
OPRD1
|
Otitis mediaDisease Category: 10.Diseases of the ear or mastoid processDisease ICD-11 Code: AA80-AB0Z |
TOP2A
|
Respiratory system diseaseDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB40-CB7Z |
TOP2A
|
Chronic myelomonocytic leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A40 |
MTOR
|
Gonococcal infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A70-1A7Z |
TOP2A
|
Spinal painDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: ME84 |
OPRD1
|
Acute diabete complicationDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A2Y |
AURKA
|
Myasthenia gravisDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C6Y |
ACHE
|
DementiaDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6D80-6D8Z |
ACHE
|
Desmoid tumourDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F7C |
APP
|
RetinopathyDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9B71 |
APP
|
Parasitic worm infestationDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1F90 |
ACHE
|
Ischaemic/haemorrhagic strokeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B20 |
THPO
|
Inborn lipid metabolism errorDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5C52 |
THRB
|
Common wartsDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1E80 |
TOP2A
|
Unspecific substance harmful effectDisease Category: 22.Injury, poisoning or certain other consequences of external causesDisease ICD-11 Code: NE6Z |
ACHE
|
HIV-infected patients with tuberculosisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1B10-1B14 |
TOP2A
|
Substance abuseDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C40 |
ACHE
|
Eexposure to noxious substances harmful effectDisease Category: 22.Injury, poisoning or certain other consequences of external causesDisease ICD-11 Code: NE61 |
BCHE
|
Anogenital wartsDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A95 |
TOP2A
|
Fungal infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1F29-1F2F |
RAB9A
|
Myocardial infarctionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA41-BA43 |
THPO
|
Malignant mesenchymal neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B5D-2B5Y |
MTOR
|
HydrocephalusDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8D64 |
MTOR
|
Opioid use disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6C43 |
OPRD1
|
PediculosisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1G00 |
ACHE
|
Urinary tract infectionDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GC08 |
TOP2A
|
Stomach cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72 |
TOP2A
|
AmyloidosisDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5D00 |
APP
|
Functional bladder disorderDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GC50 |
OPRD1
|
MigraineDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A80 |
OPRD1
|
SyphilisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A61-1A6Z |
TOP2A
|
Diagnostic imagingDisease Category: NADisease ICD-11 Code: N.A. |
APP
|
Hyper-lipoproteinaemiaDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5C80 |
THRB
|
AsthmaDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA23 |
TOP2A
|
Tonus and reflex abnormalityDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MB47 |
BCHE
|
ChemoprotectionDisease Category: NADisease ICD-11 Code: N.A. |
TOP2A
|
CoughDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MD12 |
OPRD1
|
Chronic painDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG30 |
OPRD1
|
ThrombosisDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DB61-GB90 |
TOP2A
|
Metastatic liver/bile duct neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D80 |
NCT02591030
|
Malignant neoplasms of larynx, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C23.Z |
NCT00508664
|
Neuroendocrine tumour, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH8DS0 |
NCT04602117
|
Mesotheliomas of peritoneumDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C51.2 |
NCT05449366
|
Metastatic lung neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D70 |
NCT03678883
|
Acute leukaemias of ambiguous lineageDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A61 |
NCT02419755
|
Low grade squamous intraepithelial lesion of cervix uteriDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA15.7 |
NCT00000758
|
PlagueDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1B93 |
NCT00128466
|
Heart failureDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD10-BD1Z |
NCT01534663
|
Deep vein thrombosisDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD71 |
NCT00849264
|
Infection of amniotic sac or membranesDisease Category: 18.Pregnancy, childbirth or the puerperiumDisease ICD-11 Code: JA88.1 |
NCT00879190
|
Adrenal cortical carcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH4KH2 |
NCT00786110
|
Mature B-cell leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A82 |
NCT01328236
|
Classical organic aciduriaDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5C50.E0 |
NCT00645879
|
Cervical intraepithelial neoplasiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E66 |
NCT03143491
|
Type1diabetesmellitusDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A10 |
NCT01581476
|
Diseases of coronary artery, unspecifiedDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA8Z |
NCT00124943
|
Malignant neoplasms of oropharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6A |
NCT03416153
|
Signet ring cell carcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH4546 |
NCT00217737
|
Prostate hyperplasiaDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA90 |
NCT03423979
|
NosocomialDisease Category: X.Extension CodesDisease ICD-11 Code: XB25 |
NCT01704430
|
Unspecific body region injuryDisease Category: 22.Injury, poisoning or certain other consequences of external causesDisease ICD-11 Code: ND56 |
NCT01250782
|
Becker muscular dystrophyDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C70.0 |
NCT00005574
|
Multi organ failureDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG4A |
NCT00133978
|
Burkitt-like lymphoma with 11q aberrationDisease Category: X.Extension CodesDisease ICD-11 Code: XH8NN2 |
NCT02228772
|
Periodontal diseaseDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DA0C |
NCT00010634
|
Primary neoplasms of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00 |
NCT00479765
|
Small cell carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0YB0 |
NCT02881125
|
Malignant neoplasm metastasis in bone or bone marrowDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E03 |
NCT03678883
|
Malignant trophoblastic neoplasms of placentaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C75.0 |
NCT02639650
|
Teratoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH83G5 |
NCT00104676
|
Choroid plexus tumoursDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.22 |
NCT01014767
|
Adenoid cystic carcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH4302 |
NCT00581360
|
GanglioneuroblastomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH77W7 |
NCT03786783
|
Human immunodeficiency virus type 1Disease Category: X.Extension CodesDisease ICD-11 Code: XN8LD |
NCT01435018
|
Optic nerve disorderDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9C40 |
NCT02372409
|
Osteomyelitis or osteitisDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FB84 |
NCT02099240
|
Carcinosarcoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH2W45 |
NCT00815945
|
Pilocytic astrocytomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH12D2 |
NCT02372409
|
Malignant neoplasm metastasis in female reproductive systemDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E05 |
NCT02739529
|
skin carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C31.0-2E67.40 |
NCT02218164
|
OligoastrocytomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH6F49 |
NCT02372409
|
Castlemans diseaseDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4B2Y |
NCT02228512
|
Chronic myelogenous leukaemia, BCR-ABL1-positiveDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A20.0 |
NCT01424982
|
Parathyroid carcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH7JQ0 |
NCT03181100
|
Human immunodeficiency virus disease without mention of associated disease or condition, clinical stage unspecifiedDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1C62.Z |
NCT01249443
|
Gallbladder/bile ducts/liver structural developmental anomalyDisease Category: 20.Developmental anomaliesDisease ICD-11 Code: LB20 |
NCT02392637
|
COVID-19Disease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D6Y |
NCT04909905
|
Chronic kidney diseaseDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GB61 |
NCT01353638
|
Malignant neoplasms of vulvaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C70 |
NCT00014599
|
Mucinous adenocarcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH1S75 |
NCT00068692
|
Other specified malignant neoplasms of oropharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6A.Y |
NCT01249443
|
Neoplasms of unknown behaviour of female genital organsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F96 |
NCT02182245
|
AnemiaDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3A00-3A9Z |
NCT00189371
|
Other specified amyloidosisDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5D00.Y |
NCT00030381
|
NADisease Category: NADisease ICD-11 Code: NA |
NCT00217737
|
Lymphoplasmacytic lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85.4 |
NCT00801281
|
Clostridium difficile enterocolitisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A04 |
NCT02053350
|
LiposarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B59 |
NCT05218499
|
VitreoretinopathyDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9B78 |
NCT00370760
|
coronary artery diseaseDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA8Y-BA8Z |
NCT01478126
|
Ependymoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH1511 |
NCT01498783
|
Adult T-cell leukaemia/lymphoma (HTLV-1 positive)Disease Category: X.Extension CodesDisease ICD-11 Code: XH6TE2 |
NCT00145002
|
Nausea/vomitingDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MD90 |
NCT02116530
|
Cat-scratch diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1B98 |
NCT03132116
|
Myeloproliferative neoplasm, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5HH7 |
NCT00003331
|
NeuropathyDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C0Z |
NCT02311907
|
Infectious gastroenteritis or colitis without specification of infectious agentDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A40.Z |
NCT00133406
|
Meniere diseaseDisease Category: 10.Diseases of the ear or mastoid processDisease ICD-11 Code: AB31.0 |
NCT00802529
|
Malignant neoplasm of liverDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.0 |
NCT01272557
|
Malignant neoplasms of nasal cavityDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C20 |
NCT00117572
|
Intrauterine growth restriction, unspecifiedDisease Category: 19.Certain conditions originating in the perinatal periodDisease ICD-11 Code: KA20.1Z |
NCT00005775
|
Oligodendroglioma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH7W59 |
NCT02372409
|
Basal cell carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C32 |
NCT03541252
|
Extranodal NK/T-cell lymphoma, nasal typeDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90.6 |
NCT00725231
|
Preterm newborn, unspecifiedDisease Category: 19.Certain conditions originating in the perinatal periodDisease ICD-11 Code: KA21.4Z |
NCT00005775
|
Astrocytoma, anaplasticDisease Category: X.Extension CodesDisease ICD-11 Code: XH96C7 |
NCT02372409
|
IschemiaDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B10-8B11 |
NCT02835586
|
Superficial bacterial folliculitisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1B74 |
NCT01244256
|
Squamous intraepithelial neoplasia, high gradeDisease Category: X.Extension CodesDisease ICD-11 Code: XH3EA2 |
NCT02059499
|
Cutaneous T-cell lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH1951 |
NCT02192021
|
GliosarcomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH9RC8 |
NCT04528680
|
Chalazion, unspecifiedDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9A02.0Z |
NCT02025023
|
Ventilation associated with injury or harm in therapeutic useDisease Category: 23.External causes of morbidity or mortalityDisease ICD-11 Code: PK81.0 |
NCT02515448
|
Sex cord-gonadal stromal tumour, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH9G57 |
NCT01770301
|
Other specified carcinoma in situ of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E65.Y |
NCT01891357
|
Malignant neoplasms of vaginaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C71 |
NCT00002949
|
Diabetes mellitus, type unspecifiedDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A14 |
NCT02351323
|
Allergic/hypersensitivity disorderDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4A80-4A8Z |
NCT00277043
|
Other specified malignant neoplasms of the ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.Y |
NCT02429700
|
Pleomorphic adenomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH2KC1 |
NCT00502203
|
Verrucous carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5PM0 |
NCT01249443
|
Generalised anxiety disorderDisease Category: 06.Mental, behavioural or neurodevelopmental disordersDisease ICD-11 Code: 6B00 |
NCT04274114
|
Diseases of the blood or blood-forming organs, unspecifiedDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3C0Z |
NCT04243434
|
Malignant neoplasms of other or unspecified parts of biliary tractDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C17 |
NCT05350943
|
Mature B-cell neoplasms, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A8Z |
NCT02911142
|
Coeliac diseaseDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DA95 |
TSHR
|
Myelodysplastic syndromes, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A3Z |
TSHR
|
Inflammatory bowel diseases, unspecifiedDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DD7Z |
SLCO1B3
|
Zika virus diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D48 |
ACHE
|
Osteoarthritis, unspecifiedDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FA0Z |
TOP2A
|
Idiopathic pulmonary fibrosisDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB03.4 |
SLCO1B3
|
West Nile virus infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D46 |
ACHE
|
Melanoma of skinDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30 |
APP
|
Malignant neoplasms of thyroid gland, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D10.Z |
GABRA6
|
Polycystic ovary syndromeDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A80.1 |
ITGAV
|
Ebola virus diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D60.01 |
ACHE
|
Gliomas of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.0 |
BLM
|

