Collective Molecular Activities of the Plant: Sambucus Williamsii
Plant ID: NPO17434
Plant Latin Name: Sambucus Williamsii
Taxonomy Genus: Sambucus
Taxonomy Family: Adoxaceae
Plant External Links:
NCBI TaxonomyDB:
180062
Plant-of-the-World-Online:
n.a.
Unknown.
Overview of Ingredients
47 All known Ingredients in Total
Unique ingredients have been isolated from this plant.Plant-Ingredients Associations were manually curated from publications or collected from other databases.
37 Ingredients with Acceptable Bioavailablity
Unique ingredients exhibit acceptable human oral bioavailablity, according to the criteria of SwissADME [PMID: 28256516] and HobPre [PMID: 34991690]. The criteria details:SwissADME: six descriptors are used by SwissADME to evaluate the oral bioavailability of a natural product:
☑ LIPO(Lipophility): -0.7 < XLOGP3 < +5.0
☑ SIZE: 150g/mol < MW < 500g/mol
☑ POLAR(Polarity): 20Ų < TPSA < 130Ų
☑ INSOLU(Insolubility): -6 < Log S (ESOL) < 0
☑ INSATU(Insaturation): 0.25 < Fraction Csp3 < 1
☑ FLEX(Flexibility): 0 < Num. rotatable bonds < 9
If 6 descriptors of a natural plant satisfy the above rules, it will be labeled high HOB.
HobPre: A natural plant ingredient with HobPre score >0.5 is labeled high human oral availability (HOB)
34 Ingredients with experimental-derived Activity
Unique ingredients have activity data available.Ingredient Structrual Cards
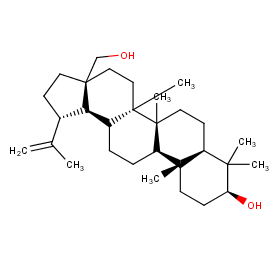
Ingredient ID: NPC95615
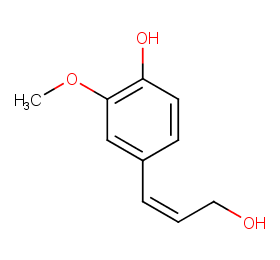
Ingredient ID: NPC78918
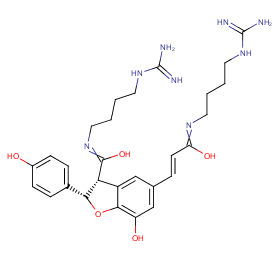
Ingredient ID: NPC48736
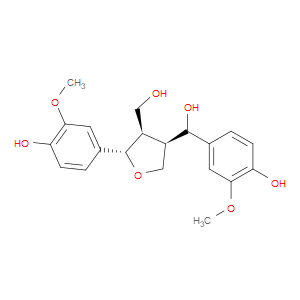
Ingredient ID: NPC485398
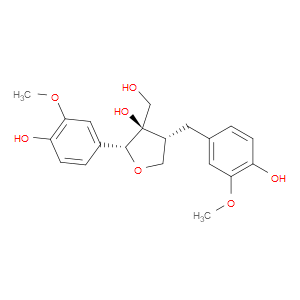
Ingredient ID: NPC485397
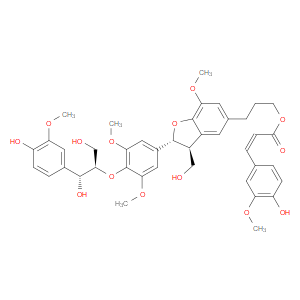
Ingredient ID: NPC485396
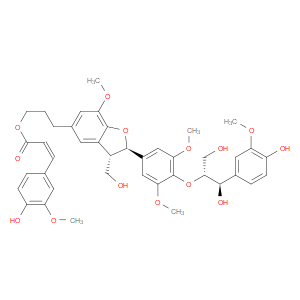
Ingredient ID: NPC485395
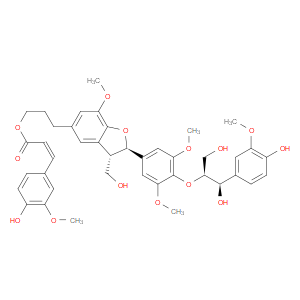
Ingredient ID: NPC485394
Ingredient ID: NPC40432
Ingredient ID: NPC38059
Ingredient ID: NPC34103
Ingredient ID: NPC3295
Ingredient ID: NPC319929
Ingredient ID: NPC311256
Ingredient ID: NPC307050
Ingredient ID: NPC29799
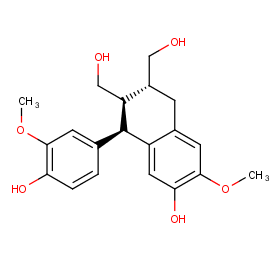
Ingredient ID: NPC286620
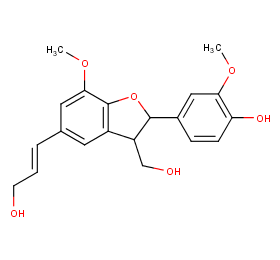
Ingredient ID: NPC273709
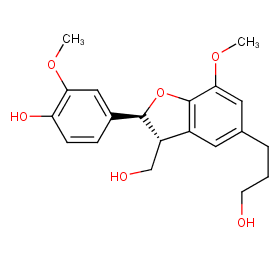
Ingredient ID: NPC263367
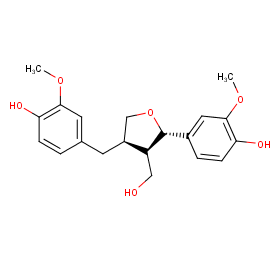
Ingredient ID: NPC257582
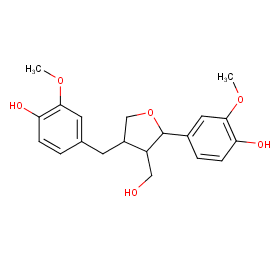
Ingredient ID: NPC23962
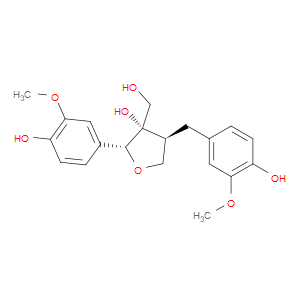
Ingredient ID: NPC23646
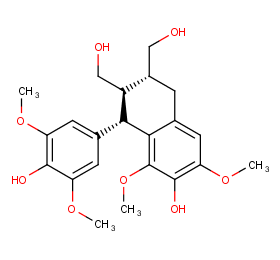
Ingredient ID: NPC230124
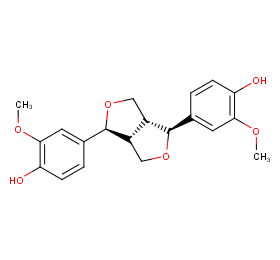
Ingredient ID: NPC228346
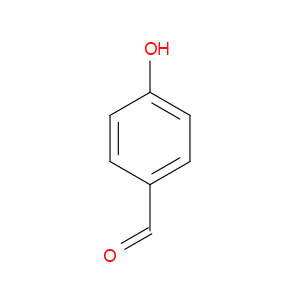
Ingredient ID: NPC219913
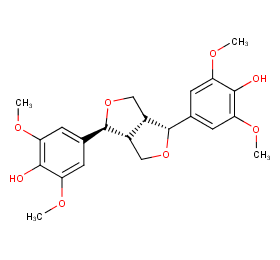
Ingredient ID: NPC214001
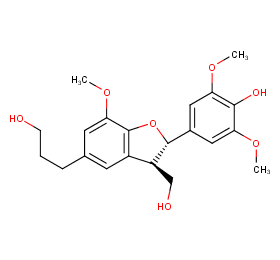
Ingredient ID: NPC202826
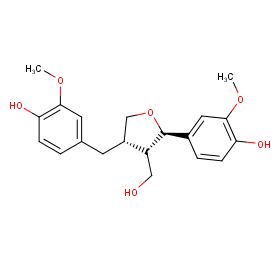
Ingredient ID: NPC187998
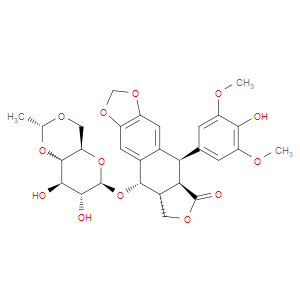
Ingredient ID: NPC185498
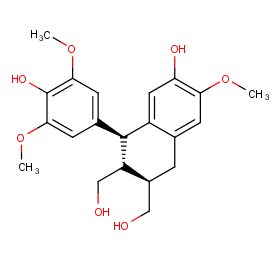
Ingredient ID: NPC178970
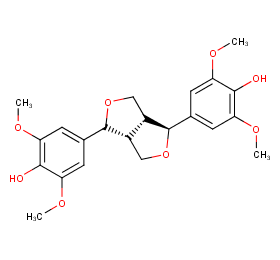
Ingredient ID: NPC165159
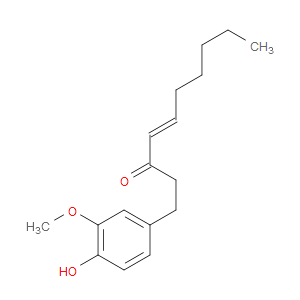
Ingredient ID: NPC164778
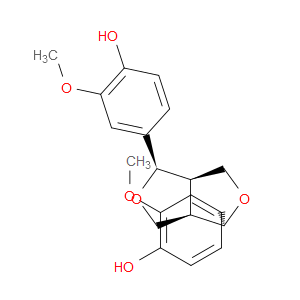
Ingredient ID: NPC161557
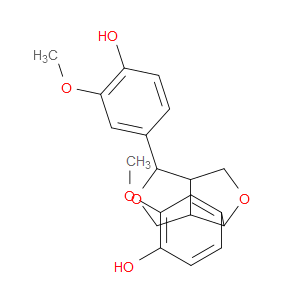
Ingredient ID: NPC158079
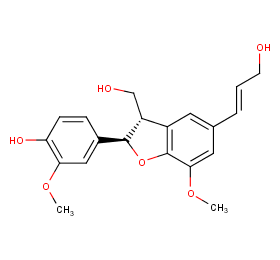
Ingredient ID: NPC156502
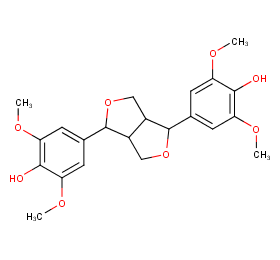
Ingredient ID: NPC153572
Ingredient ID: NPC148842

Ingredient ID: NPC143709
Ingredient ID: NPC141765
Ingredient ID: NPC139617
Ingredient ID: NPC131046
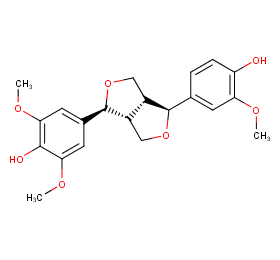
Ingredient ID: NPC126409
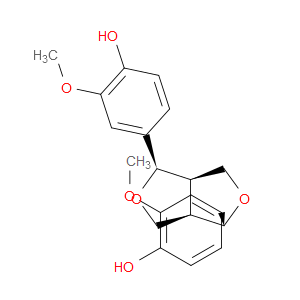
Ingredient ID: NPC115207
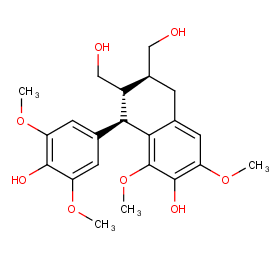
Ingredient ID: NPC114171
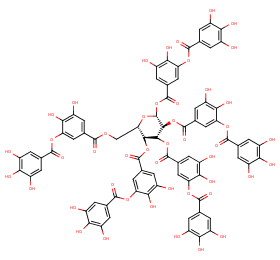
Ingredient ID: NPC113961
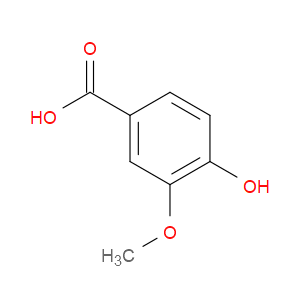
Ingredient ID: NPC111888
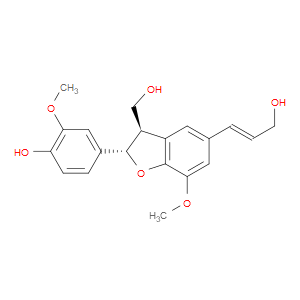
Ingredient ID: NPC10737
Classification of Human Proteins Collectively Targeted by the Plant
Detailed Information of Target Proteins
| Target Type | Protein Class | Gene ID | Protein Name | Uniprot ID | Target ChEMBL ID |
|---|---|---|---|---|---|
| Therapeutic Target | Enzyme | NOS1 | Nitric-oxide synthase, brain | P29475 | CHEMBL3568 |
| Therapeutic Target | Enzyme | NOS3 | Nitric-oxide synthase, endothelial | P29474 | CHEMBL4803 |
| Therapeutic Target | Histone acetyltransferase | NCOA3 | Nuclear receptor coactivator 3 | Q9Y6Q9 | CHEMBL1615382 |
| Therapeutic Target | Isomerase | TOP2B | DNA topoisomerase II beta | Q02880 | CHEMBL3396 |
| Therapeutic Target | Nuclear hormone receptor subfamily 3 | ESR2 | Estrogen receptor beta | Q92731 | CHEMBL242 |
| Therapeutic Target | Nuclear hormone receptor subfamily 3 | ESR1 | Estrogen receptor alpha | P03372 | CHEMBL206 |
| Therapeutic Target | Transient receptor potential channel | TRPV1 | Vanilloid receptor | Q8NER1 | CHEMBL4794 |
Clinical trials associated with plant from natural product (NP) & plant level:
| Clinical trials type | Number of clinical trials | |
|---|---|---|
| 978 | ||
| NCT ID | Title | Condition | Form in clinical use | Associated by plant or compound |
|---|---|---|---|---|
| NCT00000660 | Phase I Study of Weekly Oral VP-16 for AIDS-Associated Kaposi's Sarcoma | Kaposi's sarcoma | Etoposide (NPC185498) | |
| NCT00001339 | A Study of Combination Chemotherapy and Surgical Resection in the Treatment of Adrenocortical Carcinoma: Continuous Infusion Doxorubicin, Vincristine and Etoposide With Daily Mitotane Before and After Surgical Resection | carcinoma | Etoposide (NPC185498) | |
| NCT00001563 | EPOCH Chemotherapy +/- IL-12 for Previously Untreated and EPOCH Plus Rituximab for Previously Treated Patients With AIDS-Associated Lymphoma | Lymphoma, AIDS-Related | Etoposide (NPC185498) | |
| NCT00002461 | Combination Chemotherapy Followed by Bone Marrow or Peripheral Stem Cell Transplantation in Treating Patients With Refractory Hodgkin's Disease or Non-Hodgkin's Lymphoma | lymphoma | Etoposide (NPC185498) | |
| NCT00002471 | Combination Chemotherapy in Treating Patients With Acute B-Lymphoblastic Leukemia or Non-Hodgkin's Lymphoma | leukemia;lymphoma | Etoposide (NPC185498) | |
| NCT00002481 | Combination Chemotherapy and Radiation Therapy Plus Bone Marrow Transplantation in Treating Patients With Relapsed or Refractory Non-Hodgkin's Lymphoma | lymphoma | Etoposide (NPC185498) | |
| NCT00002488 | Combination Chemotherapy in Treating Patients With Intermediate-Grade or High-Grade Non-Hodgkin's Lymphoma Who Have Not Responded to Anthracycline-Containing Combination Chemotherapy | lymphoma | Etoposide (NPC185498) | |
| NCT00002494 | Combination Chemotherapy in Treating Patients With Non-Hodgkin's Lymphoma or Acute Lymphocytic Leukemia | leukemia;lymphoma | Etoposide (NPC185498) | |
| NCT00002509 | High-Dose Combination Chemotherapy Followed by Peripheral Stem Cell Transplantation in Treating Patients With Poor-Prognosis Breast Cancer | breast cancer | Etoposide (NPC185498) | |
| NCT00002510 | Chemotherapy and Radiation Therapy Followed by Peripheral Stem Cell Transplantation in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Etoposide (NPC185498) |
❱❱❱ Associated Human Diseases and Detailed Association Evidence
How do we define the Plant-Targeted Human Disease Association?
Associated human diseases of an individual plant are summurized based on FOUR types of association evidence, these include:
❶ Association by Therapeutic Target: Bioactive protein targets of the plant were defined in "Molecular Targets" section, target-disease associations collected from TTD database were subsequently used to build the associations between the plant and its targeted human diseases.
❷ Association by Disease Gene Reversion: Plant and a specific disease will be associated when >= 1 plant target gene overlaped with disease's DEGs.
❸ Association by Clinical Trials of Plant: Plant and a specific disease will be associated when >= 1 clinical trial (the plant is the intervetion) can be matched in ClinicalTrials.gov database.
❹ Association by Clinical Trials of Plant Ingredients: Plant and a specific disease will be associated when >= 1 clinical trial (the plant ingredient is the intervetion) can be matched in ClinicalTrials.gov database.
Associated Disease of the Plant | Association Type & Detailed Evidence |
|---|---|
Acne vulgarisDisease Category: 14.Diseases of the skinDisease ICD-11 Code: ED80 |
ESR1
|
Acquired prion diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8E01 |
ESR1
|
Acute basophilic leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60.37 |
NCT00006363,NCT00369317
|
Acute erythroid leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60.35 |
NCT00006363,NCT00003190,NCT00002798,NCT00369317
|
Acute megakaryoblastic leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60.36 |
NCT00369317,NCT00002798,NCT00006363,NCT00003190
|
Acute monocytic leukaemiaDisease Category: X.Extension CodesDisease ICD-11 Code: XH9NE2 |
NCT00006363,NCT00369317,NCT00002798,NCT00003190
|
Acute myeloid leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60 |
TOP2B
NCT00002798,NCT00151242,NCT00369317,NCT02421939,NCT02773732,NCT00186966,NCT00780104,NCT00512252,NCT01729845,NCT00003190,NCT03926624,NCT01027923,NCT01411267,NCT02638428,NCT03860844,NCT01025778,NCT03568994,NCT02626338,NCT00660036,NCT00880243,NCT03118466,NCT03182244,NCT00003190,NCT00906945,NCT03591510,NCT02070458,NCT00079482,NCT00151255,NCT00315705,NCT03504410,NCT00006363,NCT03164057,NCT00939653,NCT04326439,NCT01237808,NCT01681537,NCT03839446,NCT00703820,NCT00774046,NCT03793478,NCT02631252,NCT02349178,NCT02039726,NCT02306291,NCT02400281,NCT01677949,NCT04293562,NCT00136084,NCT01180322,NCT00146120,NCT02632708,NCT01830777,NCT02848183 |
Acute myelomonocytic leukaemiaDisease Category: X.Extension CodesDisease ICD-11 Code: XH78Y4 |
NCT00369317,NCT00002798,NCT00003190,NCT00006363
|
Adenocarcinoma of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.0 |
CASP3,NOS2
|
AdenocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D40 |
NCT01958372,NCT00020709,NCT00042835
|

