Collective Molecular Activities of the Plant: Rollinia Mucosa
Overview of Ingredients
8 All known Ingredients in Total
Unique ingredients have been isolated from this plant.Plant-Ingredients Associations were manually curated from publications or collected from other databases.
4 Ingredients with Acceptable Bioavailablity
Unique ingredients exhibit acceptable human oral bioavailablity, according to the criteria of SwissADME [PMID: 28256516] and HobPre [PMID: 34991690]. The criteria details:SwissADME: six descriptors are used by SwissADME to evaluate the oral bioavailability of a natural product:
☑ LIPO(Lipophility): -0.7 < XLOGP3 < +5.0
☑ SIZE: 150g/mol < MW < 500g/mol
☑ POLAR(Polarity): 20Ų < TPSA < 130Ų
☑ INSOLU(Insolubility): -6 < Log S (ESOL) < 0
☑ INSATU(Insaturation): 0.25 < Fraction Csp3 < 1
☑ FLEX(Flexibility): 0 < Num. rotatable bonds < 9
If 6 descriptors of a natural plant satisfy the above rules, it will be labeled high HOB.
HobPre: A natural plant ingredient with HobPre score >0.5 is labeled high human oral availability (HOB)
5 Ingredients with experimental-derived Activity
Unique ingredients have activity data available.Ingredient Structrual Cards
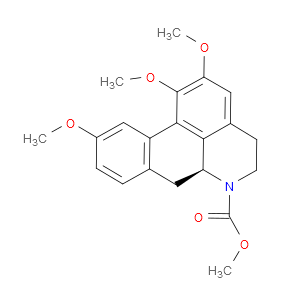
Ingredient ID: NPC96710
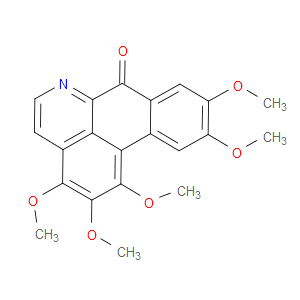
Ingredient ID: NPC90875
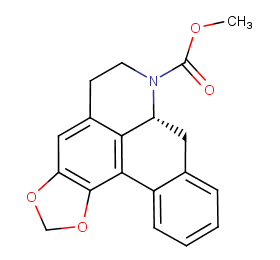
Ingredient ID: NPC74080
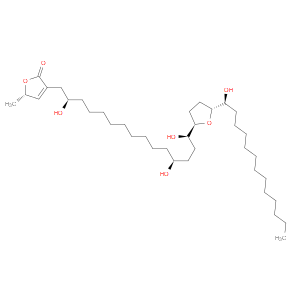
Ingredient ID: NPC485249
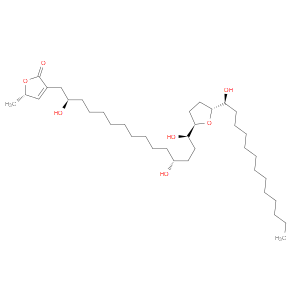
Ingredient ID: NPC485248
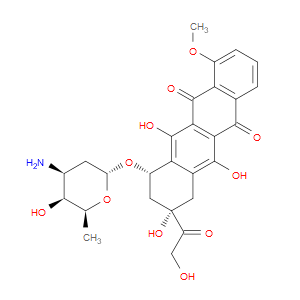
Ingredient ID: NPC261012
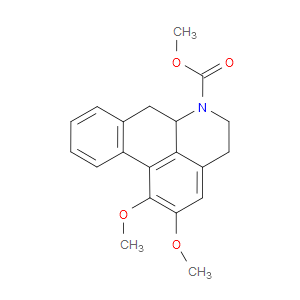
Ingredient ID: NPC17342
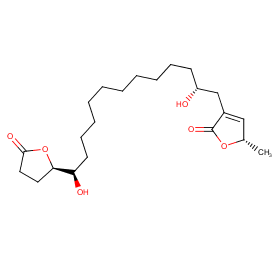
Ingredient ID: NPC112685
Classification of Human Proteins Collectively Targeted by the Plant
Detailed Information of Target Proteins
| Target Type | Protein Class | Gene ID | Protein Name | Uniprot ID | Target ChEMBL ID |
|---|---|---|---|---|---|
| Therapeutic Target | Isomerase | TOP2A | DNA topoisomerase II alpha | P11388 | CHEMBL1806 |
| Therapeutic Target | Protein Kinase | AURKA | Serine/threonine-protein kinase Aurora-A | O14965 | CHEMBL4722 |
Clinical trials associated with plant from natural product (NP) & plant level:
| Clinical trials type | Number of clinical trials | |
|---|---|---|
| 886 | ||
| NCT ID | Title | Condition | Form in clinical use | Associated by plant or compound |
|---|---|---|---|---|
| NCT02298283 | Brentuximab Vedotin as Consolidation Treatment in Patients With Stage I/II HL and PET Positivity After 2 Cycles of ABVD | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01468740 | Prospective Study on HIV-related Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT05177796 | Panitumumab and Pembrolizumab in Combination With Neoadjuvant Chemotherapy for the Treatment of Stage III-IV Triple Negative Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT02505269 | Brentuximab Vedotin Plus AD in Non-bulky Limited Stage Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04968106 | Neoadjuvant Chemotherapy and Retifanlimab in Patients With Selected Sarcomas (TORNADO) | sarcoma | Doxorubicin (NPC261012) | |
| NCT00346229 | Temperature-Sensitive Liposomal Doxorubicin and Hyperthermia in Treating Women With Locally Recurrent Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00191789 | Neoadjuvant Sequential Administration of Two Gemcitabine Combinations in Operable Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02181738 | Study of Nivolumab in Patients With Classical Hodgkin's Lymphoma (Registrational) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00634179 | A Phase I/II Trial of VR-CHOP in Lymphoma Patients | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT03817320 | PO Ixazomib in Combination With Chemotherapy for Childhood Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00976911 | AURELIA: A Study of Avastin (Bevacizumab) Added to Chemotherapy in Patients With Platinum-resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT05389423 | Pomalidomide and Dose-Adjusted EPOCH +/- Rituximab for HIV-Associated Lymphomas | Burkitts lymphoma;diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01396655 | PET in Breast Cancer Receiving Neoadjuvant Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT03598270 | Platinum-based Chemotherapy With Atezolizumab and Niraparib in Patients With Recurrent Ovarian Cancer | ovarian carcinoma | Doxorubicin (NPC261012) | |
| NCT01100944 | A Phase 1/2 Study of PXD101 (Belinostat) in Combination With Cisplatin, Doxorubicin and Cyclophosphamide in the First Line Treatment of Advanced or Recurrent Thymic, Malignancies | Thymic Carcinoma;Thymoma | Doxorubicin (NPC261012) | |
| NCT01004991 | Phase I/II Trial of R-CHOP + Azacytidine in Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00973752 | Treatment of Older Adults With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00038142 | Vincristine, Doxorubicin, Cyclophosphamide and Dexrazoxane (VACdxr) in High Risk Ewing's Sarcoma Patients | Ewing sarcoma | Doxorubicin (NPC261012) | |
| NCT04043494 | International Cooperative Treatment Protocol for Children and Adolescents With Lymphoblastic Lymphoma | lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00131027 | High-Dose Methotrexate (MTX) for Adult Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00499018 | Dose Dense Chemotherapy + Rituximab +/-Intensified High Dose Chemoimmunotherapy With Support of Peripheral Autologous Stem Cell in Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05270057 | Loncastuximab Tesirine in Combination With DA-EPOCH-R in Patients With Previously Untreated Aggressive B-cell Lymphoid Malignancies | Burkitts lymphoma | Doxorubicin (NPC261012) | |
| NCT01270373 | NeoSAMBA: Neoadjuvant: Does the Sequence of Anthracycline and Taxane Matters: Before or After? | breast cancer | Doxorubicin (NPC261012) | |
| NCT00691912 | Therapy of Metastatic Breast Cancer With Paclitaxel and Liposomal Doxorubicin | breast cancer | Doxorubicin (NPC261012) | |
| NCT03991884 | Inotuzumab Ozogamicin and Chemotherapy in Treating Patients With Recurrent or Refractory B-cell Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT03564704 | Precision Diagnosis Directing HDACi Chidamide Target Therapy for Adult T-LBL/ALL | lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT01847001 | Study of Propranolol in Newly Diagnosed Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT01779050 | Evaluation of an Anti-cancer Immunotherapy Combined With Standard Neoadjuvant Treatment in Patients With WT1-positive Primary Invasive Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00299182 | Study of AMG 531 to Evaluate the Safety & Efficacy in Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00179725 | Phase I/II Open-Label, Dose Escalation Study To Determine The Maximum Tolerated Dose And To Evaluate The Safety Profile of Lenalidomide (Revlimid® CC-5013) With Liposomal Doxorubicin In Subjects With Advanced Ovarian and Primary Peritoneal Carcinoma | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00030381 | Iododoxorubicin in Treating Patients With Primary Systemic Amyloidosis | primary systemic amyloidosis | Doxorubicin (NPC261012) | |
| NCT01210768 | A Study of Pegylated Liposomal Doxorubicin and Cyclophosphamide in Her2-negative Stage I and II Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT05428670 | The Efficacy and Safety of ZR2 Versus R-CHOP-like Regimen for Elderly Patients With Newly Diagnosed Diffuse Large B Cell Lymphoma. | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02890602 | Erythropoietin for Management of Anemia Caused by Chemotherapy | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00093444 | Heat Activated Liposomal Doxorubicin and Radiofrequency Ablation in Treating Patients With Primary or Metastatic Liver Tumors | liver cancer | Doxorubicin (NPC261012) | |
| NCT03699449 | An uMbrella Study of BIomarker-driven Targeted Therapy In Patients With Platinum-resistant Recurrent OvariaN Cancer(AMBITION) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03725059 | Study of Pembrolizumab (MK-3475) Versus Placebo in Combination With Neoadjuvant Chemotherapy & Adjuvant Endocrine Therapy in the Treatment of Early-Stage Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative (ER+/HER2-) Breast Cancer (MK-3475-756/KEYNOTE-756) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00795613 | Positron Emission Tomography (PET)-Adapted Chemotherapy In Advanced Hodgkin Lymphoma (HL) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02659930 | Pomalidomide in Combination With Liposomal Doxorubicin in People With Advanced or Refractory Kaposi Sarcoma | Kaposi's sarcoma | Doxorubicin (NPC261012) | |
| NCT04002947 | Acalabrutinib With DA-EPOCH-R or R-CHOP for People With Untreated Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01855750 | A Study of the Bruton's Tyrosine Kinase Inhibitor, PCI-32765 (Ibrutinib), in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Non-Germinal Center B-Cell Subtype of Diffuse Large B-Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02784015 | Response Based Treatment for Children With Unresectable Localized Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01804127 | Interim PET/CT Guided Cycle Numbers of R-CHOP in DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01056679 | Adriamycin, Vinblastine, DTIC and Revlimid in Elderly Hodgkin Lymphoma Patients | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02428751 | R-CHOP Versus R-CDOP as First-line Treatment for Elderly Patients With Diffuse Large-B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00577629 | Chemotherapy With Monoclonal Antibody and Radioimmunotherapy for High-Risk B-Cell Non-Hodgkins Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03150693 | Inotuzumab Ozogamicin and Frontline Chemotherapy in Treating Young Adults With Newly Diagnosed B Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01451515 | NHL16: Study For Newly Diagnosed Patients With Acute Lymphoblastic Lymphoma | lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT04021992 | GVD±R Regimen for ASCT-eligible Patients With Refractory/Relapsed DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00516191 | A Phase I/II Study of Liposomal Doxorubicin (Doxil)/Melphalan/Bortezomib (Velcade) in Relapsed/Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT05422066 | Selinexor Plus R-CHOP in High-risk GCB-subtype Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01514188 | Preliminary Efficacy and Safety of INNO-206 Compared to Doxorubicin in Advanced Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02419755 | Bortezomib and Vorinostat in Younger Patients With Refractory or Relapsed MLL Rearranged Hematologic Malignancies | acute leukemia of ambiguous lineage;acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00719472 | A Study of Rituximab Alternative Dosing Rate in Patients With Previously Untreated Diffuse Large B-cell or Follicular Non-Hodgkin's Lymphoma (RATE) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02876302 | Study Of Ruxolitinib (INCB018424) With Preoperative Chemotherapy For Triple Negative Inflammatory Breast Cancer | inflammatory breast carcinoma | Doxorubicin (NPC261012) | |
| NCT00140595 | ACVBP Plus Rituximab Versus CHOP Plus Rituximab in Patients With Diffuse Large B-cell Lymphoma and Age-adjusted IPI of 1 | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02951728 | Decitabine Plus R-CHOP in Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00002557 | Combination Chemotherapy in Patients With Advanced or Recurrent Mycosis Fungoides | lymphoma | Doxorubicin (NPC261012) | |
| NCT05467670 | Safety and Efficacy of Anti-CD47, ALX148 in Combination With Liposomal Doxorubicin and Pembrolizumab in Recurrent Platinum-resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00617591 | Pegylated Liposomal Doxorubicin, Low Freq Dexamethasone & Revlimid (Dd-R) in Newly Diagnosed Multiple Myeloma (MM) | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT03467178 | Study on Decitabine Plus Carboplatin Versus Physician's Choice Chemotherapy in Recurrent, Platinum-resistant Ovarian Cancer. | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00563953 | Caelyx as Primary Treatment for Patients With Breast Cancer and a History of Heart Disease and/or Age Over 65 Years | breast cancer | Doxorubicin (NPC261012) | |
| NCT00365365 | Safety & Efficacy of Three Docetaxel-Based Chemotherapy Regimens Plus Bevacizumab With or Without Trastuzumab for Adjuvant Treatment of Patients With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00072007 | Cladribine and Rituximab as Remission Induction Therapy Followed By Rituximab and Stem Cell Mobilization in Treating Patients With CLL | leukemia | Doxorubicin (NPC261012) | |
| NCT00861120 | Panitumumab and Pegylated Liposomal Doxorubicin for Platinum-Resistant Epithelial Ovarian Cancer With KRAS Wild-type | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00554164 | Positron Emission Tomography Guided Therapy of Aggressive Non-Hodgkin's Lymphomas | lymphoma | Doxorubicin (NPC261012) | |
| NCT01328236 | Bortezomib in Combination With Liposomal Doxorubicin and Dexamethasone to Treat Plasma Cell Leukemia | plasma cell leukemia;multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01177683 | Bendamustine in Combination With Bortezomib and Pegylated Liposomal Doxorubicin for Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT03023046 | Etoposide, Prednisone, Vincristine Sulfate, Cyclophosphamide, and Doxorubicin in Treating Patients With Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00003578 | High Dose Chemotherapy With or Without Bone Marrow Transplantation in Treating Patients With Intermediate- or High-Grade Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003907 | Chemoembolization in Treating Patients With Primary Liver Cancer or Metastases to the Liver | liver cancer | Doxorubicin (NPC261012) | |
| NCT03042819 | Study of Selinexor and Doxorubicin in Advanced Soft Tissue Sarcomas | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT04874311 | Bintrafusp Alfa and Doxorubicin Hydrochloride in Treating Patients With Advanced Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT03159897 | FIL Study on ABVD DD-DI as Upfront Therapy in HL. | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02733380 | Chidamide Combined With VDDT Regimen in the Relapse and Refractory Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00189137 | Evaluation of Side Effects and Relative Activity of Two Chemotherapy Regimens in the Treatment Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT03384654 | A Study to Evaluate the Efficacy and Safety of Daratumumab in Pediatric and Young Adult Participants Greater Than or Equal to (>=)1 and Less Than or Equal to (<=) 30 Years of Age With Relapsed/Refractory Precursor B-cell or T-cell Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00193115 | Docetaxel Followed by Doxorubicin Plus Cyclophosphamide for Node Positive or High-Risk Primary Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03618550 | Phase II Study of Second- Line Pembrolizumab Plus GVD for Relapsed or Refractory Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02275598 | Brentuximab Vedotin Followed by ABVD in Patients With Previously Untreated Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02771743 | Response-based Treatment of High-risk Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT02641847 | TA(E)C-GP Versus A(E)C-T for the High Risk TNBC Patients and Validation of the mRNA-lncRNA Signature | breast cancer | Doxorubicin (NPC261012) | |
| NCT01240629 | Doxorubicin-GnRH Agonist Conjugate AEZS-108 in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer | prostate cancer | Doxorubicin (NPC261012) | |
| NCT03975205 | To Study the Concentration Level of, Doxil, and Doxorubicin at Various Time Frames | leukemia;lymphoma | Doxorubicin (NPC261012) | |
| NCT00574080 | UARK 2006-15: A Study of Tandem Transplants With or Without Bortezomib and Thalidomide | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00479128 | Bortezomib With Gemcitabine/Doxorubicin in Patients With Urothelial Cancer and Other Solid Tumors | urethra cancer | Doxorubicin (NPC261012) | |
| NCT04012827 | Apatinib Mesylate Combined With Doxorubicin and Ifosfamide in Advanced Soft-tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00504504 | Rituximab and ABVD for Hodgkin's Patients | lymphoma | Doxorubicin (NPC261012) | |
| NCT03527628 | OPTmizing Advanced Stage HodgkIn LymphoMa patIentS Therapy | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT01004978 | Chemoembolization With or Without Sorafenib Tosylate in Treating Patients With Liver Cancer That Cannot Be Removed by Surgery | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT01116635 | Clinical Study Examining the Safety and Efficacy of Doxorubicin Drug Eluting Microspheres Transarterial Embolization in the Setting of Hepatocellular Carcinoma (HCC) | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00968253 | RAD001 Study in Treatment of Relapsed or Refractory Acute Lymphocytic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT02732015 | Rolapitant Hydrochloride in Preventing Nausea/Vomiting in Patients With Sarcoma Receiving Chemotherapy | sarcoma | Doxorubicin (NPC261012) | |
| NCT02506777 | Neoadjuvant FDC With Melatonin or Metformin for Locally Advanced Breast Cancer. | breast cancer | Doxorubicin (NPC261012) | |
| NCT01070862 | Multiple Myeloma Treated With Thalidomide Before Autotransplant or With Conventional Chemotherapy and as Consolidation/Maintenance Treatment in Young and Elderly Patients : 3 Randomized Studies. | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT04625907 | FaR-RMS: An Overarching Study for Children and Adults With Frontline and Relapsed RhabdoMyoSarcoma | rhabdomyosarcoma | Doxorubicin (NPC261012) | |
| NCT04566887 | Acalabrutinib With R-CHOP in Previously Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00336791 | Randomized Clinical Trial to Evaluate the Predictive Accuracy of a Gene Expression for Stage I-II Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05290090 | ZR2 Followed by Immunochemotherapy in Elderly Patients With Newly-diagnosed DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01246063 | Carfilzomib, Pegylated Liposomal Doxorubicin Hydrochloride, and Dexamethasone in Treating Patients With Relapsed or Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00546156 | Preoperative Dose-dense Doxorubicin and Cyclophosphamide Followed by Paclitaxel With Bevacizumab in Operable Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02566993 | Clinical Trial of Lurbinectedin (PM01183)/Doxorubicin Versus CAV or Topotecan as Treatment in Patients With Small-Cell Lung Cancer | small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT00193440 | Rituximab and Chemotherapy Followed by Ibritumomab Tiuxetan as Treatment for Low Grade Follicular Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03542669 | Study of 6b11-OCIK Injection Treatment in Patients With Recurrent Drug-resistant Ovarian Cancer | ovarian carcinoma | Doxorubicin (NPC261012) | |
| NCT02449278 | The Palliative Benefit of Involved-site Radiotherapy for Patients With Advanced-stage Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00064116 | Combination Chemotherapy With or Without Rituximab in Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01920932 | Adcetris (Brentuximab Vedotin), Combination Chemotherapy, and Radiation Therapy in Treating Younger Patients With Stage IIB, IIIB and IV Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01121406 | BI 6727 (Volasertib) Randomised Trial in Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT02531308 | Metformin in Combination With Standard Induction Therapy for Large B-cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00608803 | Phase 1 Study of ZIO-201-T in Combination With Doxorubicin in Solid Tumors | cancer | Doxorubicin (NPC261012) | |
| NCT02406092 | Safety Study of Rituximab (SC) Administered in Participants With CD20+ DLBCL or CD20+ Follicular NHL Grade 1 to 3A | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00657878 | Efficacy Study of Chemotherapy to Treat Ovarian Cancer Recurrence by Prolonging the Platinum Free Interval | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00068393 | Doxorubicin and Gemcitabine in Treating Patients With Locally Recurrent or Metastatic Unresectable Renal Cell Carcinoma | renal cell carcinoma | Doxorubicin (NPC261012) | |
| NCT00907348 | Prospective Multicenter Dose Finding Phase II Pilot Trial to Evaluate Efficacy and Safety of LR-CHOP21 for Elderly Patients With Untreated Diffuse Large B Cell Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT01358071 | Phase II Study of NGR-hTNF in Combination With Doxorubicin in Platinum-resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT05498259 | Orelabrutinib With R-CHOP-like Regimen for Patients With Newly Diagnosed Untreated Non-GCB DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00883116 | A Study of Ixabepilone as Second-line Therapy for Locally Advanced, Recurrent, or Metastatic Endometrial Cancer | endometrial cancer | Doxorubicin (NPC261012) | |
| NCT00770224 | S0801 Iodine I 131 Tositumomab, Rituximab, and Combination Chemotherapy in Previously Untreated Stage II, Stage III, or Stage IV Follicular Non-Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003782 | Combination Chemotherapy in Treating Women With Stage I, Stage II, or Stage IIIA (cT1-3, N0-1, M0) Breast Cancer and Positive Axillary Lymph Nodes | breast cancer | Doxorubicin (NPC261012) | |
| NCT04199026 | Implantable Microdevice for the Delivery of Drugs and Their Effect on Tumors in Patients With Metastatic or Recurrent Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT05318794 | Neoadjuvant Systemic and Peritoneal Chemotherapy for Advanced Gastric Cancer | gastric cancer | Doxorubicin (NPC261012) | |
| NCT01275677 | Chemotherapy With or Without Trastuzumab After Surgery in Treating Women With Invasive Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT00470301 | Tipifarnib and Combination Chemotherapy in Treating Patients With Stage II or Stage III Breast Cancer | male breast carcinoma | Doxorubicin (NPC261012) | |
| NCT02850419 | Heat-Activated Target Therapy of Local-Regional Relapse in Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT03517449 | Lenvatinib in Combination With Pembrolizumab Versus Treatment of Physician's Choice in Participants With Advanced Endometrial Cancer (MK-3475-775/E7080-G000-309 Per Merck Standard Convention [KEYNOTE-775]) | endometrial neoplasm | Doxorubicin (NPC261012) | |
| NCT02098343 | p53 Suppressor Activation in Recurrent High Grade Serous Ovarian Cancer, a Phase Ib/II Study of Systemic Carboplatin Combination Chemotherapy With or Without APR-246 | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02427620 | Ibrutinib, Rituximab, and Consolidation Chemotherapy in Treating Young Patients With Newly Diagnosed Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00312208 | Docetaxel in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00461344 | Docetaxel + Doxorubicin as Neoadjuvant Chemotherapy in Patients With Breast Cancer | breast ductal carcinoma in situ | Doxorubicin (NPC261012) | |
| NCT02475772 | A Study With Intraperitoneal Cisplatin and Doxorubicin in Recurrent Ovarian Cancer and Peritoneal Carcinomatosis | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00148681 | Preoperative Herceptin and Navelbine for Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00443677 | Treatment of Advanced Hodgkin's Disease (Stages IIB-III-IV) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03108300 | Use of Propranolol Hydrochloride in the Treatment of Metastatic STS | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02995772 | Neoadjuvant Hormonal Therapy Compared to Neoadjuvant Chemotherapy in Stage IIIB/C and IV Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT04852367 | PanDox: Targeted Doxorubicin in Pancreatic Tumours | pancreatic ductal adenocarcinoma | Doxorubicin (NPC261012) | |
| NCT01394354 | Vorinostat in Combination With Bortezomib, Doxorubicin and Dexamethasone (VBDD) in Patients With Refractory or Relapsed Multiple Myeloma (MM) | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01009801 | Transarterial Chemoembolization With Doxorubicin With or Without Everolimus in Treating Patients With Liver Cancer | liver cancer | Doxorubicin (NPC261012) | |
| NCT05371093 | Study of Axicabtagene Ciloleucel Versus Standard of Care Therapy in Participants With Relapsed/Refractory Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT01172223 | Primary Chemotherapy in Patients With HER2-positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04332822 | A Randomized, Multicenter, Phase III Trial Comparing Treatment With R-mini-CHOP With R-mini-CHP + Polatuzumab Vedotin in Patients With Diffuse Large Cell B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04776525 | Sequential Neoadjuvant Chemotherapy in Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00944047 | Evaluate Trastuzumab Plus Standard Chemotherapy Given Before Surgery in Breast Cancer Patients With Low HER 2 Expression | breast cancer | Doxorubicin (NPC261012) | |
| NCT00149214 | Preoperative Treatment of Breast Cancer With Two Different Sequential Treatment Regimens | breast cancer | Doxorubicin (NPC261012) | |
| NCT01724021 | A Study of Participant Preference With Subcutaneous Versus Intravenous MabThera/Rituxan in Participants With CD20+ Diffuse Large B-Cell Lymphoma or CD20+ Follicular Non-Hodgkin's Lymphoma Grades 1, 2 or 3a | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00822120 | S0816 Fludeoxyglucose F 18-PET/CT Imaging and Combination Chemotherapy With or Without Additional Chemotherapy and G-CSF in Treating Patients With Stage III or Stage IV Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01622439 | Valproate as First Line Therapy in Combination With Rituximab and CHOP in Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01060904 | A Phase 1 Study of Brentuximab Vedotin Combined With Multi-Agent Chemotherapy for Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00303108 | Doxil & Carboplatin Plus HER2+ in Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00809341 | R-ICE and High-Dose Cyclophosphamide With PET/CT for Diffuse Large B-Cell Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01287741 | A Study of Obinutuzumab in Combination With CHOP Chemotherapy Versus Rituximab With CHOP in Participants With CD20-Positive Diffuse Large B-Cell Lymphoma (GOYA) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01078441 | Bortezomib, Liposomal Doxorubicin Hydrochloride, Dexamethasone, and Cyclophosphamide in Treating Patients With Multiple Myeloma That Relapsed After Autologous Stem Cell Transplant | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT04047004 | Adjuvant PIPAC in Gastric Cancer Patients | gastric adenocarcinoma | Doxorubicin (NPC261012) | |
| NCT01541332 | Pomalidomide, Dexamethasone and Pegylated Liposomal Doxorubicin for Relapsed/Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00774826 | Multicentric Study, Three Randomized Arms (R-CVP vs R-CHOP vs R-FM),for Patients With Stage II-IV Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT04172259 | ACH-TH vs EC-TH as Neoadjuvant Therapy for HER2-positive EBC | breast cancer | Doxorubicin (NPC261012) | |
| NCT03553537 | Efficacy and Safety of Decitabine Plus CHOP vs CHOP in Patients With Untreated Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00613457 | Combination Chemotherapy Based on Risk of Relapse in Treating Young Patients With Acute Lymphoblastic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT01139359 | Safety Study of Darinaparsin in Combination With Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) to Treat Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00869232 | UARK 2008-02 A Trial for High-risk Myeloma Evaluating Accelerating and Sustaining Complete Remission | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT05189197 | A Study to Evaluate Efficacy and Safety of Zanubrutinib With R-CHOP in Newly Diagnosed Non-GCB DLBCL Patients With Double Expression | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01215344 | First Autologous Transplant on Minimal Residual Disease Markers in Previously Untreated Myeloma Undergoing Initial Treatment With Velcade | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00038610 | Study of Hyper-CVAD Plus Imatinib Mesylate for Philadelphia-Positive Acute Lymphocytic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT01569204 | Targeted BEACOPP Variants in Patients With Newly Diagnosed Advanced Classical Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02055820 | A Study Evaluating the Safety, Efficacy and Pharmacokinetics of Venetoclax Combined With Chemotherapy in Participants With B-Cell Non-Hodgkin's Lymphoma (NHL) and DLBCL | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01206881 | Neoadjuvant Pegylated Liposomal Doxorubicin and Cyclophosphamide +/- Trastuzumab Followed by Docetaxel in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04824092 | Tafasitamab + Lenalidomide + R-CHOP Versus R-CHOP in Newly Diagnosed High-intermediate and High Risk DLBCL Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00814541 | PAD. ICORG 05-01, V11 | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01381211 | Transarterial RAdioembolization Versus ChemoEmbolization for the Treatment of Hepatocellular Carcinoma (HCC) | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00002707 | Chemotherapy in Treating Women With Breast Cancer That Can Be Surgically Removed | breast cancer | Doxorubicin (NPC261012) | |
| NCT03571321 | Ruxolitinib and Chemotherapy in Adolescents and Young Adults With Ph-like Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00790244 | A Scandinavian Sarcoma Group Protocol for Patients With High-risk Soft Tissue Sarcoma of the Extremities and Trunk Wall | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00003421 | Combination Chemotherapy in Treating Patients With Advanced Hodgkin's Disease | lymphoma | Doxorubicin (NPC261012) | |
| NCT04443348 | Pre-op Pembro + Radiation Therapy in Breast Cancer (P-RAD) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00859495 | Trimodal Lung-Sparing Treatment of Pleural Mesothelioma | mesothelioma | Doxorubicin (NPC261012) | |
| NCT01852435 | R-CEOP-90/R-CEOP-70 Versus R-CHOP-50 in the Treatment of Diffuse Large B-cell Lymphoma and Follicular Lymphoma Grade 3B | diffuse large B-cell lymphoma;follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT00087178 | Comparison of Two Combination Chemotherapy Regimens in Treating Women With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00797472 | Study Comparing R-mabHD and a Combination of ABVD in Hodgkin's Disease | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04383743 | Pembrolizumab and Combination Chemotherapy Before Surgery for the Treatment of Muscle-Invasive Bladder Cancer | urinary bladder carcinoma | Doxorubicin (NPC261012) | |
| NCT00005867 | Combination Chemotherapy in Treating Patients With Aggressive Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00789581 | A Randomized Trial of Ixempra Versus Taxol in Adjuvant Therapy of Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01925612 | Study of Brentuximab Vedotin Combined With RCHOP or RCHP in Front-line Treatment of Patients With Diffuse Large B-cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04101812 | Pegylated Liposomal Doxorubicin, PD-1 in Treating Muscle Invasive Bladder Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT00108953 | A Research Study to Treat Patients With Advanced Hepatocellular Carcinoma | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00121992 | Docetaxel, Doxorubicin (A), Cyclophosphamide (C) (TAC) vs 5-Fluorouracil, A, C (5FAC) Breast Cancer Adjuvant Treatment | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00093795 | Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00136539 | Neoadjuvant Therapy With Herceptin and Taxol for Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01324076 | Transarterial Chemoembolization Using Doxorubicin Beads With or Without Sorafenib Tosylate in Treating Patients With Liver Cancer That Cannot Be Removed By Surgery | liver cancer | Doxorubicin (NPC261012) | |
| NCT01966133 | TACE as an Adjuvant Therapy After Hepatectomy for HCC | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT01168791 | Study of Palifosfamide-tris in Combination With Doxorubicin in Patients With Front-line Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT04301739 | to Evaluate Efficacy and Safety of HLX10 in Combination With Chemotherapy Versus Placebo in Combination With Chemotherapy as Neoadjuvant Therapy and HLX10 Versus Placebo as Adjuvant Therapy in Patients With Triple Negative Breast Cancer (TNBC) | breast cancer | Doxorubicin (NPC261012) | |
| NCT04835870 | Zanubrutinib Plus R-CHOP for Patients With Newly Diagnosed Untreated Non-GCB DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00324467 | Tailoring Treatment for B Cell Non-hodgkin's Lymphoma Based on PET Scan Results Mid Treatment | neoplasm of mature B-cells;non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03805022 | Benefit of Intensified Peri-operative Chemotherapy Within High-risk CINSARC Patients With Resectable Soft-tissue Sarcomas | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00124956 | Doxorubicin Pharmacokinetic (PK) Study | cancer | Doxorubicin (NPC261012) | |
| NCT00493870 | TAC Versus TC for Adjuvant Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00886028 | Palliative Treatment With Liposomal Doxorubicin Plus Cisplatin for Patients With Malignant Pleural Mesothelioma | malignant pleural mesothelioma | Doxorubicin (NPC261012) | |
| NCT02787239 | Clinical Study to Compare the Efficacy and Safety of Rituximab Biosimilar HLX01 and Rituximab in Combination With CHOP, in Previously Untreated Subjects With CD20+ DLBCL | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT00268853 | A Trial in Patients With Diffuse Large-B-cell Lymphoma Comparing Pixantrone Against Doxorubicin | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03669783 | Clinical Trial for Patients With a Stage IV Childhood Renal Tumor, Comparing Upfront Vincristine, Actinomycin-D and Doxorubicin (Standard Arm) With Upfront Vincristine, Carboplatin and Etoposide (Experimental Arm) | childhood kidney neoplasm | Doxorubicin (NPC261012) | |
| NCT02420717 | Ruxolitinib Phosphate or Dasatinib With Chemotherapy in Treating Patients With Relapsed or Refractory Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT04139304 | A Study of Daratumumab and Dose-Adjusted EPOCH in Plasmablastic Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01272557 | Sorafenib Plus Doxorubicin Versus Sorafenib Alone for the Treatment of Advanced Hepatocellular Carcinoma: a Randomized Phase II Trial | carcinoma of liver and intrahepatic biliary tract | Doxorubicin (NPC261012) | |
| NCT04650984 | A Study Comparing the Efficacy of L19TNF+Doxorubicin vs Doxorubicin Alone as First-line Therapy in Patients With Advanced or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00003389 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00600977 | Liposomal Anthracyclin in the Treatment of Elderly ALL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00433433 | Fludeoxyglucose F 18 PET Scan-Guided Therapy or Standard Therapy in Treating Patients With Previously Untreated Stage I or Stage II Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01992653 | A Study of Polatuzumab Vedotin in Combination With Rituximab or Obinutuzumab, Cyclophosphamide, Doxorubicin, and Prednisone in Participants With B-Cell Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00201318 | A Randomized Study in Non-Hodgkin's Lymphoma Patients Carrying Hepatitis B Surface Antigen | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00003150 | Combination Chemotherapy With or Without Monoclonal Antibody Therapy in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003896 | S9912 Combination Chemo in Stage III Ovarian Cancer, | fallopian tube cancer;ovarian cancer;peritoneum cancer | Doxorubicin (NPC261012) | |
| NCT03033914 | A(B)VD Followed by Nivolumab as Frontline Therapy for Higher Risk Patients With Classical Hodgkin Lymphoma (HL) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01848132 | Efficacy/Safety Study of R-CHOP vs Bortezomib-R-CAP for Young Patients With Diffuse Large B-cell Lymphoma With Poor IPI. | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01672671 | BRCA1-associated DNA Repair Dysfunction in Patients With Early Triple Negative Breast Cancer Treated With Neoadjuvant Platinum-based Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT03758989 | A Study of PET Adapted Therapy and Non-invasive Monitoring for Previously Untreated Limited Stage Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01490047 | Single Dose of Intravenous rhTNF-α and Liposomal Doxorubicin in Patients With Advanced Solid Tumors or Lymphomas | lymphoma | Doxorubicin (NPC261012) | |
| NCT03101748 | Neratinib and Paclitaxel With or Without Pertuzumab and Trastuzumab Before Combination Chemotherapy in Treating Patients With Metastatic or Locally Advanced Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT00659178 | Combination Study Of SB-485232 (Interleukin 18) And Doxil For Advanced Stage Epithelial Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT01882816 | Intensity-Modulated Radiation Therapy (IMRT) in the Treatment of Non-Anaplastic Non-Medullary Thyroid Cancer | thyroid cancer | Doxorubicin (NPC261012) | |
| NCT00250874 | Myocet, Docetaxel & Trastuzumab as 1st Line Treatment of Patients With HER-2/Neu Positive Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03650933 | A Study Comparing the Efficacy and Safety of G-CHOP Versus R-CHOP in Untreated Diffuse Large B-cell Lymphoma Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03639246 | Efficacy and Safety Study of AVB-S6-500 in Patients With Platinum-Resistant Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01110603 | A Study of MK-4827 in Combination With Standard Chemotherapy in Participants With Advanced Solid Tumors (MK-4827-008 AM1) | cancer | Doxorubicin (NPC261012) | |
| NCT01309789 | A Phase 1 Study of Brentuximab Vedotin Given Sequentially and Combined With Multi-Agent Chemotherapy for CD30-Positive Mature T-Cell and NK-Cell Neoplasms | anaplastic large cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01100372 | Liposome-Encapsulated Doxorubicin Citrate With or Without Gemcitabine Hydrochloride in Treating Patients With Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cavity Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Doxorubicin (NPC261012) | |
| NCT01414855 | A Study of Obinutuzumab [RO5072759 (GA101)] in Combination With CHOP Chemotherapy in Patients With Previously Untreated Advanced Diffuse Large B-Cell Lymphoma (GATHER) | lymphoma | Doxorubicin (NPC261012) | |
| NCT01251107 | Study Comparing ABVD vs BEACOPP in Advanced Hodgkin's Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03933319 | Phase Ⅱ Study of Pegylated Liposomal Doxorubicin(PLD)Plus Trastuzumab in HER-2 Positive Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05051891 | A Randomized, Open-label, Multi-center, Phase III Study of Orelabrutinib in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) vs. R-CHOP Alone in Patients With Treatment-naїve Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04729387 | Alpelisib Plus Olaparib in Platinum-resistant/Refractory, High-grade Serous Ovarian Cancer, With no Germline BRCA Mutation Detected | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02685657 | Neoadjuvant Chemotherapy Docetaxel With or Without SELUMETINIB in Patients With Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02107378 | Efficacy of DCVAC/OvCa Plus Standard of Care in Relapsed Platinum Resistant Epithelial Ovarian Carcinoma | ovarian carcinoma | Doxorubicin (NPC261012) | |
| NCT02911142 | Lenalidomide Combined With Modified DA-EPOCH and Rituximab (EPOCH-R2) in Primary Effusion Lymphoma or KSHV-associated Large Cell Lymphoma | B-cell neoplasm | Doxorubicin (NPC261012) | |
| NCT04656262 | Low Dose Continuous Cyclophosphamide vs Standard Doxorubicin in Advanced Sarcoma Elderly Patients | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01516567 | Intergroup Trial for Children or Adolescents With Primary Mediastinal Large B-Cell Lymphoma: DA-EPOCH-Rituximab Evaluation | lymphoma | Doxorubicin (NPC261012) | |
| NCT00401635 | END-1: First Line Chemotherapy for Advanced or Recurrent Endometrial Carcinoma With Carboplatin and Liposomal Doxorubicin | endometrial cancer | Doxorubicin (NPC261012) | |
| NCT04348032 | Apatinib Combined With PLD vs PLD for Platinum-resistant Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00133302 | Study of Standard CHOP Versus Biweekly CHOP in Aggressive Non-Hodgkin's Lymphoma (JCOG9809) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04980222 | A Study to Evaluate the Safety and Efficacy of Glofitamab in Combination With Rituximab (R) Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) in Circulating Tumor (ct)DNA High-Risk Patients With Untreated Diffuse Large B-Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01008150 | Phase II Randomized Trial Evaluating Neoadjuvant Therapy With Neratinib and/or Trastuzumab Followed by Postoperative Trastuzumab in Women With Locally Advanced HER2-positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03003520 | A Study of Durvalumab in Combination With R-CHOP or Lenalidomide Plus R-CHOP in Previously Untreated High-Risk Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04790903 | A Study Evaluating the Safety, Efficacy and Pharmacokinetics of Venetoclax in Combination With Polatuzumab Vedotin Plus Rituximab (R) and Cyclophosphamide, Doxorubicin, Prednisone (CHP) in Participants With Untreated BCL-2 Immunohistochemistry (IHC)-Positive Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00319865 | PAD Combination Therapy Followed by Thal/Dex for Relapsed or Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00312650 | Doxil and Gemcitabine in Recurrent Ovarian Cancer | ovarian carcinoma | Doxorubicin (NPC261012) | |
| NCT00401817 | Bevacizumab + CHOP-Rituximab in Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04762901 | LCI-BRE-MTN-NIR-001:Ph I Study of Niraparib in Combo With Standard Chemo in Metastatic Trip Neg Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00582725 | R-CHOP + GM-CSF for Previously Untreated LCL in Elderly | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03804866 | NGR-hTNF in Combination With an Anthracycline in Platinum-resistant Ovarian Cancer (NGR018) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01959490 | Trastuzumab and Pertuzumab or Bevacizumab With Combination Chemotherapy in Treating Patients With Stage II-III Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00431795 | Randomized Phase II Study of Epirubicin vs Caelyx in Pretreated Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01746238 | Bevacizumab/Doxorubicin/Radiation for Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT05020860 | Correlation of Clinical Response to Pathologic Response in Patients With Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03678883 | 9-ING-41 in Patients With Advanced Cancers | lung neoplasm;Malignant Bone Neoplasm;malignant glioma;metastasis;kidney cancer;sarcoma | Doxorubicin (NPC261012) | |
| NCT00626704 | Phase 1b/2 Study of AMG 655 With Doxorubicin for the First-Line Treatment of Unresectable Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00956930 | Chemoembolization Versus Radioembolization in Treating Patients With Liver Cancer That Cannot Be Treated With Radiofrequency Ablation Or Surgery | liver cancer | Doxorubicin (NPC261012) | |
| NCT00193037 | Doxorubicin HCI Liposome Injection Versus Weekly Docetaxel in Patients First Relapse Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03248427 | Neadjuvant Multi-agent Chemotherapy or Letrozole Plus Ribociclib in Luminal B/HER2-negative Breast Cancer. | breast cancer | Doxorubicin (NPC261012) | |
| NCT03283696 | A Study of Olaratumab (LY3012207), Doxorubicin, and Ifosfamide in Participants With Advanced or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02053597 | TRIal evalUating the Menstrual and Ovarian Function of Young Breast Cancer Patients Treated With a cycloPHosphamide-free Regimen | breast cancer | Doxorubicin (NPC261012) | |
| NCT05275777 | A Phase Ib Safety lead-in, Followed by Phase II Trial of ADG106 in Combination With Neoadjuvant Chemotherapy in HER2 Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01818063 | Carboplatin and Combination Chemotherapy With or Without Veliparib in Treating Patients With Stage IIB-IIIC Breast Cancer | triple-negative breast cancer | Doxorubicin (NPC261012) | |
| NCT01327521 | Transarterial Chemoembolization vs CyberKnife for Recurrent Hepatocellular Carcinoma | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00248248 | DOXIL for Consolidation Therapy in Ovarian Cancer. | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT01148446 | R-CHOP Versus R-mini-CEOP in Elderly Patients(>65)With DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03005015 | Lenvatinib in Second Line Endometrial Carcinoma | endometrial neoplasm | Doxorubicin (NPC261012) | |
| NCT01655693 | Efficacy and Safety Doxorubicin Transdrug Study in Patients Suffering From Advanced Hepatocellular Carcinoma | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | cutaneous melanoma;metastatic melanoma;non-small cell lung carcinoma;renal cell carcinoma;breast carcinoma;Fallopian Tube Carcinoma;ovarian carcinoma | Doxorubicin (NPC261012) | |
| NCT00189553 | Caelyx Plus Carboplatin Versus Paclitaxel Plus Carboplatin in Patients With Epithelial Ovarian Cancer in Late Relapse | fallopian tube cancer;ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03412643 | Study of Neoadjuvant Chemotherapy Plus Trastuzumab and Pertuzumab in HER2-Negative Breast Cancer Patients With Abnormal HER2 Signaling | breast cancer | Doxorubicin (NPC261012) | |
| NCT01796002 | Efficacy and Safety of Romidepsin CHOP vs CHOP in Patients With Untreated Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01650701 | A Phase 3 Open Label Randomized Study to Compare the Efficacy and Safety of Rituximab Plus Lenalidomide (CC-5013) Versus Rituximab Plus Chemotherapy Followed by Rituximab in Subjects With Previously Untreated Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT04757337 | Comparison of Oral Cyclophosphamide vs Doxorubicin in ≥65 Years Old Advanced or Metastatic Soft Tissue Sarcoma Patients | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01547741 | Docetaxel and Cyclophosphamide Compared to Anthracycline-Based Chemotherapy in Treating Women With HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01969578 | Androgen Deprivation Therapy in Advanced Salivary Gland Cancer | salivary gland cancer | Doxorubicin (NPC261012) | |
| NCT00060346 | Rituximab and Combination Chemotherapy in Treating Patients With Newly Diagnosed Waldenstrom's Macroglobulinemia | lymphoma | Doxorubicin (NPC261012) | |
| NCT04231448 | Phase III Study of Tucidinostat in Combination With R-CHOP in Patients With Newly Diagnosed Double-Expressor DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00722137 | Study of the Combination of Rituximab, Cyclophosphamide, Doxorubicin, VELCADE, and Prednisone or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01670500 | Cisplatin vs. Doxorubicin/Cyclophosphamide in BrCa | breast cancer | Doxorubicin (NPC261012) | |
| NCT02979522 | A Study of Brentuximab Vedotin + Adriamycin, Vinblastine, and Dacarbazine in Pediatric Participants With Advanced Stage Newly Diagnosed Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02499367 | Nivolumab After Induction Treatment in Triple-negative Breast Cancer (TNBC) Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT02372409 | Using MRI-Guided Laser Heat Ablation to Induce Disruption of the Peritumoral Blood Brain Barrier to Enhance Delivery and Efficacy of Treatment of Pediatric Brain Tumors | anaplastic astrocytoma;glioblastoma multiforme;oligoastrocytoma;oligodendroglioma;optic nerve glioblastoma;pilocytic astrocytoma | Doxorubicin (NPC261012) | |
| NCT00265031 | HD12 for Advanced Stages | lymphoma | Doxorubicin (NPC261012) | |
| NCT03188198 | Risk Adapted Therapy in Diffuse Large B Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02449252 | Efficacy of Consolidative Involved-site Radiotherapy for Patients With Limited-stage Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT00201708 | Dose-Dense Docetaxel Before or After Doxorubicin/Cyclophosphamide in Axillary Node-Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03329378 | Neoadjuvant Dose-Dense For Early Her2Neu Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03467373 | A Study of Glofitamab in Combination With Rituximab or Obinutuzumab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP), or Polatuzumab Vedotin Plus Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (CHP) in Participants With Non-Hodgkin Lymphomas or With DLBCL | neoplasm of mature B-cells;non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00581776 | Phase II Study of VcR-CVAD With Rituximab Consolidation and Maintenance for Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00109837 | S0333 Combination Chemotherapy in Treating Patients With Newly Diagnosed Acute Lymphoblastic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT04887961 | Reprab Study: PLD + Trabectedin Rechallenge | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00825149 | A Study of Obinutuzumab in Combination With Chemotherapy in Participants With CD20+ B-Cell Follicular Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04922567 | Efficacy and Safety of Lenalidomide Plus CHOP vs CHOP in Patients With Untreated Peripheral T-Cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01404936 | Study of a-Interferon With Adriamycin, Bleomycin, Velban, and Dacarbazine (ABVD) With Hodgkin's Disease | lymphoma | Doxorubicin (NPC261012) | |
| NCT00003215 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Newly Diagnosed Aggressive Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00455533 | Study to Assess Effectiveness of Giving Combination of Standard Chemotherapy Drugs Versus Combination of Standard Chemotherapy and New Drug Ixabepilone When Given Before Surgical Removal of Early Stage Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01659099 | GA In NEwly Diagnosed Diffuse Large B Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02677116 | A Study of Olaratumab Alone and in Combination With Standard Chemotherapies in Children With Cancer | metastasis | Doxorubicin (NPC261012) | |
| NCT00609765 | Avastin, Fluorouracil, Doxorubicin and Streptozocin in Locally Advanced and Metastatic Pancreatic Endocrine Tumors | pancreatic carcinoma | Doxorubicin (NPC261012) | |
| NCT00450801 | R-MACLO-IVAM and Thalidomide in Untreated Mantle Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00850512 | Study to Evaluate the Efficacy and Safety of Subsequent Treatment With the Zevalin (Ibritumomab Tiuxetan) in Elderly (More Than 60 Years) Patients With Diffuse Large B Cell Lymphoma After 4 Cycles of CHOP21-Rituximab (CHOP21-R) Therapy | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00722592 | Platinum Resistant Ovarian Cancer Evaluation of Doxil and Vintafolide (MK-8109, EC145) Combination Therapy (8109-009, EC-FV-04) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03192644 | The Impact on Recurrence Risk of Adjuvant Transarterial Chemoinfusion (TAI) Versus Adjuvant Transarterial Chemoembolization (TACE) for Patients With Hepatocellular Carcinoma And Portal Vein Tumor Thrombosis (PVTT) After Hepatectomy : A Random, Controlled, Stage III Clinical Trial. | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT05006664 | Brentuximab Vedotin in Combination With CHEP in Patient With PTCL | lymphoma | Doxorubicin (NPC261012) | |
| NCT00001384 | A Pilot Trial of AC (Adriamycin, Cyclophosphamide) Chemotherapy With G-CSF (Granulocyte Colony-Stimulating Factor) Followed by Infusional Taxol (Paclitaxel) as Adjuvant Treatment for High Risk Stage II and Stage III Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT05404945 | Fitness-adapted, Pembrolizumab-based Therapy for Untreated Classical Hodgkin Lymphoma Patients 60 Years of Age and Above | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00669773 | Validate Gene Expression and Proteomic Signatures Predictive of Treatment for Response for Breast Cancer Patient | breast cancer | Doxorubicin (NPC261012) | |
| NCT05008224 | Study of Safety and Efficacy of Pembrolizumab and Chemotherapy in Participants With Newly Diagnosed Classical Hodgkin Lymphoma (cHL) (MK-3475-C11/KEYNOTE-C11) | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00477412 | Bortezomib, Rituximab and Combination Chemotherapy in Treating Participants With Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04023916 | Sintilimab Plus R-CHOP as the First-line Treatment in Patients With Diffuse Large B-Cell Lymphoma. | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02131480 | Study of Doxorubicin and Trabectedin in First Line Treatment on Patients With Metastatic Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00572169 | UARK 2006-66, Total Therapy 3B: An Extension of UARK 2003-33 Total Therapy | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT03553238 | Precision Diagnosis Directing HDACi Chidamide Target Therapy for Adult ETP-ALL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT02255110 | A Japanese Trial of TH-302 in Subjects With Locally Advanced Unresectable or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00209209 | Induction Chemotherapy (R-CHOP Vs. R-FC) Followed by Interferon Maintenance Versus Rituximab Maintenance in MCL | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00688740 | Docetaxel in Node Positive Adjuvant Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05088057 | Neoadjuvant Camrelizumab Plus Chemotherapy in Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03678883 | Study of Pembrolizumab (MK-3475) Plus Chemotherapy vs Placebo Plus Chemotherapy as Neoadjuvant Therapy and Pembrolizumab vs Placebo as Adjuvant Therapy in Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-522/KEYNOTE-522) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00434031 | CETRA: Neoadjuvant Caelyx and Trastuzumab in Her-2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02063022 | Efficacy of Dose Intensification in Patients With Non-metastatic Ewing Sarcoma | Ewing sarcoma | Doxorubicin (NPC261012) | |
| NCT00725231 | Immunotherapy in Peripheral T Cell Lymphoma - the Role of Alemtuzumab in Addition to Dose Dense CHOP | angioimmunoblastic T-cell lymphoma;extranodal nasal NK/T cell lymphoma;unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03786783 | Dinutuximab, Sargramostim, and Combination Chemotherapy in Treating Patients With Newly Diagnosed High-Risk Neuroblastoma | ganglioneuroblastoma;neuroblastoma | Doxorubicin (NPC261012) | |
| NCT00004010 | Combination Chemotherapy and Radiation Therapy in Treating Children With Previously Untreated Stage II, Stage III, or Stage IV Hodgkin's Disease | lymphoma | Doxorubicin (NPC261012) | |
| NCT00715208 | Phase 2 Study of VELCADE (Bortezomib) in Patients With Relapsed Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02488564 | A Study of Liposomal Doxorubicin + Docetaxel + Trastuzumab + Metformin in Operable and Locally Advanced HER2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05099666 | Lurbinectedin + Doxorubicin In Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT01214668 | Dose-Escalation Study of LY573636-sodium and Liposomal Doxorubicin in Patients With Advanced Solid Tumors | neoplasm | Doxorubicin (NPC261012) | |
| NCT03129828 | Ibrutinib, Bortezomib and Rituximab-CHOP for the Treatment of Elderly Patients With CD20+ DLBCL, IPI ≥ 2 | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04546620 | Acalabrutinib in Combination With R-CHOP for Previously Untreated Diffuse Large B-cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02228512 | Study of Pomalidomide Combined With Modified DA-EPOCH and Rituximab in KSHV-Associated Lymphomas | Castleman disease;diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00184002 | Doxorubicin (Doxil) Combined With Rituxan, Cyclophosphamide, Vincristine and Prednisone in Newly Diagnosed Aggressive Non-Hodgkin's Lymphomas | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00791947 | A Nordic Phase II Study of PTCL Based on Dose-intensive Induction and High-dose Consolidation With ASCT | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03571308 | A Combination of Acalabrutinib With R-CHOP for Patient Diffuse Large B-cell Lymphoma (DLBCL) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03515200 | Treatment With Combination Chemotherapy for Relapsed or Refractory Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01854255 | Intraperitoneal Aerosol Chemotherapy in Gastric Cancer | gastric cancer | Doxorubicin (NPC261012) | |
| NCT01046825 | Mature B-Cell Lymphoma And Leukemia Study III | lymphoma | Doxorubicin (NPC261012) | |
| NCT01324180 | Vincristine, Dexamethasone, Doxorubicin, and PEG-asparaginase (VPLD) and Metformin for Relapsed Childhood Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00210379 | Phase II Study of Combined Modality Treatment in Primary Testicular Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00174655 | BIG 02/98 Docetaxel - Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT01831505 | Feasibility of Assessing Lymphoma Response to Precise Local Injection of Candidate Chemotherapy Agents | lymphoma | Doxorubicin (NPC261012) | |
| NCT02192021 | Micro Needle Array-Doxorubicin (MNA-D) in Patients With Cutaneous T-cell Lymphoma (CTCL) | Cutaneous T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00815945 | Multicenter Trial With PegLiposomal Doxorubicin and Carboplatin Combination Chemotherapy in Gynecological Sarcomas and Mixed Epithelial-Mesenchymal Tumors | carcinosarcoma;leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00636441 | Trial to Evaluate Genomic Expression Profiles to Direct Preoperative Chemotherapy in Early Stage Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00145002 | A Study for Aggressive Adult T-cell Leukemia-lymphoma (ATLL) | adult T-cell leukemia/lymphoma | Doxorubicin (NPC261012) | |
| NCT01415336 | AX Versus AC as Adjuvant Treatment for Node-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01812369 | Perioperative Chemotherapy for Patients With Locally Advanced Bladder Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT00148317 | Phase II Study of Velcade, Decadron, and Doxil Followed by Cyclophosphamide in Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT03609047 | Adjuvant Palbociclib in Elderly Patients With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02359162 | Efficacy and Safety Study of P-Gemox vs.EPOCH as First-line Chemotherapy to Treat NK/T-cell Lymphoma With Early Stage | lymphoma | Doxorubicin (NPC261012) | |
| NCT05093920 | Role of DEB-TACE Versus c-TACE in Treatment of HCC | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT02186834 | Selinexor (KPT-330) and Liposomal Doxorubicin For Relapsed and Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00194753 | Adjuvant Therapy for High-Risk Breast Cancer With Wkly Adriamycin & Oral Cytoxan With G-CSF for 12 Wks | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT02753881 | Pharmacokinetics of Doxorubicin in cTACE of Liver Cancer | liver cancer | Doxorubicin (NPC261012) | |
| NCT00005584 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03542266 | CC486-CHOP in Patients With Previously Untreated Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00506155 | Neoadjuvant Chemotherapy With Methotrexate, Vinblastine, Adriamycin and Cisplatin (M-VAC) Plus Avastin in Patients With Urothelial Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT01176799 | Randomized Study of Doxorubicin and Cyclophosphamide With or Without Intermittent Sunitinib in the First-line Treatment of Locally Advanced or Metastatic Breast Cancer Patients With Measurable Primary Breast Tumor | breast cancer | Doxorubicin (NPC261012) | |
| NCT02633137 | Sequential Chemotherapy and Lenalidomide Followed by Rituximab and Lenalidomide Maintenance for Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00550771 | German Preoperative Adriamycin Docetaxel Study | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT02049905 | Phase 3 Study to Treat Patients With Soft Tissue Sarcomas | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT05448820 | YH001 Plus Envafolimab With or Without Doxorubicin in Patients With Advanced or Metastatic Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT03497702 | Neo-adjuvant Chemotherapy With Letrozole in Patients With Estrogen Receptor Positive/HER-2 Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00144807 | ACVBP Plus Rituximab in Patients Aged From 18 to 59 Years With High-risk Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03197935 | A Study to Investigate Atezolizumab and Chemotherapy Compared With Placebo and Chemotherapy in the Neoadjuvant Setting in Participants With Early Stage Triple Negative Breast Cancer | triple-negative breast cancer | Doxorubicin (NPC261012) | |
| NCT02494713 | Hormonal Therapy and Chemotherapy Followed by Prostatectomy in Patients With Prostate Cancer | prostate cancer | Doxorubicin (NPC261012) | |
| NCT01516580 | Intergroup Randomized Trial for Children or Adolescents With B-Cell Non Hodgkin Lymphoma or B-Acute Leukemia: Rituximab Evaluation in High Risk Patients | leukemia;non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00206466 | Biologic Correlative Taxotere/AC | breast cancer | Doxorubicin (NPC261012) | |
| NCT01321008 | Stage I/II Nasal NK Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02631109 | L-DEP Regimen as a Salvage Therapy for Refractory Epstein Barr Virus-induced Hemophagocytic Lymphohistiocytosis | hemophagocytic syndrome | Doxorubicin (NPC261012) | |
| NCT01802749 | Bevacizumab Beyond Progression in Platinum Sensitive Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03755804 | Pediatric Classical Hodgkin Lymphoma Consortium Study: cHOD17 | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00669877 | Rituximab and Hyper-CVAD (Cyclophosphamide, Vincristine, Adriamycin, and Dexamethasone) for Burkitt's and Burkitt's -Like Leukemia/Lymphoma | Burkitts lymphoma | Doxorubicin (NPC261012) | |
| NCT00913835 | A Study of Liposomal Doxorubicin With or Without Olaratumab (IMC-3G3) in Platinum-Refractory or Resistant Advanced Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT00165087 | Treatment of Childhood Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03617432 | Chidamide Combined With CHOPE Regimen for Peripheral T-cell Lymphoma Patients | neoplasm | Doxorubicin (NPC261012) | |
| NCT00691236 | Evaluation of Zoledronic Acid as a Single Agent or as an Adjuvant to Chemotherapy in High Grade Osteosarcoma | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT00049595 | Comparison of Two Combination Chemotherapy Regimens in Treating Patients With Stage III or Stage IV Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03004287 | 2015-12: A Study Exploring the Use of Early and Late Consolidation/Maintenance Therapy | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00183742 | Trial of Liposomal Doxorubicin (Doxil) and Weekly Docetaxel (Taxotere) | carcinoma | Doxorubicin (NPC261012) | |
| NCT03698227 | OlaReDo - Olaratumab and Rechallenge With Doxorubicin in Soft Tissue Sarcoma Patients | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT04540692 | Evaluation of Sequencing of Anthracyclines and Taxanes for Locally Advanced HER2-negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00002565 | Combination Chemotherapy in Treating Patients With Intermediate-Grade or Immunoblastic Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03880695 | Anlotinib Hydrochloride Combined With Liposomal Doxorubicin in the Treatment of Locally Advanced or Metastatic Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT00635193 | Efficacy and Safety Study of M200(Volociximab in Combination With Liposomal Doxorubicin) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01204801 | Randomized Study of Combination Chemotherapy With or Without Focused Microwave Thermotherapy Before Surgery in Treating Women With Large Breast Cancer Tumors | breast cancer | Doxorubicin (NPC261012) | |
| NCT00186888 | Study of Treatment for Patients With Cancer of the Eye -Retinoblastoma | retinoblastoma | Doxorubicin (NPC261012) | |
| NCT02812654 | Ifosfamide, Doxorubicin and Hypofractionated Radiotherapy in Neoadjuvant Sarcoma Treatment | sarcoma | Doxorubicin (NPC261012) | |
| NCT00369681 | Phase 2 Study of Rituximab-ABVD in Classical Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00129389 | FAC Versus FAC Plus Weekly Paclitaxel as Adjuvant Treatment of Node Negative High Risk Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT02790580 | Dose-dense Doxorubicin/Cyclophosphamide With Intermittent Low-dose Sunitinib in Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT01712490 | A Frontline Therapy Trial in Participants With Advanced Classical Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03349281 | Pevonedistat With VXLD Chemotherapy for Adolescent/Young Adults With Relapsed/Refractory ALL or Lymphoblastic NHL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT05456022 | Therapeutic Efficacy of Quercetin Versus Its Encapsulated Nanoparticle on Tongue Squamous Cell Carcinoma Cell Line | mouth neoplasm | Doxorubicin (NPC261012) | |
| NCT05112536 | Trilaciclib, a CDK4/6 Inhibitor, in Patients With Early-Stage Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00278109 | Partial Breast Irradiation With Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT00841945 | Treatment of Aggressive Localized Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01354522 | TAC Versus TCX as Adjuvant Treatment for Node-Positive Her2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01809379 | Intraperitoneal Aerosol High-pressure Chemotherapy for Women With Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01455532 | A Dose Escalation Study of Iniparib as a Single Agent and in Combination in Solid Tumors | neoplasm | Doxorubicin (NPC261012) | |
| NCT01871766 | Risk-Adapted Focal Proton Beam Radiation and/or Surgery in Patients With Low, Intermediate and High Risk Rhabdomyosarcoma Receiving Standard or Intensified Chemotherapy | rhabdomyosarcoma | Doxorubicin (NPC261012) | |
| NCT04307576 | A Treatment Study Protocol for Participants 0-45 Years With Acute Lymphoblastic Leukaemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01783535 | Protocol for the Study and Treatment of Participants With Intraocular Retinoblastoma | retinoblastoma | Doxorubicin (NPC261012) | |
| NCT00174707 | Study of Docetaxel in Breast Cancer Patients | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00169130 | ACVBP Followed by ASCT in Patients With BCL-2 Positive Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01104298 | Doxorubicin vs. Trabectedin Plus Doxorubicin in Non Operable and/or Metastatic STS | sarcoma | Doxorubicin (NPC261012) | |
| NCT01285765 | Evaluate a Treatment Adapted to the PET Response Compared to a Standard Treatment, for Low Risk DLBCL CD 20+ Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01865110 | R-CHOP + R-HAD vs R-CHOP Followed by Maintenance Lenalidomide + Rituximab vs Rituximab for Older Patients With MCL | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03285607 | MCS110 Combined With Neoadjuvant Doxorubicin, Cyclophosphamide, and Weekly Paclitaxel in Patients With Hormone-Receptor Positive and HER2- Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00180908 | Comparison of High-Dose Methotrexate (HDM) Plus Doxorubicin to HDM Plus Etoposide-Ifosfamide in Osteosarcoma Children | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT02938442 | Vaccination of Triple Negative Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT00024102 | Comparison of Combination Chemotherapy Regimens in Treating Older Women Who Have Undergone Surgery for Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00408408 | Chemotherapy With or Without Bevacizumab in Treating Women With Stage I, Stage II, or Stage IIIA Breast Cancer That Can Be Removed By Surgery | breast cancer | Doxorubicin (NPC261012) | |
| NCT00923936 | Pilot Study of Liposomal Doxorubicin Combined With Bevacizumab Followed by Bevacizumab Monotherapy in Adults With Advanced Kaposi s Sarcoma | Kaposi's sarcoma | Doxorubicin (NPC261012) | |
| NCT03301350 | Neoadjuvant Carbo/Paclitaxel Followed by Doxorubicin/Cyclophosphamide in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02605694 | Duvelisib With Rituximab vs R-CHOP in Subjects With Relapsed/Refractory Follicular Lymphoma (FRESCO) | lymphoma | Doxorubicin (NPC261012) | |
| NCT03646123 | Clinical Trial of Brentuximab Vedotin in Classical Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00266799 | The Efficacy and Safety of Pegylated Liposomal Doxorubicin Compared With Capecitabine as First Line Chemotherapy for Metastatic Breast Cancer (P04445/MK-2746-071) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00559845 | A Study of Avastin (Bevacizumab) in Patients With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01319981 | Hyper-CVAD With Liposomal Vincristine in Acute Lymphoblastic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT00212082 | Gene Expression Profiles in Predicting Chemotherapy Response in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00258960 | Caelyx, Cyclophosphamide and Herceptin in Patients With Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02867566 | A Study Comparing the Efficacy and Safety Between I-CHOP and R-CHOP in Untreated CD20-Positive Diffuse Large B-cell Lymphoma Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01087424 | Mini-CHOP and Rituximab in Patients Aged Over 80 Years | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01705691 | Comparison of Neoadjuvant Chemotherapy With Weekly Paclitaxel or Eribulin Followed by A/C in Women With Locally Advanced HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02858258 | ASCT After a Rituximab/Ibrutinib/Ara-c Containing iNduction in Generalized Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00455897 | CHOP-Rituximab Augmented With GM-CSF in Patients With Previously Untreated Diffuse Large B Cell Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00476190 | ALL Adult Consortium Trial: Adult ALL Trial | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00912444 | Neoadjuvant Treatment of Docetaxel, Anthracycline and Cyclophosphamide (TAC) Versus Docetaxel and Cyclophosphamide (TC) in Triple-Negative or Her2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04081389 | Chemokine Modulation Therapy and Standard Chemotherapy Before Surgery for the Treatment of Early Stage Triple Negative Breast Cancer | breast carcinoma in situ | Doxorubicin (NPC261012) | |
| NCT04584112 | A Study of the Safety, Efficacy, and Pharmacokinetics of Tiragolumab in Combination With Atezolizumab and Chemotherapy in Participants With Triple-Negative Breast Cancer | triple-negative breast cancer | Doxorubicin (NPC261012) | |
| NCT00486759 | A Study of Bevacizumab (Avastin) in Combination With Rituximab (MabThera) and CHOP (Cyclophosphamide, Hydroxydaunorubicin [Doxorubicin], Oncovin [Vincristine], Prednisone) Chemotherapy in Patients With Diffuse Large B-cell Lymphoma | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT03677141 | A Phase Ib/II Study Investigating the Safety, Tolerability, Pharmacokinetics, and Efficacy of Mosunetuzumab (BTCT4465A) in Combination With CHOP or CHP-Polatuzumab Vedotin in Participants With B-Cell Non-Hodgkin Lymphoma | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT00102609 | A Safety Study Utilizing Yondelis and Doxorubicin in Patients With a Type of Cancer Called Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01646034 | High Dose Chemotherapy in Oligo-metastatic Homologous Recombination Deficient Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02471820 | Lenalidomide & Adriamycin & Dexamethasone (RAD) in Newly Diagnosed, Multiple Myeloma Patients | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00512980 | PVAG-14 Pilot for Intermediate Stages Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00755261 | Phase II Study of Doxorubicin and Avastin® in Sarcoma. | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT03726879 | A Study To Evaluate the Efficacy and Safety Of Atezolizumab or Placebo in Combination With Neoadjuvant Doxorubicin + Cyclophosphamide Followed By Paclitaxel + Trastuzumab + Pertuzumab In Early Her2-Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00736320 | HD16 for Early Stage Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00586846 | Phase II Study of Chemotherapy and Pamidronate for the Treatment of Newly Diagnosed Osteosarcoma | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT00083538 | Study of Tumor Antigen-Pulsed Autologous Dendritic Cell Vaccination Administrated Subcutaneously or Intranodally | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT03536039 | RCHOP Chemoimmunotherapy Preceded BY BBB Permeabilization by t-NGR Necrosis Factor | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00006721 | S0016 Combination Chemotherapy With Monoclonal Antibody Therapy in Newly Diagnosed Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04159818 | Immune Induction Strategies to Improve Response to Immune Checkpoint Blockade in Triple Negative Breast Cancer (TNBC) Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT02623972 | A Phase 2 Study of Eribulin Followed by AC as Preoperative Therapy for HER2-negative Inflammatory Breast Cancer | inflammatory breast carcinoma | Doxorubicin (NPC261012) | |
| NCT01777152 | ECHELON-2: A Comparison of Brentuximab Vedotin and CHP With Standard-of-care CHOP in the Treatment of Patients With CD30-positive Mature T-cell Lymphomas | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02845882 | LBL-2016 for Children or Adolescents in China | lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00920153 | Three Different Therapy Regimens in Treating Patients With Previously Untreated Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT05159193 | Neoadjuvant Treatment Pegylated Liposomal Doxorubicin Plus Cyclophosphamide Sequential Docetaxel Plus Trastuzumab and Pertuzumab Versus Docetaxel Plus Carboplatin Combined With Trastuzumab and Pertuzumab in HER-2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01704716 | High Risk Neuroblastoma Study 1.8 of SIOP-Europe (SIOPEN) | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT00931918 | Study to Assess the Effectiveness of RCHOP With or Without VELCADE in Previously Untreated Non-Germinal Center B-Cell-like Diffuse Large B-Cell Lymphoma Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02897700 | A Randomized Trial of Chemotherapy in Surgical Patients With Infiltrating Ductal Carcinoma of Breast | breast cancer | Doxorubicin (NPC261012) | |
| NCT01227941 | MK-4827 in Combination With Pegylated Liposomal Doxorubicin in Participants With Advanced Solid Tumors and Ovarian Cancer (MK-4827-011) | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00052936 | Combination Chemotherapy With or Without Rituximab in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01940497 | A Study of the Safety of Subcutaneously Administered Trastuzumab (Herceptin) in Participants With Early and Locally Advanced Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04077905 | Pegylated Liposomal Doxorubicin as a Induction Therapy for Lymphoma Induced Hemophagocytic Lymphohistiocytosis. | hemophagocytic syndrome | Doxorubicin (NPC261012) | |
| NCT02292979 | Brentuximab Vedotin Associated With Chemotherapy in Untreated Patients With Hodgkin Lymphoma. | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00793377 | Neoadjuvant Doxorubicin/Cyclophosphamide Followed by Docetaxel (AC-Doc) Versus Dose-Dense Doxorubicin and Docetaxel (ADoc) in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03399747 | Abbreviated 3 Cycles of Rituximab Plus CHOP(Cyclophosphamide, Adriamycin, Vincristine, and Prednisolone) Immunochemotherapy in Patients With Completely Excised LocalizedGastrointestinal CD(Cluster of Differentiation Antigen)20(+) Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00305084 | Study of NGR-hTNF in Combination With Doxorubicin in Solid Tumors | cancer | Doxorubicin (NPC261012) | |
| NCT00523380 | Efficacy Study of Recombinant Interleukin-21 in the Treatment of Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03335241 | Study of Fludarabine With Pegylated Liposomal Doxorubicin Versus Pegylated Liposomal Doxorubicin Alone In Patients With Platinum Resistant/Refractory Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00265018 | HD10 for Early Stages | lymphoma | Doxorubicin (NPC261012) | |
| NCT00988195 | Study of Pegylated Human Recombinant Arginase for Liver Cancer | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT04679064 | Trial on NIraparib-TSR-042 (Dostarlimab) vs Physician's Choice CHEmotherapy in Recurrent, Ovarian, Fallopian Tube or Primary Peritoneal Cancer Patients Not Candidate for Platinum Retreatment | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00578864 | Protracted Etoposide During Induction Therapy for High Risk Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT00780039 | A Study to Evaluate the Safety and Efficacy of Caelyx in Combination With Carboplatin in Patients With Ovarian Cancer Recurrent Within Six to Twelve Months After Initial Carboplatin and Paclitaxel Chemotherapy (P03625) | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT00003352 | Combination Chemotherapy in Treating Women With Stage IIIB or Stage IV Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01424982 | Combination Chemotherapy and Ponatinib Hydrochloride in Treating Patients With Acute Lymphoblastic Leukemia | Blast Phase Chronic Myelogenous Leukemia, BCR-ABL1 Positive | Doxorubicin (NPC261012) | |
| NCT03274492 | A Study Comparing the Efficacy and Safety of Polatuzumab Vedotin With Rituximab-Cyclophosphamide, Doxorubicin, and Prednisone (R-CHP) Versus Rituximab-Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Participants With Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01498588 | Trial of Eribulin Followed by Doxorubicin & Cyclophosphamide for Her2-negative, Locally Advanced Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT02125344 | A Phase III Trial Comparing Two Dose-dense, Dose-intensified Approaches (ETC and PM(Cb)) for Neoadjuvant Treatment of Patients With High-risk Early Breast Cancer (GeparOcto) | inflammatory breast carcinoma | Doxorubicin (NPC261012) | |
| NCT03020030 | Treatment of Newly Diagnosed Acute Lymphoblastic Leukemia in Children and Adolescents | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00355199 | Comparison of HD Chemotherapy Followed by Auto-transplant and R-CHOP in High Risk Patients With DLBCL. | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00004031 | SWOG-9704 Chemoradiotherapy and Peripheral Stem Cell Transplantation Compared With Combination Chemotherapy in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT05210374 | Disulfiram With Copper Gluconate and Liposomal Doxorubicin in Treatment-Refractory Sarcomas | sarcoma | Doxorubicin (NPC261012) | |
| NCT01227408 | Neoadjuvant Doxorubicin, Polyglutamate Paclitaxel, Capecitabine and Metronomic Chemotherapy in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03971409 | Avelumab With Binimetinib, Sacituzumab Govitecan, or Liposomal Doxorubicin in Treating Patients With Stage IV or Unresectable, Recurrent Triple Negative Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT00580333 | Preoperative Cisplatin and Bevacizumab in ER-, PR-, HER2 Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00448266 | Intensified IAA With PBPC Support in Breast Tumors With Evidence of a HRD | breast cancer | Doxorubicin (NPC261012) | |
| NCT00187161 | Treatment of Burkitt Lymphoma/Leukemia and B Large Cell NHL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00126191 | Intensive Chemotherapy and Rituximab in the Treatment of Burkitt Lymphoma | Burkitts lymphoma | Doxorubicin (NPC261012) | |
| NCT04895358 | 9-ING-41 in Patients With Advanced Cancers | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00798252 | Ascending Multiple-Dose Study of Brivanib Alaninate in Combination With Chemotherapeutic Agents in Subjects With Advanced Cancers | cancer | Doxorubicin (NPC261012) | |
| NCT01492881 | Study of Vorinostat With Doxil and Bortezomib for Patients With Relapsed/Refractory Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01120171 | Myocet Plus Endoxan for Older Patients With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00426127 | Docetaxel and Liposomal Doxorubicin Chemotherapy With Enoxaparin in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Doxorubicin (NPC261012) | |
| NCT02472353 | Use of Metformin to Reduce Cardiac Toxicity in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00945724 | Safety and Feasibility Study of Combination of State of Art Chemoimmunotherapy, Intensive Central Nervous System Prophylaxis and Scrotal Irradiation to Treat Primary Diffuse Large B-cell Lymphoma of Testis | lymphoma | Doxorubicin (NPC261012) | |
| NCT04884035 | Study of Safety and Efficacy of Iberdomide (CC-220) and CC-99282 Combined With R-CHOP to Treat Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04224337 | Phase II Study of Durvalumab ,Doxorubicin, and Ifosfamide in Pulmonary Sarcomatoid Carcinoma | non-small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT02918747 | PEG-ASP+Gemoxd vs. PEG-ASP+CHOP as First-line Chemotherapy to Treatment NK/T-cell Lymphoma With Early Stage | lymphoma | Doxorubicin (NPC261012) | |
| NCT03937830 | Combined Treatment of Durvalumab, Bevacizumab, Tremelimumab and Transarterial Chemoembolization (TACE) in Subjects With Hepatocellular Carcinoma or Biliary Tract Carcinoma | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00930605 | The Effectiveness of Alemtuzumab Given in Combination With CHOP and ESHAP in Patients Newly Diagnosed With Peripheral T-Cell Lymphoma (PTCL) | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00002105 | Randomized, Comparative Trial of DOX-SL (Stealth Liposomal Doxorubicin Hydrochloride) Versus Bleomycin and Vincristine in the Treatment of AIDS-Related Kaposi's Sarcoma | Kaposi's sarcoma | Doxorubicin (NPC261012) | |
| NCT03596281 | Pembrolizumab in Combination With Bevacizumab and Pegylated Liposomal Doxorubicin in Patients With Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT01415765 | MLN4924 Compared With MLN4924 Plus Chemotherapy for Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03017326 | Paediatric Hepatic International Tumour Trial | Hepatoblastoma | Doxorubicin (NPC261012) | |
| NCT02617485 | MabionCD20® Compared to MabThera® in Lymphoma Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00925821 | Lenalidomide-Adriamycin-Dexamethasone (RAD) Induction Followed by Stem Cell Transplant in Newly Diagnosed Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00135499 | R-ACVBP Versus R-CHOP in Patients Aged 60-65 With Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03071926 | Metronomic PLD in Patients With Primary Endocrine Resistant ABC | breast cancer | Doxorubicin (NPC261012) | |
| NCT01990352 | Correlate BRCA1 Protein Expression With Response to DNA Damaging Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT04908787 | A Phase III Study of BD0801 Combined With Chemotherapy in Recurrent, Platinum-resistant Epithelial Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00215943 | Phase III Randomized Trial of Thalidomide/Dexamethasone Versus Vincristine+Adriamycin+Dexamethasone (VAD) | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT04910126 | Camrelizumab Plus Doxorubicin for the First Line Treatment of Adcanced Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01014767 | Intercontinental Multidisciplinary Registry and Treatment Optimization Study for Choroid Plexus Tumors | brain cancer;choroid plexus neoplasm | Doxorubicin (NPC261012) | |
| NCT04263584 | Copanlisib in Combination With Rituximab and CHOP Chemotherapy in Patients With Previously Untreated DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05238064 | Parsaclisib in Combination With CHOP in Participants With Previously Untreated PTCL | mature T-cell and NK-cell non-Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00718484 | A Study of Palifosfamide Tris Plus Doxorubicin Versus Doxorubicin in Unresectable or Metastatic Soft-tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT04164368 | Lenalidomide Combined With R-CHOP(R2-CHOP) in Newly Diagnosed Double-expressor Diffuse Large B-Cell Lymphoma Patients | lymphoma | Doxorubicin (NPC261012) | |
| NCT00094497 | Trial in Locally Advanced and Metastatic Adrenocortical Carcinoma Treatment (FIRM-ACT) | carcinoma | Doxorubicin (NPC261012) | |
| NCT03145558 | TATE Versus TACE in Intermediate Stage HCC | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00002318 | A Comparison of DOX-SL Versus Adriamycin Plus Bleomycin Plus Vincristine in the Treatment of Severe AIDS-Related Kaposi's Sarcoma | Kaposi's sarcoma | Doxorubicin (NPC261012) | |
| NCT01000285 | EPOCH Chemotherapy and Bortezomib for Associated T-Cell Leukemia Lymphoma | T-cell acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00147225 | AMG 531 in Patients With Advanced Malignancy Receiving Treatment With Carboplatin | cancer | Doxorubicin (NPC261012) | |
| NCT00849472 | A Study to Evaluate Safety and Efficacy of Caelyx in Combination With Cyclophosphamide in the Treatment of Metastatic Breast Cancer (P02948) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT03949634 | Cardiac Safety and Efficacy for Early-stage Breast Cancer Patients Treated With Pegylated Liposomal Doxorubicin(PLD) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00808639 | Dose-Dense MVAC With Pegfilgrastim Support in Subjects With Muscle-Invasive Urothelial Carcinoma | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT00254592 | Neoadjuvant Treatment of Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03994107 | Pegylated Liposomal Doxorubicin Plus Albumin-Bound Paclitaxel and Trastuzumab in HER-2 Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01637532 | Feasibility of the Combination of Chemotherapy (Carbo/Caelyx or Carbo/Doxorubicin) With Tocilizumab (mAb IL-6R) and Peg-Intron in Patients With Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT04038489 | COX Inhibition and Biomarkers During Neoadjuvant Chemoendocrine Therapy for ER+, HER2- Stage I-III Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00903656 | Lapatinib Plus Caelyx in Patients With Advanced Metastatic Breast Cancer Following Failure of Trastuzumab Therapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003165 | Doxorubicin in Treating Women With Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00801281 | First-line R-CVP vs R-CHOP Induction Immunochemotherapy for Indolent Lymphoma and R Maintenance. | chronic lymphocytic leukemia;follicular lymphoma;lymphoplasmacytic lymphoma | Doxorubicin (NPC261012) | |
| NCT02116530 | Antiemetic Therapy With or Without Olanzapine in Preventing Chemotherapy-Induced Nausea and Vomiting in Patients With Cancer Receiving Highly Emetogenic Chemotherapy | Nausea and vomiting | Doxorubicin (NPC261012) | |
| NCT01329627 | Feasibility Study of Metronomic Chemotherapy for Locally Advanced HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01666444 | VTX-2337 and Pegylated Liposomal Doxorubicin (PLD) in Patients With Recurrent or Persistent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00001339 | A Study of Combination Chemotherapy and Surgical Resection in the Treatment of Adrenocortical Carcinoma: Continuous Infusion Doxorubicin, Vincristine and Etoposide With Daily Mitotane Before and After Surgical Resection | carcinoma | Doxorubicin (NPC261012) | |
| NCT04478292 | A Multi-institutional Study for Treatment of Children With Newly Diagnosed Hepatoblastoma Using a Modified PHITT Strategy | Hepatoblastoma | Doxorubicin (NPC261012) | |
| NCT00721747 | Taxotere®, Followed by Myocet® and Cyclophosphamide First Line Treatment in HER2 Neg Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03595592 | Neoadjuvant Treatment of HER2 Positive Early High-risk and Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00866749 | Augmented Berlin-Frankfurt-Munster (BFM) Therapy for Adolescent/Young Adults With Acute Lymphoblastic Leukemia or Acute Lymphoblastic Lymphoma | lymphoid leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT01605526 | A Study of RO5045337 in Combination With Doxorubicin in Patients With Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT00887536 | A Clinical Trial Comparing the Combination of TC Plus Bevacizumab to TC Alone and to TAC for Women With Node-Positive or High-Risk Node-Negative, HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00290498 | Study of Rituximab-HCVAD Alternating With Rituximab-Methotrexate-Cytarabine Versus Standard Rituximab-CHOP Every 21 Days for Patients With Newly Diagnosed High Risk Aggressive B-Cell Non-Hodgkin's Lymphomas in Patients 60 Years Old or Younger | lymphoma | Doxorubicin (NPC261012) | |
| NCT00465673 | Liposomal Doxorubicin (Lipo-Dox) in Patients With Brain Metastasis From Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01796197 | Paclitaxel + Trastuzumab + Pertuzumab as Pre-Op for Inflammatory BrCa | breast cancer | Doxorubicin (NPC261012) | |
| NCT01678664 | Everolimus After (Chemo)Embolization for Liver Metastases From Digestive Endocrine Tumors | neuroendocrine neoplasm | Doxorubicin (NPC261012) | |
| NCT04535713 | GALLANT: Metronomic Gemcitabine, Doxorubicin, Docetaxel and Nivolumab for Advanced Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT02783599 | A Study of Olaratumab (LY3012207) in Participants With Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT03712202 | Brentuximab Vedotin and Nivolumab in Treating Patients With Early Stage Classic Hodgkin Lymphoma | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT02562378 | T-DM1 and Non-pegylated Liposomal Doxorubicin in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00101101 | Universal Granulocyte Macrophage-colony Stimulating Factor (GM-CSF)-Producing and GM.CD40L for Autologous Tumor Vaccine in Mantle Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04996160 | Palbociclib in Combination With Chemotherapy in Pediatric Patients With Relapsed or Refractory Acute Lymphoblastic Leukemia (RELPALL2) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT04745949 | PACIFIC: Primary Mediastinal Large B-cell Lymphoma Treated With Antibody Therapy, Checkpoint Inhibitor in Frontline With ImmunoChemotherapy | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT02032277 | A Study Evaluating Safety and Efficacy of the Addition of ABT-888 Plus Carboplatin Versus the Addition of Carboplatin to Standard Chemotherapy Versus Standard Chemotherapy in Subjects With Early Stage Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03742986 | Trial of Nivolumab With Chemotherapy as Neoadjuvant Treatment in Inflammatory Breast Cancer (IBC) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00890656 | Study of Augmented Hyper-CVAD in Acute Lymphoblastic Leukemia Salvage | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00404066 | Phase 2 Neoadjuvant Doxorubicin and Cyclophosphamide -> Docetaxel With Lapatinib in Stage II/III Her2Neu+ Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00016406 | S0012 Doxorubicin, Cyclophosphamide, and Paclitaxel With or Without Filgrastim in Treating Women With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003541 | Combination Chemotherapy, Radiation Therapy, and Peripheral Stem Cell Transplantation in Treating Patients With Stage III or Stage IV Mantle Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00001498 | A Pilot Trial of Sequential Chemotherapy With Antimetabolite Induction, High-Dose Alkylating Agent Consolidation With Peripheral Blood Progenitor Cell Support, and Intensification With Paclitaxel and Doxorubicin for Patients With High-Risk Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01719835 | CHOP vs GEM-P in 1st Line Treatment of T-cell Lymphoma, Multicentre Phase II Study | anaplastic large cell lymphoma;angioimmunoblastic T-cell lymphoma;unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01746173 | CHOEP + High Dose Therapy + Auto SCT for T-Cell Lymphoma | T-cell non-Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00145639 | Osteosarcoma1999-A Study Of Intensive Chemotherapy for Osteosarcoma | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT05075460 | Tucidinostat, Azacitidine Combined With CHOP Versus CHOP in Patients With Untreated Peripheral T-cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT05113251 | Trastuzumab Deruxtecan (T-DXd) Alone or in Sequence With THP, Versus Standard Treatment (ddAC-THP), in HER2-positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02792491 | Phase II Prospective Trial of Addition of Rituximab to Reduced Dose CHOP Chemotherapy in DLBC L Patients Aged 65 Years and Over | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01969032 | Induction Preoperative Chemotherapy for Patients With Locally Advanced Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01459887 | Study of Recombinant Human-Mouse Chimeric Anti-CD20 Monoclonal Antibody to Treat Non-hodgkin's Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04529772 | A Combination of Acalabrutinib With R-CHOP in Subjects With Previously Untreated Non-GCB DLBCL (ACE-LY-312) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01131364 | Combination Therapy of F16IL2 and Doxorubicin in Solid Tumour Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT00703170 | Phase I Study of DOXIL and Temsirolimus in Resistant Solid Malignancies | cancer | Doxorubicin (NPC261012) | |
| NCT00051311 | Modified Stem Cell Transplant Procedure to Treat Patients With Blood and Immune System Cancers | hematopoietic and lymphoid cell neoplasm | Doxorubicin (NPC261012) | |
| NCT00379574 | Bortezomib Plus CHOP Every 2 Weeks for Advanced Stage DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00225173 | Combination Chemotherapy +/- Radiation in High Risk Hodgkin's Disease | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02228772 | Phase I Study of MLN 9708 in Addition to Chemotherapy for the Treatment of Acute Lymphoblastic Leukemia in Older Adults | B-cell acute lymphoblastic leukemia;lymphoblastic lymphoma;T-cell acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00484341 | Phase II Study of NGR-hTNF in Combination With Doxorubicin in Patients Affected by Soft Tissue Sarcomas. | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01527422 | Cyclophosphamide, Doxorubicin, Vincristine, Prednisone, Rituximab Pateinets With Aggresive NHL | lymphoma | Doxorubicin (NPC261012) | |
| NCT00290732 | Liposomal Doxorubicin Before Mastectomy in Treating Women With Invasive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05303792 | Testing the Combination of Inotuzumab Ozogamicin and Lower Dose Chemotherapy Compared to Usual Chemotherapy for Adults With B-Cell Acute Lymphoblastic Leukemia or B-Cell Lymphoblastic Lymphoma | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT04243616 | Cemiplimab in High Risk or Locally Advanced Hormone Receptor Positive HER2 Negative or Triple-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00209222 | Efficacy of R-CHOP vs R-CHOP/R-DHAP in Untreated MCL | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01652261 | Very Early FDG-PET/CT-response Adapted Therapy for Advanced Hodgkin Lymphoma (H11) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00041132 | S0213 Chemotherapy Plus Rituximab in Treating Patients With Mantle Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00635726 | Methotrexate, Vinblastine, Doxorubicin and Cisplatin (MVAC) Followed by Gemcitabine Plus Cisplatin (GEM+CDDP) in Locally Advanced or Metastatic Bladder Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT01027910 | PCI-24781 in Combination With Doxorubicin to Treat Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT04996004 | A Study to Learn About the Study Medicine (Called Ontorpacept or TTI-621) Given Alone and in Combination With Doxorubicin in People With Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00574236 | Phase II Trial of Bortezomib and Doxorubicin in Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02753647 | Chidamide Plus R-CHOP in Elderly DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03493854 | A Study to Evaluate the Pharmacokinetics, Efficacy, and Safety of Subcutaneous Administration of the Fixed-Dose Combination of Pertuzumab and Trastuzumab in Combination With Chemotherapy in Participants With HER2-Positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03056001 | Safety, Tolerability, and Efficacy of Doxorubicin and Pembrolizumab for Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT03036488 | Safety and Efficacy Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy as Neoadjuvant Treatment for Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-173/KEYNOTE-173) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00568464 | Study on VCD/IE in the Patients With Ewing's Sarcoma Family of Tumors (ESFT) | Ewing sarcoma | Doxorubicin (NPC261012) | |
| NCT01356680 | HD17 for Intermediate Stage Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00779129 | A Phase I, Open-label, Study of Pazopanib in Combination With Epirubicin or Doxorubicin for Advanced Solid Tumors | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT03860844 | Isatuximab in Combination With Chemotherapy in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia or Acute Myeloid Leukemia | acute myeloid leukemia;acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01861951 | A Trial Comparing Two Medications as First Treatment in Elderly Patients With Metastatic or Advanced Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00135135 | Therapy for Children With Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT00750815 | Cyclophosphamide, VELCADE, DOXIL, and Dexamethasone, (CVDD) in Newly Diagnosed Patients With Multiple Myeloma (MM) | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT02177695 | S1314, Co-expression Extrapolation (COXEN) Program to Predict Chemotherapy Response in Patients With Bladder Cancer | urinary bladder cancer | Doxorubicin (NPC261012) | |
| NCT02096588 | Detection and Prevention of Anthracycline-Related Cardiac Toxicity With Concurrent Simvastatin | breast cancer | Doxorubicin (NPC261012) | |
| NCT03018626 | R-ACVBP and DA-EPOCH-R in Patients With Non-GCB DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02413320 | Neoadjuvant Study of Two Platinum Regimens in Triple Negative Breast Cancer | triple-negative breast cancer | Doxorubicin (NPC261012) | |
| NCT01040871 | Study of the Combination of VELCADE, Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Non-Germinal Center B-Cell Subtype of Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02865811 | Pembrolizumab Combined With PLD For Recurrent Platinum Resistant Ovarian, Fallopian Tube Or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Doxorubicin (NPC261012) | |
| NCT02734771 | A Study of Brentuximab Vedotin, Rituximab, and Dose Attenuated CHP in Elderly Patients With Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00816959 | Study Evaluating the Effect of R-mabHDI in Lymphocytic Predominant Hodgkin's Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04083963 | Phase 2 Trial of Neoadjuvant Weekly Carboplatin Plus Paclitaxel in Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00038558 | Prophylactic Use of Filgrastim SD/01 in Patients With Hodgkin's Disease Receiving ABVD Chemotherapy | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT05234684 | A Study of Orelabrutinib Plus R-CHOP in Treatment-naïve Patients With MCD Subtype Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02483247 | A Study of BBI503 in Combination With Selected Anti-Cancer Therapeutics in Adult Patients With Advanced Cancer | cancer | Doxorubicin (NPC261012) | |
| NCT02626455 | Study of Copanlisib in Combination With Standard Immunochemotherapy in Relapsed Indolent Non-Hodgkin's Lymphoma (iNHL) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00361621 | Ph II CHOP+Velcade in Mediastinal LBCL | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT03007147 | Imatinib Mesylate and Combination Chemotherapy in Treating Patients With Newly Diagnosed Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia | T-cell acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03498716 | A Study Comparing Atezolizumab (Anti PD-L1 Antibody) In Combination With Adjuvant Anthracycline/Taxane-Based Chemotherapy Versus Chemotherapy Alone In Patients With Operable Triple-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01481194 | ACVDL Treatment for Patients With Newly Diagnosed Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT02054559 | R-CHOP Alone vs. R-CHOP Plus Radiotherapy for Localized CD20+ DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03233347 | Doxorubicin, Vinblastine, Dacarbazine, Brentuximab Vedotin, and Nivolumab in Treating Patients With Stage I-II Hodgkin Lymphoma | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT03225924 | Study of Entospletinib (ENTO) in Newly Diagnosed DLBCL Patients With aaIPI>=1 Treated by Chemiotherapy | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01379989 | INOVATYON STUDY -International, Randomized Study in Patients With Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00203372 | Neoadjuvant TAC Plus or Minus Bevacizumab(AVF3299) | breast cancer | Doxorubicin (NPC261012) | |
| NCT02997358 | Study Comparing Efficacy of Doxorubicin With Trabectedin Followed by Trabectedin Versus Doxorubicine in Patients With Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT01974440 | A Study of PCI-32765 (Ibrutinib) in Combination With Either Bendamustine and Rituximab or Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Participants With Previously Treated Indolent Non-Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00484432 | Study of NGR-hTNF in Combination With Doxorubicin in Patients Affected by Advanced or Metastatic Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02132949 | A Study Evaluating Pertuzumab (Perjeta) Combined With Trastuzumab (Herceptin) and Standard Anthracycline-based Chemotherapy in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Locally Advanced, Inflammatory, or Early-stage Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02822157 | Circulating Tumor DNA Guiding (Olaparib) Lynparza® Treatment in Ovarian Cancer | malignant epithelial tumor of ovary | Doxorubicin (NPC261012) | |
| NCT03952572 | Efficacy and Safety of CDOP vs CHOP for Newly Diagnosed Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04661007 | To Assess the Safety and Tolerability of Tafasitamab Alone or in Combination With Other Drugs in Japanese Participants With Non-Hodgkins Lymphoma (NHL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04358341 | Pegliposomal Doxorubicin and 5-fluorouracil as Second Line Therapy for Metastatic Gastric Cancer | gastric cancer | Doxorubicin (NPC261012) | |
| NCT00136435 | A Study in Adults With Untreated Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00471965 | Oxaliplatin + 5-FluoroUracil/LeucoVorin (5-FU/LV) (FOLFOX4) Versus Doxorubicin as Palliative Chemotherapy in Advanced Hepatocellular Carcinoma Patients | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00502411 | Post-Operative Chemoradiation for Extremity & Trunk Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01101594 | A Study of hLL1-DOX (Milatuzumab-Doxorubicin Antibody-Drug Conjugate) in Patients With Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT04745832 | Phase 3 Study of Zandelisib (ME-401) in Combination With Rituximab in Patients With iNHL - (COASTAL) | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02431559 | Phase 1/2 Study of Motolimod, Doxorubicin, and Durvalumab in Recurrent, Platinum-Resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02661503 | HD21 for Advanced Stages | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT01669239 | Study of Neoadjuvant Myocet®, Paclitaxel, Pertuzumab, and Trastuzumab in HER2-positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01839097 | Phase 1 Dose Finding Study of Belinostat for Treatment of Patients With Peripheral T-cell Lymphoma (PTCL) | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00484601 | Ifosfamide and Doxorubicin in Patients With Refractory Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Doxorubicin (NPC261012) | |
| NCT00021255 | Combination Chemotherapy With or Without Trastuzumab in Treating Women With Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT02215876 | Neoadjuvant Doxorubicin/Cyclophosphamide Followed by Eribulin Chemotherapy (ACE) in Operable HER2-negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00366106 | Alternative Schedule of Velcade/Dexamethasone Plus Doxil for Patients With Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01186328 | EZN-3042 Administered With Re-induction Chemotherapy in Children With Relapsed Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00288431 | Safety, Tolerability and Maximum Tolerated Dose of Oral AP23573 in Combination With Doxorubicin (8669-015) | sarcoma | Doxorubicin (NPC261012) | |
| NCT00441168 | Velcade (Bortezomib), Adriamycin Dexamethasone (PAD) or Vincristine Adriamycin Dexamethasone in Second Line Treatment of Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00057382 | T138067 Versus Doxorubicin in Chemotherapy-Naive, Unresectable, Hepatocellular Carcinoma Patients | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT01887587 | Vincristine, Doxorubicin, And Dexamethasone + Ixazomib in Acute Lymphoblastic Leukemia (ALL), Lymphoblastic Lymphoma Or Mixed Phenotype Acute Leukemia | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00126243 | Efficacy of Rituximab and Cyclophosphamide, Doxorubicin, Vincristine, Prednisone (CHOP) in Patients With HIV Associated Non-Hodgkin's Lymphoma | Lymphoma, AIDS-Related | Doxorubicin (NPC261012) | |
| NCT03719430 | APX005M and Doxorubicin in Advanced Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01332968 | A Study of Obinutuzumab (RO5072759) Plus Chemotherapy in Comparison With Rituximab Plus Chemotherapy Followed by Obinutuzumab or Rituximab Maintenance in Patients With Untreated Advanced Indolent Non-Hodgkin's Lymphoma (GALLIUM) | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00687440 | Phase II Trial to Compare the Safety of Two Chemotherapy Plus Trastuzumab Regimens as Adjuvant Therapy for HER2-positive Breast Cancer (Study P05048) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT03517137 | Very Early PET-response Adapted Targeted Therapy for Advanced Hodgkin Lymphoma: a Single -Arm Phase II Study | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01057069 | Neo Adjuvant Chemotherapy in Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00722293 | A Study to Determine the Activity of Caelyx With Trastuzumab and Docetaxel in the Treatment of Metastatic Breast Cancer (Study P03679) | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT01281943 | Study of Pegylated Liposomal Doxorubicin and Temsirolimus in Patients With Advanced Hepatocellular Cancer | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT01935492 | 8 Continuous vs 8 Intermittent Cycles in First and Second Line in HER2/Neu Neg Metastatic Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT01220128 | Treatment With Pazopanib for Neoadjuvant Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT02751918 | Phase Ib Study of Anetumab Ravtansine in Combination With Pegylated Liposomal Doxorubicin in Patients With Recurrent Mesothelin-expressing Platinum-resistant Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT04032964 | Dose Finding Study of L19TNF and Doxorubicin in Patients With STS | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01333423 | Maximum Tolerated Dose (MTD) of Liposomal Doxorubicin in Combination With Seliciclib for Patients With Metastatic Triple Negative Breast Cancer (TNBC) | breast cancer | Doxorubicin (NPC261012) | |
| NCT05218499 | Brightline-1: A Study to Compare BI 907828 With Doxorubicin in People With a Type of Cancer Called Dedifferentiated Liposarcoma | dedifferentiated liposarcoma | Doxorubicin (NPC261012) | |
| NCT00123929 | Genetic Expression and Prediction of Response to Neoadjuvant Docetaxel or Doxorubicin in Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00543829 | Study of Suitable Schedule of Docetaxel,Anthracycline and Cyclophosphamide in Adjuvant Therapy of Beast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00350948 | Phase 3 Randomized Study of Telcyta + Doxorubicin Versus Doxorubicin in Platinum Refractory or Resistant Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT04660799 | A Study on Pharmacokinetics (PK), Efficacy and Safety of Subcutaneous (SC) Versus Intravenous (IV) Rituximab, in Combination With CHOP (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone) in Previously Untreated Participants With CD20 Positive Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02994251 | A Trial of Systemic Chemotherapy in Combination With Conventional Transarterial Chemoembolization in Patients With Advanced Intra-Hepatic Cholangiocarcinoma | cholangiocarcinoma | Doxorubicin (NPC261012) | |
| NCT01649856 | A Study of Subcutaneous Versus Intravenous MabThera/Rituxan (Rituximab) in Combination With CHOP Chemotherapy in Patients With Previously Untreated CD20-Positive Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00204568 | Trofosfamide Versus Adriamycin in Elderly Patients With Soft Tissue Sarcoma (STS) | sarcoma | Doxorubicin (NPC261012) | |
| NCT04517435 | ME-401 and R-CHOP in Newly Diagnosed Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03539328 | Study on MK-3475 Plus Chemotherapy Versus Chemotherapy Alone in Recurrent, Platinum-resistant Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00871013 | Trial for Patients Not Qualifying for TT4 and TT5 Protocols Because of Prior Therapy | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00808899 | Neuroblastoma Protocol 2008: Therapy for Children With Advanced Stage High Risk Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT01090128 | Study of Neoadjuvant Chemotherapy With Nanoparticle Albumin Bound Paclitaxel, Doxorubicin and Cyclophosphamide (NAC) in Patients With Stages II-III Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00590785 | Phase III Comparison of Adjuvant Chemotherapy W/High-Dose Cyclophosphamide Plus Doxorubicin (AC) vs Sequential Doxorubicin Fol by Cyclophosphamide (A-C) in High Risk Breast Cancer Patients With 0-3 Positive Nodes (Intergroup, CALGB 9394) | breast cancer | Doxorubicin (NPC261012) | |
| NCT00530101 | The Magnetic Resonance Imaging Evaluation of Doxorubicin Cardiotoxicity | breast cancer | Doxorubicin (NPC261012) | |
| NCT00796120 | An Efficacy and Safety Study of Trabectedin Versus Doxorubicin-Based Chemotherapy in Participants With Translocation-Related Sarcomas (TRS) | sarcoma | Doxorubicin (NPC261012) | |
| NCT00499122 | NOV-002, Doxorubicin, Cyclophosphamide, and Docetaxel in Women With Newly Diagnosed Stage II or IIIC Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01784120 | A Phase II Trial of Doxorubicin and Genexol-PM in Patients With Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00206518 | Taxotere and Adriamycin/Cytoxan (AC) Validation in Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT00003784 | S9911, Combination Chemotherapy Plus Monoclonal Antibody Therapy in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00146562 | Pegfilgrastim and Darbepoetin Alfa in Support of Adjuvant Chemotherapy for Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00429299 | Wkly Taxol x 12 | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00581360 | Phase II Trial of Doxorubicin and Bortezomib in Patients With Incurable Adenoid Cystic Carcinoma of the Head and Neck | adenoid cystic carcinoma | Doxorubicin (NPC261012) | |
| NCT00083915 | DTPACE Followed by Tandem Transplant With Melphalan (MEL) 200 Versus MEL/Dexamethasone/Thalidomide (DT) Platinol/Adriamycin/Etoposide (PACE) Hybrid and DTPACE Consolidation | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00137111 | Therapy for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00451178 | A Study of Participants With Lymphoma Who Take R-CHOP and Enzastaurin Compared to Participants Who Take R-CHOP Only | lymphoma | Doxorubicin (NPC261012) | |
| NCT00801580 | My-HyperCVAD in the Treatment of Relapsed Refractory Adult Acute Lymphoid Leukemia | lymphoid leukemia | Doxorubicin (NPC261012) | |
| NCT00083551 | UARK 98-026 TT II: Multiple Myeloma Evaluating Anti-Angiogenesis With Thalidomide and Post-Transplant Consolidation Chemotherapy | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01702129 | Anti-EGFR Immunoliposomes in Solid Tumors | neoplasm | Doxorubicin (NPC261012) | |
| NCT02261805 | A Phase I/II Study of Ganetespib in Combination With Doxorubicin | small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT02445404 | Compare Efficacy of CHOP Versus Fractionated ICED in Transplant-eligible Patients With Previously Untreated PTCL | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00041210 | Combination Chemotherapy in Treating Patients With Previously Untreated Advanced Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00893516 | CD4 in Combination With CHOP in Treating Non-cutaneous Peripheral TCell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00628251 | Dose-finding Study Comparing Efficacy and Safety of a PARP Inhibitor Against Doxil in BRCA+ve Advanced Ovarian Cancer | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT02362165 | CyBorD vs. PAD in the Treatment of Newly Diagnosed Multiple Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01440088 | A Trial of TH-302 in Combination With Doxorubicin Versus Doxorubicin Alone to Treat Patients With Locally Advanced Unresectable or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT03201471 | Chidamide With R-CHOP Regimen for DLBCL Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00574587 | Trial for Locally Advanced Breast Cancer Using Vorinostat Plus Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT00326456 | MITO-2: A Study Comparing 2 Chemotherapy Regimens (Carboplatin/Liposomal Doxorubicin vs Carboplatin/Paclitaxel) in Patients With Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00102219 | A Study of Pemetrexed Plus Doxorubicin Given to Patients With Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00525642 | Pilot Study of the Safety & Efficacy of Two Docetaxel-Based Regimens Plus Bevacizumab for the Adjuvant Treatment of Subjects With Node Positive or High Risk Node Negative Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT03023358 | Compared the Efficacy and Safety of CDOP Combined With Chidamide and CDOP in de Novo Peripheral T Cell Lymphoma Patients | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00854568 | Comparison Study of Doxorubicin Versus Epirubicin-induced Cardiotoxicity in Patients With DLBCL | lymphoma | Doxorubicin (NPC261012) | |
| NCT02605915 | Safety and Pharmacokinetics of Atezolizumab Combination Treatments in Participants With HER2-Positive and HER2-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02379585 | Fasting on Newly Diagnosed Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04685616 | Brentuximab Vedotin in Early Stage Hodgkin Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02670317 | Phase II Study About Combination CHOP-21, Obinutuzumab and Ibrutinib in Untreated Young High Risk DLBCL Patients. | lymphoma | Doxorubicin (NPC261012) | |
| NCT04227990 | Plinabulin iv Solution in Prevention of TAC Induced Neutropenia | neutropenia | Doxorubicin (NPC261012) | |
| NCT01857934 | Therapy for Children With Advanced Stage Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT00004112 | Combination Chemotherapy With or Without Rituximab in Treating Patients With Newly Diagnosed Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03802071 | A Study of Durvalumab in Combination With Doxorubicin for Advanced Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT00003853 | 4'-Iodo-4'-Deoxydoxorubicin in Treating Patients With Primary Systemic Amyloidosis | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00439296 | ABT-751 With Chemotherapy for Relapsed Pediatric ALL | childhood acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00186849 | Therapy for Children With Advanced Stage High Risk Neuroblastoma | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT04638790 | First Line Chemotherapy for Classical Hodgkin Lymphoma in Russia (HL-Russia-1) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01696032 | SGI-110 in Combination With Carboplatin in Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00394251 | Study of Dose-dense Adriamycin Plus Cytoxan (AC) Followed by Either ABI-007 (Abraxane) or Taxol With Bevacizumab as Adjuvant Therapy for Patients With Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00911183 | Diffuse Large B Cell Non-Hodgkin's Lymphoma in the Vulnerable/Frail Elderly. A Multicentric Randomized Phase II Trial | lymphoma | Doxorubicin (NPC261012) | |
| NCT03340376 | Doxorubicin Alone Versus Atezolizumab Alone Versus Doxorubicin and Atezolizumab in Recurrent Cervical Cancer | cervical cancer | Doxorubicin (NPC261012) | |
| NCT00524810 | Liposomal Doxorubicin and Docetaxel in Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01593020 | Neoadjuvant Study of Sequential Eribulin Followed by FAC Compared to Sequential Paclitaxel Followed by FEC in Early Stage Breast Cancer Not Overexpressing HER-2 | breast cancer | Doxorubicin (NPC261012) | |
| NCT00365417 | Therapy With Bevacizumab (BEV), Doxorubicin, and Cyclophosphamide Followed by BEV, Docetaxel, and Capecitabine Before Surgery Followed by BEV Alone After Surgery for Women With Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02903004 | Trial on Trabectedin (ET-743) vs Clinician's Choice Chemotherapy in Recurrent Ovarian, Primary Peritoneal or Fallopian Tube Cancers of BRCA Mutated or BRCAness Phenotype Patients | ovarian neoplasm | Doxorubicin (NPC261012) | |
| NCT00758732 | Docetaxel/Carboplatin Versus Docetaxel/Caelyx in Pretreated Patients With Ovarian Carcinoma | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00003595 | Combination Chemotherapy With or Without Monoclonal Antibody Therapy in Treating Patients With Previously Untreated HIV-Associated Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00314977 | Sequential vs Upfront Intensified Neoadjuvant Chemotherapy in Patients With Large Resectable or Locally Advanced Breast Cancer. | breast cancer | Doxorubicin (NPC261012) | |
| NCT00017160 | Combination Chemotherapy, Radiation Therapy, and Surgery in Treating Patients With Primary or Recurrent Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02606305 | Study of Mirvetuximab Soravtansine in Combination With Bevacizumab, Carboplatin, Pegylated Liposomal Doxorubicin, Pembrolizumab, or Bevacizumab + Carboplatin in Participants With Folate Receptor Alpha (FRα) Positive Advanced Epithelial Ovarian Cancer, Primary Peritoneal, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00217425 | Bevacizumab and Combination Chemotherapy in Treating Patients With Peripheral T-Cell Lymphoma or Natural Killer Cell Neoplasms | lymphoma | Doxorubicin (NPC261012) | |
| NCT00903630 | Lenalidomide and Doxorubicin Hydrochloride Liposome in Recurrent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Doxorubicin (NPC261012) | |
| NCT01016054 | A Study of the Safety and Pharmacokinetics of AGS-8M4 Given in Combination With Chemotherapy in Women With Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02398240 | Brentuximab for Newly Diagnosed Hodgkin Disease | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00787527 | SAHA + CHOP in Untreated T-cell Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02449265 | Efficacy of Consolidative Involved-site Radiotherapy for Patients With Limited-stage Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00549848 | Total Therapy Study XVI for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03420014 | Treatment of Metastatic Soft Tissue Sarcoma (STS) Patients (FIBROSARC USA) | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT01858207 | Combine TACE and RFA Versus RFA Monotherapy in Unilobar HCC of 3.1 to 7 cm Patient | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT02855359 | Denintuzumab Mafodotin (SGN-CD19A) Combined With RCHOP or RCHP Versus RCHOP Alone in Diffuse Large B-Cell Lymphoma or Follicular Lymphoma | diffuse large B-cell lymphoma;follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT01885013 | Myocet + Cyclophosphamide + Metformin Vs Myocet + Cyclophosphamide in 1st Line Treatment of HER2 Neg. Metastatic Breast Cancer Patients | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT04594798 | A Study of Polatuzumab Vedotin, Rituximab and Dose Attenuated CHP in Older Patients With DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04765228 | Pegylated Liposomal Doxorubicin Combined With Anlotinib for Neoadjuvant Treatment of Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT04213794 | Heated Intra-peritoneal Chemotherapy With Doxorubicin and Cisplatin for the Treatment of Resectable, Refractory, or Recurrent Abdominal or Pelvic Tumors in Pediatric Patients, T.O.A.S.T. I.T. Study | rhabdomyosarcoma | Doxorubicin (NPC261012) | |
| NCT00082095 | To Compare Treatment With Doxorubicin or Capecitabine for Metastatic Breast Cancer in Women 60 Years and Older | breast cancer | Doxorubicin (NPC261012) | |
| NCT00734877 | UARK 2013-13, Total Therapy 4B - Formerly 2008-01 - A Phase III Trial for Low Risk Myeloma | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00944801 | Pegylated Liposomal Doxorubicine and Prolonged Temozolomide in Addition to Radiotherapy in Newly Diagnosed Glioblastoma | glioblastoma multiforme | Doxorubicin (NPC261012) | |
| NCT00176293 | Randomized Phase II Trial of Doxil With or Without Dexamethasone for Metastatic Hormone Refractory Prostate Cancer | prostate cancer | Doxorubicin (NPC261012) | |
| NCT01864109 | Irinotecan and Temozolomide in Combination With Existing High Dose Alkylator Based Chemotherapy for Treatment of Patients With Newly Diagnosed Ewing Sarcoma | Ewing sarcoma | Doxorubicin (NPC261012) | |
| NCT02838225 | DA Versus DAC as Postoperative Adjuvant Treatment for Early-stage Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05018520 | The Safety and Effectiveness of 4R-CHOP+4R vs 6R-CHOP+2R in Newly Diagnosed Patients With DLBCL in Low Risk | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00486668 | A Study of AC Followed by a Combination of Paclitaxel Plus Trastuzumab or Lapatinib or Both Given Before Surgery to Patients With Operable HER2 Positive Invasive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00877006 | Study of Bendamustine Hydrochloride and Rituximab (BR) Compared With R-CVP or R-CHOP in the First-Line Treatment of Patients With Advanced Indolent Non-Hodgkin's Lymphoma (NHL) or Mantle Cell Lymphoma (MCL) - Referred to as the BRIGHT Study | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00542191 | Phase II Trial of Neoadjuvant Metronomic Chemotherapy in Triple-Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00264953 | HD11 for Intermediate Stages | lymphoma | Doxorubicin (NPC261012) | |
| NCT00990860 | Study in Asia of the Combination of TACE With Sorafenib in HCC Patients | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT04322318 | A Study of Combination Chemotherapy for Patients With Newly Diagnosed DAWT and Relapsed FHWT | kidney Wilms tumor | Doxorubicin (NPC261012) | |
| NCT02772822 | A Study Comparing the Efficiency and Safety of S-CHOP(Cyclophosphamide, Hydroxydaunomycin, Oncovin, and Prednisone) Versus R-CHOP in Untreated CD20(Cluster of Differentiation Antigen 20)-Positive DLBCL Patients | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05179733 | The Efficacy and Safety of ZR2 Versus R-miniCHOP in the Treatment of Unfit or Frail de Novo Diffuse Large B-cell Lymphoma Patients Aged Older Than or Equal to 70 Years | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT05097248 | Camrelizumab in Combination With PLD and Losartan in Patients With TNBC Who Have Received ≦ 1 Line of Chemotherapy | breast cancer | Doxorubicin (NPC261012) | |
| NCT04203641 | L-DOS47 Plus Doxorubicin in Advanced Pancreatic Cancer | pancreatic carcinoma | Doxorubicin (NPC261012) | |
| NCT00610792 | Phase 2 Study of Twice Weekly VELCADE and CAELYX in Patients With Ovarian Cancer Failing Platinum Containing Regimens | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT02978495 | Neoadjuvant Carboplatin in Triple Negative Breast Cancer | Hereditary breast and ovarian cancer syndrome | Doxorubicin (NPC261012) | |
| NCT00262990 | Patupilone Versus Doxorubicin in Patients With Ovarian, Primary Fallopian, or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneal neoplasm | Doxorubicin (NPC261012) | |
| NCT03853044 | Study Evaluating the Safety and Efficacy of C-CHOP in Untreated Subjects With Angioimmunoblastic T Cell Lymphoma | angioimmunoblastic T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04025593 | Biomarker Guided Treatment in DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01523977 | Everolimus With Multiagent Re-Induction Chemotherapy in Pediatric Patients With ALL | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00083226 | Doxorubicin and Bortezomib in Treating Patients With Liver Cancer | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00026208 | Combination Chemotherapy Plus Low-Dose Radiation Therapy in Treating Patients With Stage I or Stage IIA Hodgkin's Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01964391 | A Study of Participant Satisfaction and Safety With Subcutaneously Administered Trastuzumab (Herceptin) in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05207514 | Compare the Efficacy and the Safety of Doxorubicin and Cyclophosphamide Followed by Taxotere Versus Doxorubicin and Cyclophosphamide Nanoxel M as Neoadjuvant Chemotherapy in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04859465 | Albumin-bound Paclitaxel Combined With Liposomal Doxorubicin in the Treatment of Advanced or Unresectable Angiosarcoma | angiosarcoma | Doxorubicin (NPC261012) | |
| NCT00129376 | Doxorubicin and Cyclophosphamide (AC) Followed by Weekly Docetaxel as Neoadjuvant Treatment of Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT03808480 | Nivolumab After Cyclophosphamide and Doxorubicin Induction Therapy in NSCLC With PD-L1<10% | non-small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT01358747 | Study of a Treatment Driven by Early PET Response to a Treatment Not Monitored by Early PET in Patients With AA Stage 3-4 or 2B HL | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00777673 | Preoperative Chemotherapy in Triple Negative Invasive Breast Cancer That Can be Removed by Surgery. | breast cancer | Doxorubicin (NPC261012) | |
| NCT01767155 | Zoptarelin Doxorubicin (AEZS 108) as Second Line Therapy for Endometrial Cancer | endometrial cancer | Doxorubicin (NPC261012) | |
| NCT04221035 | High-Risk Neuroblastoma Study 2 of SIOP-Europa-Neuroblastoma (SIOPEN) | neuroblastoma | Doxorubicin (NPC261012) | |
| NCT02710734 | Risk Enabled Therapy After Initiating Neoadjuvant Chemotherapy for Bladder Cancer (RETAIN) | urinary bladder carcinoma | Doxorubicin (NPC261012) | |
| NCT01358877 | A Study of Pertuzumab in Addition to Chemotherapy and Trastuzumab as Adjuvant Therapy in Participants With Human Epidermal Growth Receptor 2 (HER2)-Positive Primary Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00446030 | Neoadjuvant Study With Chemotherapy, Lapatinib And Trastuzumab In Breast Cancer | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT02861222 | Myocet® in Children With Relapsed or Refractory Non-brainstem Malignant Glioma | malignant glioma | Doxorubicin (NPC261012) | |
| NCT00060385 | Combination Chemotherapy With or Without Etoposide in Treating Older Patients With Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT04506554 | A Study of Risk Enabled Therapy After Neoadjuvant Immunochemotherapy for Bladder Cancer | urinary bladder carcinoma | Doxorubicin (NPC261012) | |
| NCT05386524 | Sintilimab and Bevacizumab Biosimilar Combined With PLD in mTNBC | breast cancer | Doxorubicin (NPC261012) | |
| NCT04293393 | Neoadjuvant Study Chemotherapy vs Letrozole + Abemaciclib in HR+/HER2- High/Intermediate Risk Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT02012088 | Clinical Trial to Evaluate R-COMP Versus R-CHOP in Newly Diagnosed Patients With Non-localised Diffuse Large B-cell Lymphoma (DLBCL)/Follicular Lymphoma Grade IIIb | lymphoma | Doxorubicin (NPC261012) | |
| NCT04498793 | Study of Tislelizumab Plus Chemotherapy vs Chemotherapy as Perioperative Treatment in Participants With HER2 Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00256243 | Neoadjuvant Biweekly Treatment Followed by Weekly Treatment of Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00440726 | Bortezomib With Chemotherapy for Relapsed Pediatric Acute Lymphoblastic Leukemia (ALL) | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT00878800 | A Phase I/II Clinical Trial of PXD101 in Combination With Doxorubicin in Patients With Soft Tissue Sarcomas | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01840592 | Sorafenib Plus Doxorubicin in Patients With Advanced Hepatocellular Carcinoma With Disease Progression on Sorafenib | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT04480099 | Targeted Drug Combined With CHOP in the Treatment of Newly Diagnosed Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT04024462 | A Two-Arm Study to Evaluate the Pharmacokinetics, Efficacy, and Safety of Subcutaneous Administration of the Fixed-Dose Combination of Pertuzumab and Trastuzumab in Combination With Chemotherapy in Chinese Participants With HER2-Positive Early Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00970385 | Study About Treatment of Newly Diagnosed Non Cutaneous Peripheral T Cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00706953 | A Study of Bortezomib and Pegylated Liposomal Doxorubicin in Patients With Relapsed Multiple Myeloma Previously Treated With Bortezomib | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT00536393 | Treatment of Disseminated High Grade Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02958163 | Clinical Trial Comparing TACE With TACE + SABR in Stage BCLC B HCC (HepSTAR) | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT04569032 | A Study of Brentuximab Vedotin and CHP in Frontline Treatment of PTCL With Less Than 10% CD30 Expression | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02414568 | Bendamustine Study in Classical Hodgkin Lymphoma Patients Over 60 Treated by Prednisone, Vinblastine and Doxorubicin | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00004179 | Combination Chemotherapy With or Without Rituximab in Treating Patients With Relapsed Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT03407144 | Safety and Efficacy of Pembrolizumab (MK-3475) in Children and Young Adults With Classical Hodgkin Lymphoma (MK-3475-667/KEYNOTE-667) | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT01476787 | Combined Rituximab and Lenalidomide Treatment for Untreated Patients With Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT01258634 | A Study of Pre-Operative Treatment of Newly-Diagnosed, Surgically-Resectable Osteosarcoma With Doxorubicin, Ifosfamide, Etoposide, and Cisplatin With Early Metabolic Assessment of Response | osteosarcoma | Doxorubicin (NPC261012) | |
| NCT05200312 | A Phase II Study of Zanubrutinib, Lenalidomide Plus R-CHOP as the First-line Treatment for Diffused Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00136565 | Study of Bortezomib Combined With ACVBP in Peripheral T-cell Lymphoma | unspecified peripheral T-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00507962 | Cisplatin HAI Study in Patients With Advanced Cancer and Dominant Liver Involvement | cancer | Doxorubicin (NPC261012) | |
| NCT03437070 | Trabectedin, Doxorubicin and Olaratumab in Patients With Metastatic or Recurrent Leiomyosarcoma | leiomyosarcoma | Doxorubicin (NPC261012) | |
| NCT04974996 | A Study to Evaluate the Tolerability, Safety, Pharmacokinetics, and Antitumor Activity of Loncastuximab Tesirine in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Participants With Previously Untreated Diffuse Large B-cell Lymphoma (LOTIS-8) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00022945 | Safety and Efficacy Study of Iodine-131 Anti-B1 Antibody Plus CHOP For Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02412670 | Chemotherapy Before Surgery in Treating Patients With High Grade Upper Urinary Tract Cancer | urothelial carcinoma | Doxorubicin (NPC261012) | |
| NCT00949325 | Safety and Efficacy Study of Torisel and Liposomal Doxorubicin for Patients With Recurrent Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT03294577 | Plinabulin vs. Pegfilgrastim in Prevention of TAC Induced Neutropenia | neutropenia | Doxorubicin (NPC261012) | |
| NCT03485118 | RHCACD20MA (HS006) Combined With CHOP (Hi-CHOP) in Patients With Previously Untreated Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03575520 | Safety and Efficacy of Pegteograstim in Korean Breast Cancer Patients | breast cancer | Doxorubicin (NPC261012) | |
| NCT04780464 | A 3 Arm Randomized Study on Health-related QoL of Elderly Patients With Advanced Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00877071 | LC Drug Eluting Bead for Treatment of Liver Cancer Which Cannot be Surgically Removed | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT00974324 | Endostar Combined With CHOP Regimen as First Line Chemotherapy for Peripheral T Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00784537 | High-dose Chemotherapy and Stem Cell Transplantation, in Patients PET-2 Positive, After 2 Courses of ABVD and Comparison of RT Versus no RT in PET-2 Negative Patients | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT05100628 | A Study of NOX66 Plus Doxorubicin in Anthracycline-naïve, Adult Patients With Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT01390584 | Chemotherapy Based on PET Scan in Treating Patients With Stage I or Stage II Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00483509 | Study of NGR-hTNF in Combination With Doxorubicin in Patients Affected by Metastatic Small Cell Lung Carcinoma | small cell lung carcinoma | Doxorubicin (NPC261012) | |
| NCT00575406 | Multicentre Study to Determine the Cardiotoxicity of R-CHOP Compared to R-COMP in Patients With Diffuse Large B-Cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT03647072 | PPI Versus Histamine Antagnists as Adjuvant to Chemotherapy | lymphoma | Doxorubicin (NPC261012) | |
| NCT02377752 | A Study of Olaratumab in Japanese Participants With Advanced Cancer | neoplasm | Doxorubicin (NPC261012) | |
| NCT00204646 | Neoadjuvant Adriamycin and Ifosfamide Plus High-Dose ICE in Patients With Soft Tissue Sarcoma (STS) | sarcoma | Doxorubicin (NPC261012) | |
| NCT00671658 | Modified Hyper-CVAD (Cyclophosphamide, Vincristine, Adriamycin, and Dexamethasone) Program for Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03317457 | Durvalumab and Tremelimumab Compared to Doxorubicin in Patients With Advanced or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT00054665 | PS-341 Alone and PS-341 Plus EPOCH Chemotherapy to Treat Non-Hodgkin's Lymphoma | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT05058404 | Shortened vs Standard Chemotherapy Combined With Immunotherapy for the Initial Treatment of Patients With High Tumor Burden Follicular Lymphoma | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02247869 | Dose-dense ABVD First Line Therapy in Early Stage Unfavorable Hodgkin's Lymphoma | Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT00005800 | Doxorubicin and Docetaxel in Treating Women With Stage III Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02527772 | Liposomal Doxorubicin Plus Gemcitabine Versus Oxaliplatin Plus Fluorouracil/Leucovorin for Hepatocellular Carcinoma | hepatocellular carcinoma | Doxorubicin (NPC261012) | |
| NCT03117751 | Total Therapy XVII for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia and Lymphoma | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT05498896 | Investigate the Contribution of Ipatasertib to Neoadjuvant Chemotherapy Plus Atezolizumab in TNBC | breast cancer | Doxorubicin (NPC261012) | |
| NCT00450385 | Genes in Predicting Outcome of Patients With DLBCL Treated With Rituximab and Combination Chemotherapy (R-CHOP) | lymphoma | Doxorubicin (NPC261012) | |
| NCT02326025 | A Study of Olaratumab and Doxorubicin in Participants With Advanced Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT01100359 | Liposome-Encapsulated Doxorubicin Citrate and Carboplatin in Treating Patients With Advanced or Metastatic Recurrent Endometrial Cancer | endometrial cancer | Doxorubicin (NPC261012) | |
| NCT00819221 | AZD2281 in Combination With Liposomal Doxorubicin in Advanced Solid Tumours | neoplasm | Doxorubicin (NPC261012) | |
| NCT03409198 | Phase IIb Study Evaluating Immunogenic Chemotherapy Combined With Ipilimumab and Nivolumab in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT03004833 | Nivolumab and AVD in Early-stage Unfavorable Classical Hodgkin Lymphoma | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT00494780 | Ofatumumab (Humax-CD20) With CHOP (Cyclophosphamide,Doxorubicin, Vincristine, Predisolone) in Follicular Lymphoma (FL) Patients | follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT02881086 | Optimization of Therapy in Adult Patients With Newly Diagnosed Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma by Individualised, Targeted and Intensified Treatment | acute lymphoblastic leukemia;lymphoblastic lymphoma | Doxorubicin (NPC261012) | |
| NCT00118209 | Rituximab and Combination Chemotherapy in Treating Patients With Diffuse Large B-Cell Non-Hodgkin's Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT02451943 | A Study of Doxorubicin Plus Olaratumab (LY3012207) in Participants With Advanced or Metastatic Soft Tissue Sarcoma | soft tissue sarcoma | Doxorubicin (NPC261012) | |
| NCT02421588 | Clinical Trial of Lurbinectedin (PM01183) in Platinum Resistant Ovarian Cancer Patients | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00004067 | Doxorubicin and Cyclophosphamide Plus Paclitaxel With or Without Trastuzumab in Treating Women With Node-Positive Breast Cancer That Overexpresses HER2 | breast cancer | Doxorubicin (NPC261012) | |
| NCT01851733 | MRI-Guided Laser Surgery and Doxorubicin Hydrochloride in Treating Patients With Recurrent Glioblastoma Multiforme | glioblastoma multiforme | Doxorubicin (NPC261012) | |
| NCT00211185 | A Study of ONTAK and CHOP in Newly Diagnosed, Peripheral T-Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01788137 | A Study of Improving the Efficacy of Treatment in High Risk T Cell Lymphoma Patients | T-cell acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT05346107 | PLD-cyclophosphamide-Nab-P Continuously Combined With Dual HER2 Blockage for HER2-positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT02889523 | Study of Tazemetostat in Newly Diagnosed Diffuse Large B Cell and Follicular Lymphoma Patients Treated by Chemiotherapy | diffuse large B-cell lymphoma;follicular lymphoma | Doxorubicin (NPC261012) | |
| NCT05453500 | Chemotherapy (DA-EPOCH+/-R) and Targeted Therapy (Tafasitamab) for the Treatment of Newly-Diagnosed Philadelphia Chromosome Negative B Acute Lymphoblastic Leukemia | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT01148628 | Dose-finding Study of CAELYXTM and RAD001 in Patients With Advanced Solid Tumors | neoplasm | Doxorubicin (NPC261012) | |
| NCT00001300 | A Randomized Study of the Effect of Adjuvant Chemotherapy With Doxorubicin and Ifosfamide With Mesna in the Treatment of High-Grade Adult Extremity Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT03425656 | Comparing Efficacy and Safety of AryoGen Pharmed Biosimilar Trastuzumab (AryoTrust) Versus Herceptin® in Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01113957 | A Trial of ABT-888 in Combination With Temozolomide Versus Pegylated Liposomal Doxorubicin Alone in Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00577993 | Fludarabine, Mitoxantrone, and Dexamethasone (FND) Plus Rituximab for Lymphoma Patients | lymphoma | Doxorubicin (NPC261012) | |
| NCT03161132 | Resistant Ovarian Cancer, Olaparib and Liposomal Doxorubicin | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT03643276 | Treatment Protocol for Children and Adolescents With Acute Lymphoblastic Leukemia - AIEOP-BFM ALL 2017 | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT03164993 | Atezolizumab Combined With Immunogenic Chemotherapy in Patients With Metastatic Triple-negative Breast Cancer | breast carcinoma | Doxorubicin (NPC261012) | |
| NCT01009970 | Study With Rituximab, Cyclophosphamide, Doxorubicin Liposomal (Myocet®), Vincristine, Prednisone, (R-COMP) to Treat Non-Hodgkin's Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02723994 | A Phase 2 Study of Ruxolitinib With Chemotherapy in Children With Acute Lymphoblastic Leukemia | leukemia | Doxorubicin (NPC261012) | |
| NCT01118026 | Response-Based Therapy Assessed By PET Scan in Treating Patients With Bulky Stage I and Stage II Classical Hodgkin Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT00878254 | Rituximab and Combination Chemotherapy in Treating Patients With Previously Untreated Mantle Cell Lymphoma | Mantle cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02596971 | A Study of Atezolizumab in Combination With Either Obinutuzumab Plus Bendamustine or Obinutuzumab Plus (+) Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) in Participants With Follicular Lymphoma (FL) or Rituximab + CHOP in Participants With Diffuse Large B-Cell Lymphoma (DLBCL) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT00165178 | Treatment of Acute Lymphoblastic Leukemia in Children | acute lymphoblastic leukemia | Doxorubicin (NPC261012) | |
| NCT04243434 | PK Study on Ready-to-Use Injection (VSLI-RTU) 1 Vial & 3 Vial Formulation Marqibo® in Hematological Malignant Patients | Abnormality of blood and blood-forming tissues | Doxorubicin (NPC261012) | |
| NCT02448537 | A Phase II Multi-Strata Study of PM01183 as a Single Agent or in Combination With Conventional Chemotherapy in Metastatic and/or Unresectable Sarcomas | sarcoma | Doxorubicin (NPC261012) | |
| NCT01705158 | Myocet ® - Carboplatine in Ovarian Cancer in Relapse, Sensitive to the Platinum | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT00634205 | Phase II Study of Valproate and Doxorubicin in Malignant Mesothelioma | mesothelioma | Doxorubicin (NPC261012) | |
| NCT01998906 | A Study of Herceptin (Trastuzumab) in Combination Chemotherapy in Women With Locally Advanced Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT04877275 | ATG-010(Selinexor) in Combination With Chemotherapy in RRMM | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01185964 | A Study of Olaratumab in Soft Tissue Sarcoma | sarcoma | Doxorubicin (NPC261012) | |
| NCT01548573 | Tandem Auto Transplantation in Myeloma Patients With <12 Months of Prior Treatment | multiple myeloma | Doxorubicin (NPC261012) | |
| NCT01622361 | Premenopausal Patient With Hormone Responsive, HER2 Negative, Lymph Node Positive Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT05201248 | A Study to Evaluate Adverse Events and Change in Disease Activity of Subcutaneous (SC) Epcoritamab As Monotherapy or Combined With Standard of Care Therapies in Adult Participants in China With B-Cell Non-Hodgkin Lymphoma | neoplasm of mature B-cells | Doxorubicin (NPC261012) | |
| NCT04624984 | PD-1 Inhibitor or PD-1 Inhibitor Plus GVD for Relapsed/Refractory CHL | classic Hodgkin lymphoma | Doxorubicin (NPC261012) | |
| NCT01889069 | A Study to Evaluate Safety, Efficacy and Pharmacokinetics of Rituximab (MabThera/Rituxan) in Participants With Diffuse Large B Cell Lymphoma (DLBCL) or Follicular Lymphoma (FL) | lymphoma | Doxorubicin (NPC261012) | |
| NCT03943901 | Split-Dose R-CHOP for Older Adults With DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01388621 | Carboplatin-based Chemotherapy With or Without Panitumumab in Platinum-sensitive Recurrent Ovarian Cancer | ovarian cancer | Doxorubicin (NPC261012) | |
| NCT05406401 | A Study of Zilovertamab Vedotin (MK-2140) in Combination With Cyclophosphamide, Doxorubicin, and Prednisone Plus Rituximab or Rituximab Biosimilar (Truxima) (R-CHP) in Participants With Diffuse Large B-Cell Lymphoma (DLBCL) (MK-2140-007) | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02529852 | A Phase I/II Study of Lenalidomide and Obinutuzumab With CHOP for Diffuse Large B Cell Lymphoma | lymphoma | Doxorubicin (NPC261012) | |
| NCT01200758 | A Study of Rituximab (MabThera) Subcutaneous (SC) Versus Rituximab (MabThera) Intravenous in Participannts With Follicular Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT02285062 | Efficacy and Safety Study of Lenalidomide Plus R-CHOP Chemotherapy Versus Placebo Plus R-CHOP Chemotherapy in Untreated ABC Type Diffuse Large B-cell Lymphoma | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT02560051 | Hormone Therapy Plus Chemotherapy as Initial Treatment for Local Failures or Advanced Prostate Cancer | prostate adenocarcinoma | Doxorubicin (NPC261012) | |
| NCT02622074 | Effect of Trastuzumab on Disease Free Survival in Early Stage HER2-Negative Breast Cancer Patients With ERBB2 Expressing Disseminated Tumor Cells | breast neoplasm | Doxorubicin (NPC261012) | |
| NCT00591851 | Phase II Study of Dose-Dense Doxurubicin and Cyclophosphamide (AC) Followed By Paclitaxel With Trastuzumab in HER2/ NEU-Amplified Breast Cancer: Feasibility | breast cancer | Doxorubicin (NPC261012) | |
| NCT03059615 | A Phase 2a, Open-Label, Two Stage Study of Nerofe or Nerofe With Doxorubicin in Subjects With AML or MDS | acute myeloid leukemia;myelodysplastic syndrome | Doxorubicin (NPC261012) | |
| NCT05351346 | Genotype-guided Treatment in DLBCL | diffuse large B-cell lymphoma | Doxorubicin (NPC261012) | |
| NCT01966471 | A Study of Trastuzumab Emtansine (Kadcyla) Plus Pertuzumab (Perjeta) Following Anthracyclines in Comparison With Trastuzumab (Herceptin) Plus Pertuzumab and a Taxane Following Anthracyclines as Adjuvant Therapy in Participants With Operable HER2-Positive Primary Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00323323 | CHOP and Campath-1H in Previously Untreated Aggressive T/NK-Cell Lymphomas | non-Hodgkins lymphoma | Doxorubicin (NPC261012) | |
| NCT04664972 | The TP Regimen in the Treatment of Early Triple Negative Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT01279291 | Study of Anti-HB-EGF Antibody KHK2866 in Subjects With Advanced Solid Tumors and Ovarian Cancer | Fallopian Tube Carcinoma;ovarian neoplasm;peritoneal neoplasm | Doxorubicin (NPC261012) | |
| NCT03023124 | Study With Trabectedin Versus Adriamycin Plus Dacarbazine, in Patients With Advanced Solitary Fibrous Tumor | neoplasm | Doxorubicin (NPC261012) | |
| NCT02299999 | SAFIR02_Breast - Efficacy of Genome Analysis as a Therapeutic Decision Tool for Patients With Metastatic Breast Cancer | breast cancer | Doxorubicin (NPC261012) | |
| NCT00169156 | A Phase II Study of Rituximab Combined With CHOP in T-cell Angio-immunoblastic Lymphoma | angioimmunoblastic T-cell lymphoma | Doxorubicin (NPC261012) |
❱❱❱ Associated Human Diseases and Detailed Association Evidence
How do we define the Plant-Targeted Human Disease Association?
Associated human diseases of an individual plant are summurized based on FOUR types of association evidence, these include:
❶ Association by Therapeutic Target: Bioactive protein targets of the plant were defined in "Molecular Targets" section, target-disease associations collected from TTD database were subsequently used to build the associations between the plant and its targeted human diseases.
❷ Association by Disease Gene Reversion: Plant and a specific disease will be associated when >= 1 plant target gene overlaped with disease's DEGs.
❸ Association by Clinical Trials of Plant: Plant and a specific disease will be associated when >= 1 clinical trial (the plant is the intervetion) can be matched in ClinicalTrials.gov database.
❹ Association by Clinical Trials of Plant Ingredients: Plant and a specific disease will be associated when >= 1 clinical trial (the plant ingredient is the intervetion) can be matched in ClinicalTrials.gov database.
Associated Disease of the Plant |
Association Type & Detailed Evidence |
|---|---|
Diffuse large B-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A81 |
TOP2A
NCT00169130,NCT05179733,NCT02449265,NCT05389423,NCT01852435,NCT05428670,NCT00140595,NCT04529772,NCT04002947,NCT02596971,NCT02449278,NCT04661007,NCT01009970,NCT01848132,NCT00379574,NCT03758989,NCT02772822,NCT05406401,NCT01148446,NCT04517435,NCT02753647,NCT04835870,NCT04594798,NCT01004991,NCT04790903,NCT04139304,NCT03485118,NCT02889523,NCT04546620,NCT01622439,NCT05290090,NCT00582725,NCT03274492,NCT00931918,NCT02617485,NCT02285062,NCT04021992,NCT01925612,NCT01804127,NCT03129828,NCT01285765,NCT02855359,NCT01724021,NCT02734771,NCT02951728,NCT03018626,NCT01415765,NCT01659099,NCT05200312,NCT02531308,NCT02428751,NCT00850512,NCT04660799,NCT02867566,NCT01649856,NCT03201471,NCT03536039,NCT01287741,NCT01040871,NCT04025593,NCT04824092,NCT04974996,NCT03399747,NCT02054559,NCT04263584,NCT02890602,NCT00268853,NCT02792491,NCT00135499,NCT04023916,NCT01459887,NCT00499018,NCT03943901,NCT00144807,NCT03650933,NCT05498259,NCT03003520,NCT02228512,NCT00575406,NCT05189197,NCT04332822,NCT05234684,NCT05018520,NCT02733380,NCT05351346,NCT00355199,NCT04231448,NCT01087424,NCT03225924,NCT05422066 |
Mature B-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85 |
TOP2A
NCT00054665,NCT04745949,NCT00324467,NCT03467373,NCT02787239,NCT00486759,NCT03677141,NCT05201248 |
Unspecified malignant neoplasms of unspecified sitesDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D4Z |
NCT00798252,NCT00608803,NCT00507962,NCT01110603,NCT00305084,NCT00147225,NCT00124956,NCT02483247,NCT00703170
|
Classical Hodgkin lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B30.1 |
NCT03233347,NCT05008224,NCT03712202,NCT03527628,NCT02414568,NCT05404945,NCT03004833,NCT02661503,NCT04624984
|
Neuroblastoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH85Z0 |
NCT01857934,NCT02771743,NCT03786783,NCT00808899,NCT00186849,NCT00135135,NCT04221035,NCT00578864,NCT01704716
|
Mature T-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90 |
HIF1A,TOP2A
NCT01414855,NCT03975205,NCT00770224,NCT00004010,NCT00974324,NCT00451178,NCT00003215,NCT01139359,NCT00003595,NCT00210379,NCT00920153,NCT00064116,NCT03188198,NCT00264953,NCT00911183,NCT00003421,NCT02605694,NCT00003784,NCT00041210,NCT01974440,NCT00536393,NCT00005584,NCT00003578,NCT05006664,NCT01992653,NCT04980222,NCT00004179,NCT01889069,NCT01516567,NCT00005867,NCT02359162,NCT00854568,NCT04164368,NCT00265031,NCT01321008,NCT00369681,NCT00052936,NCT05075460,NCT01404936,NCT00060346,NCT00101101,NCT00450385,NCT00002557,NCT00217425,NCT02529852,NCT00577993,NCT00049595,NCT00002565,NCT01831505,NCT00290498,NCT00787527,NCT01855750,NCT00211185,NCT00893516,NCT00577629,NCT00945724,NCT04884035,NCT01527422,NCT00004031,NCT00822120,NCT00299182,NCT01046825,NCT00504504,NCT00841945,NCT01390584,NCT00006721,NCT00003389,NCT00450801,NCT00041132,NCT00003541,NCT02670317,NCT01490047,NCT03647072,NCT00060385,NCT00554164,NCT00433433,NCT02918747,NCT00118209,NCT00265018,NCT01118026,NCT02012088,NCT00003150,NCT00004112 |
Liver cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12 |
TOP2A,AURKA
NCT00093444,NCT02753881,NCT00003907,NCT01009801,NCT01324076,NCT00956930 |
Fallopian tube cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C74 |
NCT01100372,NCT02606305,NCT00189553,NCT00903630,NCT01666444,NCT00003896,NCT00262990,NCT02865811
|
NeoplasmsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 02 |
NCT01214668,NCT02377752,NCT01455532,NCT01702129,NCT03023124,NCT01148628,NCT03617432,NCT00819221
|
LeukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60-2B33 |
NCT00038610,NCT03975205,NCT01319981,NCT00072007,NCT00613457,NCT02723994,NCT00109837,NCT01516580
|
LeiomyosarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B58 |
NCT04996004,NCT02131480,NCT02997358,NCT03420014,NCT03437070,NCT05099666,NCT00815945
|
Malignant neoplasms of bladder, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94.Z |
NCT00635726,NCT01812369,NCT00808639,NCT00506155,NCT04101812,NCT02177695
|
Kaposi sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B57 |
TOP2A
NCT00923936,NCT00002318,NCT02659930,NCT00002105 |
Carcinomas of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.0 |
NCT03598270,NCT02107378,NCT02419495,NCT00312650,NCT03542669
|
OsteosarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B51 |
NCT01258634,NCT00145639,NCT00691236,NCT00180908,NCT00586846
|
Ovarian cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73 |
TOP2A
NCT03467178,NCT00635193,NCT03804866,NCT00189553,NCT00976911,NCT01696032,NCT01637532,NCT01113957,NCT01802749,NCT00861120,NCT00903630,NCT03639246,NCT04908787,NCT04348032,NCT00003896,NCT02475772,NCT04729387,NCT04887961,NCT01016054,NCT01388621,NCT03161132,NCT01379989,NCT00262990,NCT01666444,NCT02098343,NCT00610792,NCT02431559,NCT01705158,NCT03699449,NCT03539328,NCT00657878,NCT00484432,NCT00326456,NCT00722592,NCT00523380,NCT02421588,NCT00179725,NCT03335241,NCT03596281,NCT02606305,NCT01227941,NCT04679064,NCT05467670,NCT02865811,NCT00758732,NCT01358071,NCT01100372,NCT01809379 |
Solid tumour/cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00-2F9Z |
HIF1A,AURKA,EPAS1,TOP2A
|
Brain cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00 |
HIF1A,TOP2A,EPAS1
NCT01014767 |
Prostate cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C82 |
AURKA
NCT00176293,NCT01240629,NCT02494713 |
Acute myeloid leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60 |
TOP2A,AURKA
NCT03860844,NCT03059615 |
Burkitt lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH4KA9 |
NCT05389423,NCT00126191,NCT05270057,NCT00669877
|
Neuroendocrine carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C34 |
NCT01678664,NCT00001339,NCT00183742,NCT00094497
|
Neoplasms of unknown behaviour of urinary organsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F98 |
NCT02710734,NCT03669783,NCT04506554,NCT04383743
|
Malignant neoplasms of retroperitoneumDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C50 |
NCT02865811,NCT01100372,NCT00003896,NCT00903630
|
Angioimmunoblastic T-cell lymphomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH1J86 |
NCT00725231,NCT00169156,NCT03853044,NCT01719835
|
Endometrial cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76 |
NCT00401635,NCT01767155,NCT00883116,NCT01100359
|
Malignant neoplasms of corpus uteri, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.Z |
TOP2A,AURKA
NCT03517449,NCT03005015 |
Adult T-cell lymphoma or leukaemia, human T-cell lymphotropic virus type 1-associatedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90.5 |
NCT03007147,NCT01000285,NCT02228772,NCT01788137
|
Ewing sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B52 |
NCT01864109,NCT02063022,NCT00038142,NCT00568464
|
HepatoblastomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.01 |
TOP2A,AURKA
NCT03017326,NCT04478292 |
Hepatocellular carcinoma of liverDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.02 |
TOP2A,AURKA,TOP2A,AURKA
|
Precursor cell lymphoblastic leukaemia, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5J37 |
NCT03023046,NCT00968253,NCT03150693,NCT00671658,NCT00137111,NCT01186328,NCT05453500,NCT00476190,NCT00439296,NCT03117751,NCT00187161,NCT00131027,NCT03515200,NCT03571321,NCT03384654,NCT00165178,NCT00165087,NCT02420717,NCT01887587,NCT02419755,NCT03643276,NCT03991884,NCT00136435,NCT00549848,NCT03553238,NCT05303792,NCT04307576,NCT04996160,NCT00600977,NCT00890656,NCT01523977,NCT03817320,NCT00440726,NCT00973752,NCT03860844,NCT02881086,NCT03349281,NCT03020030,NCT01324180
|
Soft tissue disorderDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FB56 |
NCT03042819,NCT01168791,NCT00718484,NCT02451943,NCT04780464,NCT04910126,NCT02049905,NCT03317457,NCT05448820,NCT00502411,NCT00755261,NCT04032964,NCT04012827,NCT00017160,NCT03056001,NCT01861951,NCT03283696,NCT02783599,NCT03108300,NCT00878800,NCT00790244,NCT02255110,NCT00626704,NCT00102609,NCT04765228,NCT04776525,NCT04757337,NCT05100628,NCT01514188,NCT04656262,NCT03719430,NCT04874311,NCT03805022,NCT03698227,NCT02784015,NCT01440088,NCT04650984,NCT00484341
|
Multiple myelomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A83 |
NCT03004287,NCT01078441,NCT01492881,NCT00003853,NCT00925821,NCT01541332,NCT01215344,NCT00083551,NCT00574080,NCT01246063,NCT02186834,NCT04877275,NCT00319865,NCT00083538,NCT00734877,NCT00516191,NCT00869232,NCT00572169,NCT01328236,NCT00814541,NCT01394354,NCT00617591,NCT01070862,NCT01481194,NCT00215943,NCT00750815,NCT00148317,NCT02362165,NCT00366106,NCT01101594,NCT00441168,NCT02471820,NCT00871013,NCT00083915,NCT01177683,NCT00706953,NCT01548573
|
Hodgkin lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B30 |
NCT01356680,NCT00512980,NCT00816959,NCT04638790,NCT02275598,NCT03407144,NCT01056679,NCT03646123,NCT03618550,NCT02181738,NCT00784537,NCT00797472,NCT01468740,NCT01251107,NCT01652261,NCT01358747,NCT02292979,NCT01920932,NCT03159897,NCT02398240,NCT03755804,NCT02505269,NCT01712490,NCT03033914,NCT00225173,NCT03517137,NCT02979522,NCT01569204,NCT00736320,NCT04685616,NCT00038558,NCT01060904,NCT02298283,NCT00795613,NCT00443677,NCT00026208,NCT02247869
|
Malignant haematopoietic neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B33 |
TOP2A,AURKA
NCT00051311 |
Renal cell carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90 |
EPAS1
NCT02419495,NCT00068393 |
Small cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.1 |
NCT00483509,NCT02566993,NCT02261805
|
Non-small cell lung cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25 |
NCT03808480,NCT02419495,NCT04224337
|
Malignant neoplasm of pancreas, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.Z |
NCT04203641,NCT00426127,NCT00609765
|
Malignant neoplasms of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72 |
NCT01854255,NCT05318794,NCT04358341
|
Rhabdomyosarcoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0GA1 |
NCT01871766,NCT04213794,NCT04625907
|
Malignant neoplasm metastasis, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E2Z |
NCT02419495,NCT02677116,NCT03678883
|
Glioblastoma, primary, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0MB1 |
NCT00944801,NCT02372409,NCT01851733
|
Inflammatory carcinoma of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C62 |
NCT02125344,NCT02876302,NCT02623972
|
Pleural mesotheliomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C26 |
NCT00886028,NCT00859495,NCT00634205
|
Other specified malignant neoplasms of kidney, except renal pelvisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90.Y |
TOP2A,TOP2A
NCT04322318 |
Adenocarcinoma of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.0 |
TOP2A,AURKA
NCT04047004 |
Diffuse large B-cell lymphomasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A81 |
TOP2A,EPAS1,AURKA
|
Mesothelioma of pleuraDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C26.0 |
TOP2A,AURKA,DHCR7
|
Urothelial carcinoma of bladderDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94.2 |
TOP2A,AURKA,DHCR7
|
Renal cell carcinoma, chromophobe typeDisease Category: X.Extension CodesDisease ICD-11 Code: XH6153 |
TOP2A,AURKA,DHCR7
|
Malignant neoplasms of adrenal glandDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D11 |
TOP2A,AURKA,DHCR7
|
Pleomorphic sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B54 |
NCT01027910,NCT02326025,NCT05210374,NCT03880695,NCT02812654,NCT00204646,NCT01104298,NCT01185964,NCT00288431,NCT00204568,NCT00796120,NCT02448537,NCT04199026,NCT01605526,NCT04968106,NCT03678883,NCT03802071,NCT01746238,NCT00949325,NCT04535713,NCT00001300,NCT02732015,NCT00189137
|
Hepatocellular carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH4W48 |
NCT00471965,NCT00083226,NCT03192644,NCT01116635,NCT02527772,NCT01840592,NCT01858207,NCT01381211,NCT00108953,NCT03145558,NCT00990860,NCT02958163,NCT01004978,NCT05093920,NCT03937830,NCT00988195,NCT00057382,NCT01655693,NCT01327521,NCT00877071,NCT01281943,NCT01966133
|
Malignant lymphoma, not elsewhere classifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B33.5 |
NCT02406092,NCT00184002,NCT00719472,NCT00201318,NCT00193440,NCT05238064,NCT02626455,NCT01516580,NCT00455897,NCT00133302,NCT00825149,NCT00324467,NCT01777152,NCT03467373,NCT03571308,NCT01200758,NCT01332968,NCT00809341,NCT01746173,NCT00361621,NCT02055820,NCT00323323
|
Neoplasms of unknown behaviour of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F95 |
NCT00525642,NCT00174655,NCT00687440,NCT00543829,NCT03036488,NCT00174707,NCT00779129,NCT00021255,NCT02622074,NCT03678883,NCT01220128,NCT00429299,NCT01779050,NCT04895358,NCT00550771,NCT00194753,NCT00446030,NCT00849472,NCT00722293,NCT00121992
|
Adrenomedullary hyperfunctionDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A75 |
EPAS1,HIF1A
|
Lung cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25 |
AURKA,TOP2A
|
Primary haemophagocytic lymphohistiocytosisDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4A01.23 |
NCT02631109,NCT04077905
|
Malignant neoplasms of fallopian tube, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C74.Z |
NCT02419495,NCT01279291
|
Lymphoid leukaemia, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH7Q12 |
NCT00866749,NCT00801580
|
Peritoneal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C51 |
NCT01279291,NCT00262990
|
NeutropeniaDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4B00 |
NCT04227990,NCT03294577
|
Anaplastic large cell lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH1LC0 |
NCT01309789,NCT01719835
|
Glioma, malignantDisease Category: X.Extension CodesDisease ICD-11 Code: XH4RQ3 |
NCT02861222,NCT03678883
|
RetinoblastomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D02.2 |
NCT00186888,NCT01783535
|
Carcinosarcoma of uterusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.43 |
TOP2A,DHCR7
|
Malignant neoplasms of biliary tract, distal bile ductDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C15 |
TOP2A,AURKA
|
Squamous cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.2 |
TOP2A,AURKA
|
Adenocarcinoma of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.0 |
AURKA,DHCR7
|
Other specified malignant neoplasms of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.Y |
TOP2A,AURKA
|
Malignant neoplasms of oesophagusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B70 |
TOP2A,AURKA
|
Adenocarcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.0 |
TOP2A,AURKA
|
Invasive carcinoma of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C61 |
TOP2A,AURKA
|
Breast cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C60-2C6Y |
AURKA,TOP2A
NCT02938442,NCT00912444,NCT02685657,NCT00123929,NCT00669773,NCT01998906,NCT01131364,NCT00016406,NCT03497702,NCT02132949,NCT00431795,NCT00636441,NCT01672671,NCT00721747,NCT03933319,NCT01784120,NCT01057069,NCT00455533,NCT00574236,NCT05088057,NCT03425656,NCT02499367,NCT00250874,NCT03575520,NCT05207514,NCT00206466,NCT01204801,NCT00254592,NCT03071926,NCT05346107,NCT00002707,NCT01396655,NCT00493870,NCT01210768,NCT00944047,NCT05112536,NCT01818063,NCT00688740,NCT03197935,NCT00206518,NCT01333423,NCT00149214,NCT03726879,NCT03285607,NCT02562378,NCT00793377,NCT04243616,NCT02379585,NCT00003782,NCT00258960,NCT00136539,NCT03329378,NCT01796197,NCT05386524,NCT00789581,NCT00404066,NCT01227408,NCT00524810,NCT03409198,NCT02215876,NCT03725059,NCT01358877,NCT04293393,NCT00365365,NCT02053597,NCT00193115,NCT04498793,NCT00203372,NCT01959490,NCT03742986,NCT04664972,NCT01646034,NCT00303108,NCT05097248,NCT02641847,NCT00212082,NCT00001498,NCT04584112,NCT01990352,NCT01593020,NCT01964391,NCT04172259,NCT01705691,NCT00903656,NCT04024462,NCT01090128,NCT04038489,NCT00148681,NCT04159818,NCT00129376,NCT00542191,NCT02605915,NCT00278109,NCT00580333,NCT00256243,NCT00003352,NCT00591851,NCT02506777,NCT04443348,NCT04540692,NCT00201708,NCT00093795,NCT02488564,NCT01622361,NCT00365417,NCT05113251,NCT00434031,NCT01120171,NCT00486668,NCT00563953,NCT02032277,NCT04301739,NCT00546156,NCT02897700,NCT00408408,NCT05275777,NCT01176799,NCT01206881,NCT02790580,NCT03609047,NCT00082095,NCT03994107,NCT01172223,NCT00777673,NCT00024102,NCT03493854,NCT01966471,NCT00001384,NCT01940497,NCT01008150,NCT00887536,NCT00312208,NCT01669239,NCT03949634,NCT01547741,NCT00191789,NCT00005800,NCT00559845,NCT02472353,NCT02850419,NCT02838225,NCT03498716,NCT01270373,NCT04762901,NCT00590785,NCT00346229,NCT00290732,NCT00465673,NCT00530101,NCT02299999,NCT01415336,NCT00266799,NCT05159193,NCT05498896,NCT02995772,NCT03412643,NCT00193037,NCT00146562,NCT01847001,NCT02096588,NCT01354522,NCT00102219,NCT00499122,NCT01969032,NCT00448266,NCT00087178,NCT03595592,NCT00336791,NCT00004067,NCT03301350,NCT00394251,NCT03248427,NCT00129389,NCT00003165,NCT00574587,NCT05020860,NCT02413320,NCT01670500,NCT00691912,NCT04083963,NCT01329627,NCT00314977 |
Follicular lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A80 |
TOP2A
NCT05058404,NCT02889523,NCT02449252,NCT00774826,NCT01476787,NCT00634179,NCT01852435,NCT00801281,NCT05371093,NCT04745832,NCT01650701,NCT00715208,NCT02855359,NCT00494780,NCT00907348 |
Peripheral T-cell lymphoma, not otherwise specifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90.C |
NCT00725231,NCT04922567,NCT01719835,NCT04480099,NCT00136565,NCT03542266,NCT01796002,NCT04569032,NCT03023358,NCT00791947,NCT00930605,NCT01839097,NCT03952572,NCT03553537,NCT00970385,NCT02445404
|
Mantle cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85.5 |
NCT00209222,NCT00477412,NCT00022945,NCT00878254,NCT02858258,NCT00581776,NCT05051891,NCT00209209,NCT01865110,NCT00722137,NCT02633137,NCT00401817,NCT00877006,NCT04566887,NCT02427620
|
Precursor B-lymphoblastic neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A70 |
NCT03991884,NCT01451515,NCT02228772,NCT05303792,NCT03564704,NCT03817320,NCT04043494,NCT02845882,NCT03023046,NCT02881086,NCT01887587,NCT00866749
|
Breast in situ carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E65 |
NCT05177796,NCT03164993,NCT01498588,NCT01935492,NCT04081389,NCT01885013,NCT02419495,NCT03971409,NCT01275677,NCT03101748
|
Malignant neoplasms of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73 |
NCT01121406,NCT00780039,NCT02903004,NCT00350948,NCT01279291,NCT00659178,NCT00628251,NCT02751918,NCT00913835,NCT00248248
|
Otitis mediaDisease Category: 10.Diseases of the ear or mastoid processDisease ICD-11 Code: AA80-AB0Z |
TOP2A
|
SyphilisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A61-1A6Z |
TOP2A
|
Urinary tract infectionDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GC08 |
TOP2A
|
Alzheimer diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A20 |
TOP2A
|
ChemoprotectionDisease Category: NADisease ICD-11 Code: N.A. |
TOP2A
|
HIV-infected patients with tuberculosisDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1B10-1B14 |
TOP2A
|
Pancreatic cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10 |
TOP2A
|
Pulmonary hypertensionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BB01 |
HIF1A
|
ThrombosisDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DB61-GB90 |
TOP2A
|
AsthmaDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA23 |
TOP2A
|
Acute diabete complicationDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A2Y |
AURKA
|
Otitis externaDisease Category: 10.Diseases of the ear or mastoid processDisease ICD-11 Code: AA00-AA13 |
TOP2A
|
PsoriasisDisease Category: 14.Diseases of the skinDisease ICD-11 Code: EA90 |
TOP2A
|
Bladder cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94 |
TOP2A
|
Multiple sclerosisDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A40 |
TOP2A
|
Stomach cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72 |
TOP2A
|
Common wartsDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1E80 |
TOP2A
|
Respiratory system diseaseDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB40-CB7Z |
TOP2A
|
Bacterial infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A00-1C4Z |
TOP2A
|
Gonococcal infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A70-1A7Z |
TOP2A
|
Anogenital wartsDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1A95 |
TOP2A
|
Colorectal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B91 |
TOP2A
|
LiposarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B59 |
NCT05218499
|
Urethral cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C93 |
NCT00479128
|
Cervical cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C77 |
NCT03340376
|
Malignant neoplasms of nasopharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6B |
NCT00484601
|
Adult T-cell leukaemia/lymphoma (HTLV-1 positive)Disease Category: X.Extension CodesDisease ICD-11 Code: XH6TE2 |
NCT00145002
|
Lymphoplasmacytic lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A85.4 |
NCT00801281
|
OligoastrocytomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH6F49 |
NCT02372409
|
Adenocarcinoma of prostateDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C82.0 |
NCT02560051
|
Malignant neoplasms of kidney, except renal pelvisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90 |
NCT03678883
|
Carcinosarcoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH2W45 |
NCT00815945
|
Other specified amyloidosisDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5D00.Y |
NCT00030381
|
Oligodendroglioma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH7W59 |
NCT02372409
|
Extranodal NK/T-cell lymphoma, nasal typeDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90.6 |
NCT00725231
|
Ductal carcinoma in situ of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E65.2 |
NCT00461344
|
Burkitt-like lymphoma with 11q aberrationDisease Category: X.Extension CodesDisease ICD-11 Code: XH8NN2 |
NCT02228772
|
Malignant neoplasm metastasis in bone or bone marrowDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E03 |
NCT03678883
|
Hereditary breast and ovarian cancer syndromeDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C65 |
NCT02978495
|
Transitional cell carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH8EH1 |
NCT02412670
|
Malignant mixed epithelial mesenchymal tumour of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B5D.0 |
NCT02822157
|
ThymomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C27 |
NCT01100944
|
Melanoma of skin, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30.Z |
NCT02419495
|
Cutaneous T-cell lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH1951 |
NCT02192021
|
Mature B-cell leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A82 |
NCT01328236
|
Diseases of the blood or blood-forming organs, unspecifiedDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3C0Z |
NCT04243434
|
Mature B-cell neoplasms, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A8Z |
NCT02911142
|
AdenocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D40 |
NCT04852367
|
GanglioneuroblastomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH77W7 |
NCT03786783
|
Chronic lymphocytic leukaemia or small lymphocytic lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A82.0 |
NCT00801281
|
Malignant neoplasms of other or unspecified parts of mouthDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B66 |
NCT05456022
|
AngiosarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B56 |
NCT04859465
|
Pilocytic astrocytomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH12D2 |
NCT02372409
|
Choroid plexus tumoursDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.22 |
NCT01014767
|
Castlemans diseaseDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4B2Y |
NCT02228512
|
Malignant neoplasm of liverDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.0 |
NCT01272557
|
Acute leukaemias of ambiguous lineageDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A61 |
NCT02419755
|
Myelodysplastic syndromeDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A37 |
NCT03059615
|
Adenoid cystic carcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH4302 |
NCT00581360
|
Nausea/vomitingDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MD90 |
NCT02116530
|
Metastatic lung neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D70 |
NCT03678883
|
Chronic myelogenous leukaemia, BCR-ABL1-positiveDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A20.0 |
NCT01424982
|
Thymic carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH6AK2 |
NCT01100944
|
CholangiocarcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH7M15 |
NCT02994251
|
Carcinoma of male breastDisease Category: X.Extension CodesDisease ICD-11 Code: XH5KW8 |
NCT00470301
|
Malignant lymphoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5FJ5 |
NCT00126243
|
Optic nerve disorderDisease Category: 09.Diseases of the visual systemDisease ICD-11 Code: 9C40 |
NCT02372409
|
Thyroid cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D10 |
NCT01882816
|
Astrocytoma, anaplasticDisease Category: X.Extension CodesDisease ICD-11 Code: XH96C7 |
NCT02372409
|
Mixed tumour, malignant, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0V86 |
NCT01969578
|
Serous cystadenoma,borderline malignancy of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.4 |
AURKA
|
PheochromocytomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH3854 |
EPAS1
|
Osteoarthritis, unspecifiedDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FA0Z |
TOP2A
|
Malignant neoplasm metastasis in lymph nodes of head, face or neckDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D60.0 |
AURKA
|
Myotonic dystrophyDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C71.0 |
TOP2A
|
Systemic lupus erythematosusDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4A40.0 |
EPAS1
|

