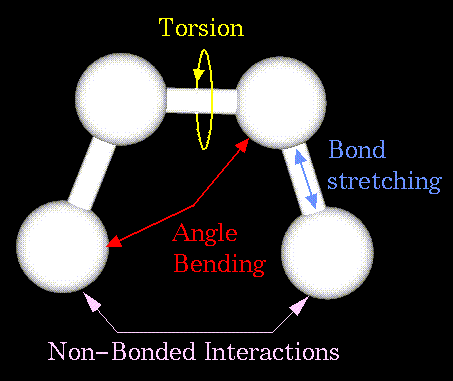
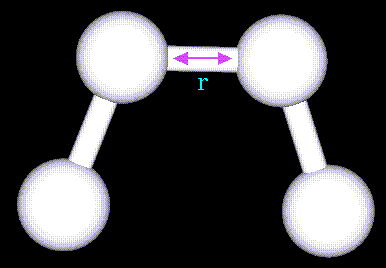
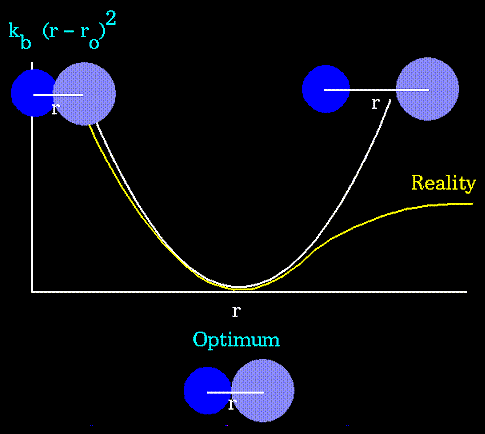
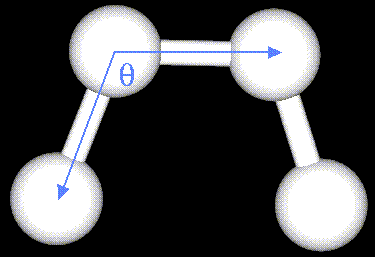
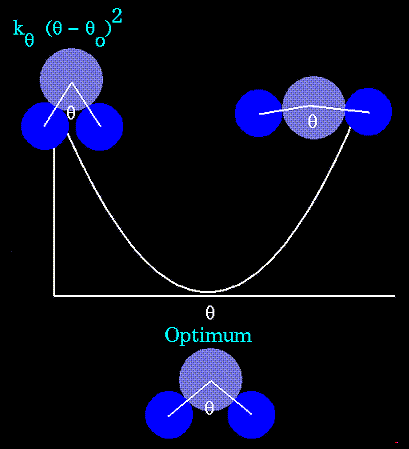
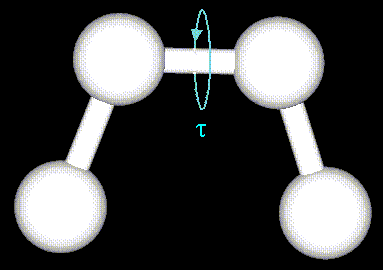
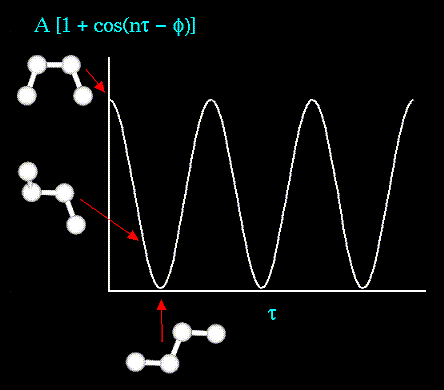
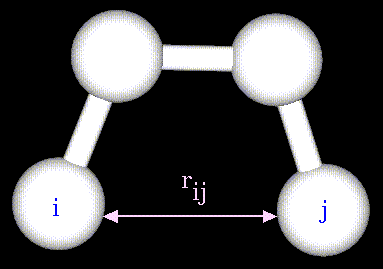
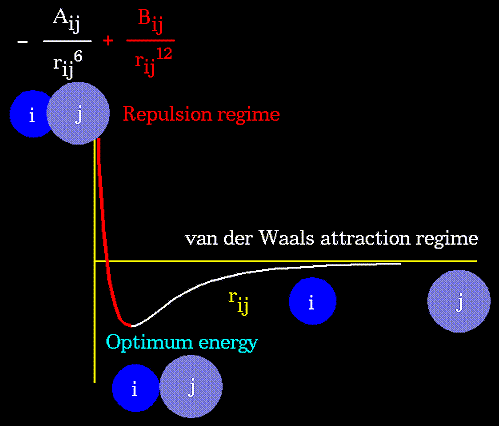
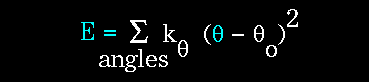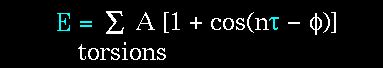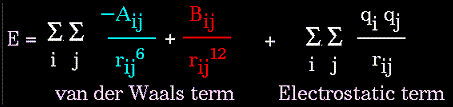- Atoms are not rigidly positioned.
- External and internal forces can induce atomic motions.
- Some motions have chemical effect.
Example:
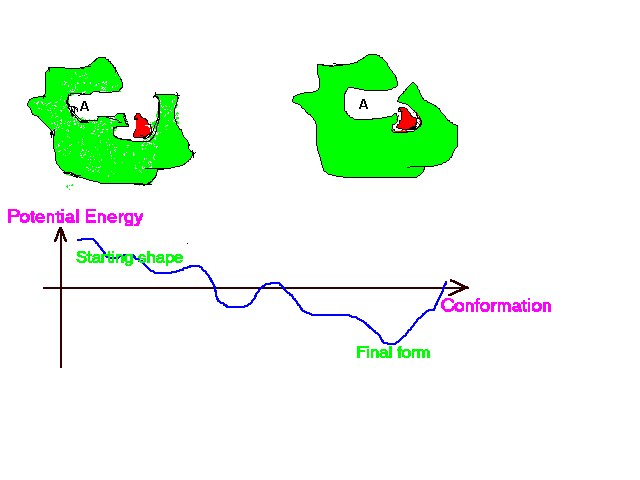
Binding of a drug to a protein can induce an atomic motion in protein that leads to the change of the 3D shape of that protein.
This shape change makes a chemically important cavity inaccessible to other molecules.
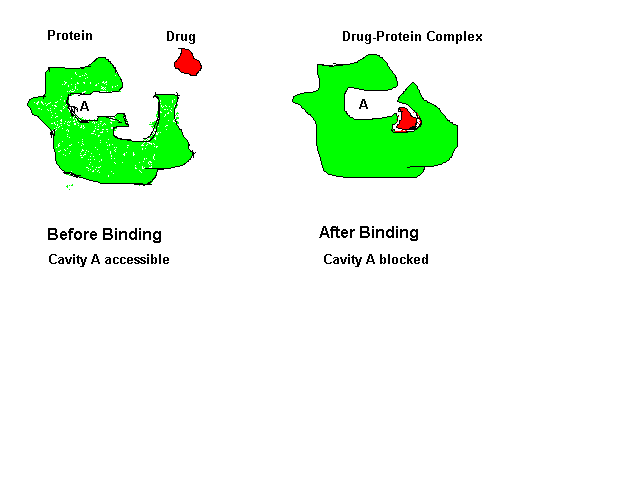
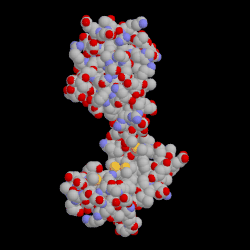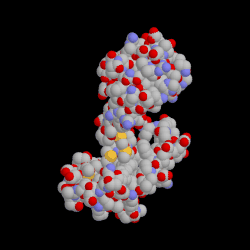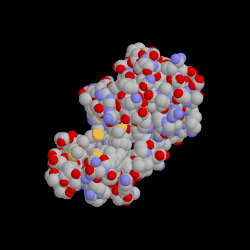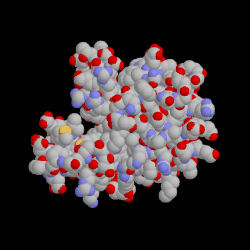
Drug Binding Induced Conformation Change in Protein.
- Energy measures the ability to do work.
- Motion is associated with energy.
- There are two types of energy:
- Kinetic energy -- motional energy.
- Potential Energy -- "positional" energy.
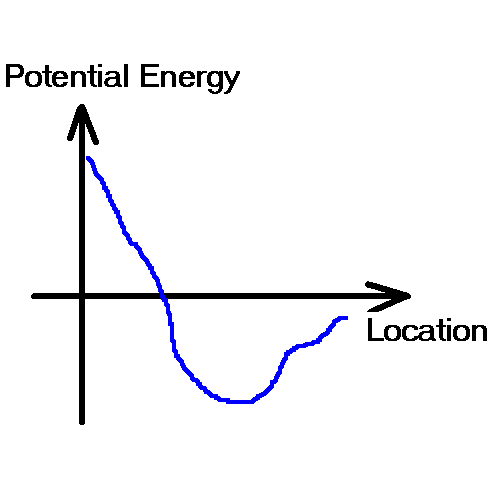
Potential energy description:

Water falls from higher ground to lower ground. In physics such a phenomenon is modeled by potential energy description:
Objects move from higher potential
energy place to lower potential energy place.