Collective Molecular Activities of the Plant: Kleinhovia Hospita
Overview of Ingredients
19 All known Ingredients in Total
Unique ingredients have been isolated from this plant.Plant-Ingredients Associations were manually curated from publications or collected from other databases.
7 Ingredients with Acceptable Bioavailablity
Unique ingredients exhibit acceptable human oral bioavailablity, according to the criteria of SwissADME [PMID: 28256516] and HobPre [PMID: 34991690]. The criteria details:SwissADME: six descriptors are used by SwissADME to evaluate the oral bioavailability of a natural product:
☑ LIPO(Lipophility): -0.7 < XLOGP3 < +5.0
☑ SIZE: 150g/mol < MW < 500g/mol
☑ POLAR(Polarity): 20Ų < TPSA < 130Ų
☑ INSOLU(Insolubility): -6 < Log S (ESOL) < 0
☑ INSATU(Insaturation): 0.25 < Fraction Csp3 < 1
☑ FLEX(Flexibility): 0 < Num. rotatable bonds < 9
If 6 descriptors of a natural plant satisfy the above rules, it will be labeled high HOB.
HobPre: A natural plant ingredient with HobPre score >0.5 is labeled high human oral availability (HOB)
15 Ingredients with experimental-derived Activity
Unique ingredients have activity data available.Ingredient Structrual Cards
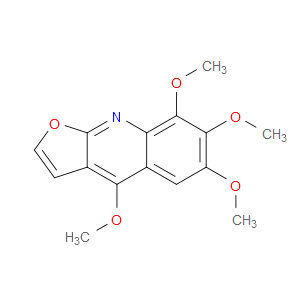
Ingredient ID: NPC82681

Ingredient ID: NPC69408

Ingredient ID: NPC483448

Ingredient ID: NPC483447

Ingredient ID: NPC483445

Ingredient ID: NPC483444

Ingredient ID: NPC483443

Ingredient ID: NPC483442

Ingredient ID: NPC483441
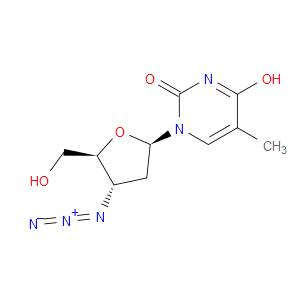
Ingredient ID: NPC478793
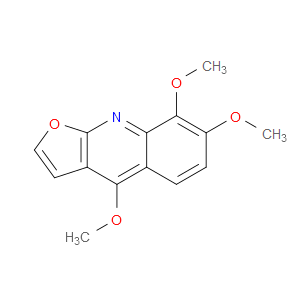
Ingredient ID: NPC34770
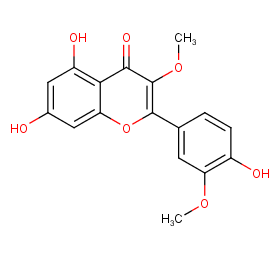
Ingredient ID: NPC279989
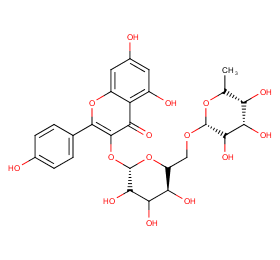
Ingredient ID: NPC230861
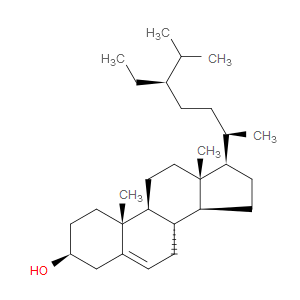
Ingredient ID: NPC230301
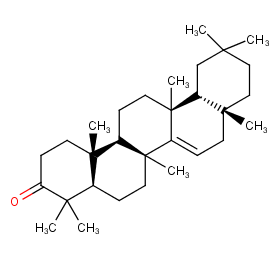
Ingredient ID: NPC220210
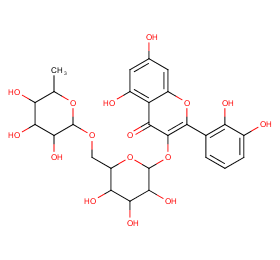
Ingredient ID: NPC218109
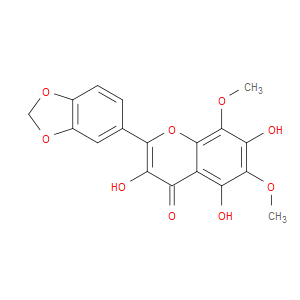
Ingredient ID: NPC211390
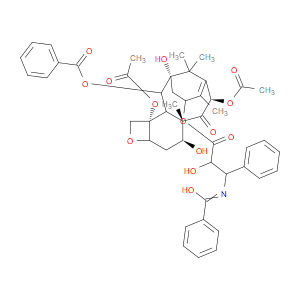
Ingredient ID: NPC208553

Ingredient ID: NPC127790
Classification of Human Proteins Collectively Targeted by the Plant
Detailed Information of Target Proteins
| Target Type | Protein Class | Gene ID | Protein Name | Uniprot ID | Target ChEMBL ID |
|---|---|---|---|---|---|
| Cytochrome P450 | Cytochrome P450 family 1 | CYP1A2 | Cytochrome P450 1A2 | P05177 | CHEMBL3356 |
| Therapeutic Target | Hydrolase | RAB9A | Ras-related protein Rab-9A | P51151 | CHEMBL1293294 |
| Therapeutic Target | Nuclear hormone receptor subfamily 1 | THRB | Thyroid hormone receptor beta-1 | P10828 | CHEMBL1947 |
| Therapeutic Target | Protein Kinase | MTOR | Serine/threonine-protein kinase mTOR | P42345 | CHEMBL2842 |
| Drug Transporter | SLC superfamily of solute carriers | SLCO1B3 | Solute carrier organic anion transporter family member 1B3 | Q9NPD5 | CHEMBL1743121 |
| Drug Transporter | SLC superfamily of solute carriers | SLCO1B1 | Solute carrier organic anion transporter family member 1B1 | Q9Y6L6 | CHEMBL1697668 |
| Therapeutic Target | Structural protein | LMNA | Prelamin-A/C | P02545 | CHEMBL1293235 |
| Therapeutic Target | Structural protein | TUBB3 | Tubulin beta-3 chain | Q13509 | CHEMBL2597 |
| Therapeutic Target | Unclassified protein | GMNN | Geminin | O75496 | CHEMBL1293278 |
| Therapeutic Target | Unclassified protein | PMP22 | Peripheral myelin protein 22 | Q01453 | CHEMBL1293298 |
Clinical trials associated with plant from natural product (NP) & plant level:
| Clinical trials type | Number of clinical trials | |
|---|---|---|
| 2100 | ||
| NCT ID | Title | Condition | Form in clinical use | Associated by plant or compound |
|---|---|---|---|---|
| NCT00006120 | Docetaxel or Paclitaxel in Treating Women With Unresectable Locally Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04584112 | A Study of the Safety, Efficacy, and Pharmacokinetics of Tiragolumab in Combination With Atezolizumab and Chemotherapy in Participants With Triple-Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT01739894 | Feasibility Study of Intraperitoneal Paclitaxel | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04766359 | Paclitaxel (Albumin-bound) Combined With Radiotherapy for the Treatment of Early Stage Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT03534713 | Induction Chemotherapy Followed by Standard Therapy in Cervical Cancer With Aortic Lymph Node Spread | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02570893 | A Phase III Trail of Adjuvant Chemoradiotherapy Versus Adjuvant Radiotherapy in Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00004202 | Combination Chemotherapy, Radiation Therapy, and RSR13 in Treating Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00021060 | Combination Chemotherapy With or Without Bevacizumab in Treating Patients With Advanced, Metastatic, or Recurrent Non-Small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00003930 | Radiation Therapy and Combination Chemotherapy in Treating Patients With Stage II or Stage III Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT02430311 | The Pharmacokinetics and Safety of Olaparib Alone and With Paclitaxel in Chinese Patients With Advanced Solid Tumour. | neoplasm | Paclitaxel (NPC208553) | |
| NCT01179269 | Pazopanib and Paclitaxel for Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04056949 | Efficacy and Safety of IBI308 and Paclitaxel/Albumin Paclitaxel for SCLC Patients Who Failed Etoposide Chemotherapy | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00825201 | A Phase I/II Study of Paclitaxel Plus Carboplatin Plus Vorinostat in Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003539 | Paclitaxel Plus Monoclonal Antibody Therapy in Treating Women With Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01578551 | Study of Metformin Plus Paclitaxel/Carboplatin/Bevacizumab in Patients With Adenocarcinoma. | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03603184 | Atezolizumab Trial in Endometrial Cancer - AtTEnd | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00448591 | A Study of Avastin (Bevacizumab) Plus Taxane-Based Therapy in Patients With Locally Recurrent or Metastatic Breast Cancer. | breast cancer | Paclitaxel (NPC208553) | |
| NCT00499603 | Paclitaxel Followed by FEC Versus Paclitaxel and RAD001 Followed by FEC In Women With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01820754 | Evaluation of Circulating T Cells and Tumor Infiltrating Lymphocytes (TILs) During / After Pre-Surgery Chemotherapy in Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02713386 | Pembrolizumab, Carboplatin, and Paclitaxel in Treating Patients With Stage III-IV Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT02020707 | Nab-Paclitaxel and Bevacizumab in Treating Patients With Unresectable Stage IV Melanoma or Gynecological Cancers | cervical adenocarcinoma;cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT04694183 | The Conversion Therapy of Chemotherapy Plus Camrelizumab in Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01755897 | A Multicenter, Prospective, Randomized Trial of Adjuvant Chemotherapy for Early-Stage Cervical Cancer Patients | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00394251 | Study of Dose-dense Adriamycin Plus Cytoxan (AC) Followed by Either ABI-007 (Abraxane) or Taxol With Bevacizumab as Adjuvant Therapy for Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05244993 | Study of Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy for HR+/HER2- Locally Recurrent Inoperable or Metastatic Breast Cancer (MK-3475-B49/KEYNOTE-B49) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00154726 | Paclitaxel-HDFL for Locally Advanced and Recurrent/Metastatic Gastric Cancers | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00391118 | Comparing Two Treatments for Ovarian Cancer: Standard Chemotherapy Plus Enzastaurin, or Placebo ("Sugar Pill") | Fallopian Tube Carcinoma;ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT01069328 | Dose Escalating Study With BAY43-9006 With Carboplatin, Paclitaxel and Bevacizumab in Untreated Stage IIIb Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01935492 | 8 Continuous vs 8 Intermittent Cycles in First and Second Line in HER2/Neu Neg Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00394082 | ABI-007 In Combination With Bevacizumab in Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05489848 | Chemotherapy vs Chemoradiotherapy for Post-operative Endometrial Cancer Patients With P53-mutation | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00998192 | A Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin in Patients With Squamous Cell Carcinoma of the Lung | squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003927 | Combination Chemotherapy, Amifostine, and Peripheral Stem Cell Transplantation in Treating Patients With Stage II, Stage III, or Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00088530 | BBR 2778 for Relapsed, Aggressive Non-Hodgkin's Lymphoma (NHL) | non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT00176267 | Paclitaxel, Carboplatin And Low Dose Radiation As Induction Therapy In Locally Advanced Head And Neck Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01170663 | A Study of Paclitaxel With or Without Ramucirumab (IMC-1211B) in Metastatic Gastric Adenocarcinoma | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00591851 | Phase II Study of Dose-Dense Doxurubicin and Cyclophosphamide (AC) Followed By Paclitaxel With Trastuzumab in HER2/ NEU-Amplified Breast Cancer: Feasibility | breast cancer | Paclitaxel (NPC208553) | |
| NCT00622466 | Sorafenib and Paclitaxel in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04354961 | Almonertinib Versus Paclitaxel Plus Carboplatin as First-line Treatment in Patients With EGFR Mutation Positive Locally Advanced or Metastatic Pulmonary Adenosquamous Carcinoma (ARISE) | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00933803 | Hydroxychloroquine, Carboplatin, Paclitaxel, and Bevacizumab in Recurrent Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02611700 | Nimotuzumab in Combined With Paclitaxel and Cisplatin for Treatment of Metastatic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02243007 | Phase II Study of Preoperative FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel in Patients With Resectable Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00526890 | Carboplatin, Paclitaxel, Selenomethionine, and Radiation Therapy in Treating Patients With Stage III Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT05233696 | Radiotherapy in Combo With Chemo and Immunotherapy in Patients With PD-L1 Positive Metastatic TNBC | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00434226 | A Study of Sunitinib in Combination With Bevacizumab, Carboplatin, and Paclitaxel in Patients With Advanced Non-Small Cell Lung Cancer (SABRE-L) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00679029 | Combination Chemotherapy and Bevacizumab in Treating Women With HER2/Neu-Negative Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00280787 | Induction Chemotherapy Using Paclitaxel, Carboplatin, CPT-11 With Pegfilgrastim | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01711970 | SGI-110 in Combination With Carboplatin in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00347412 | Study of NOV-002 in Combination With Chemotherapy to Treat Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00277043 | A Phase III Randomized Trial Assessing the Utility of a Test Dose Program With Taxanes | allergic disease | Paclitaxel (NPC208553) | |
| NCT01444547 | A Study on Predictive Value of ERCC1 in Esophageal Cancer Patients Treated With Paclitaxel and Cisplatin | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04449549 | Rapid Analysis and Response Evaluation of Combination Anti-Neoplastic Agents in Rare Tumors (RARE CANCER) Trial: RARE 1 Nilotinib and Paclitaxel | neoplasm | Paclitaxel (NPC208553) | |
| NCT03766607 | Trastuzumab Beyond Progression in HER2 Positive Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00062010 | Interferon Alfa, Isotretinoin, and Paclitaxel in Treating Patients With Recurrent Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03872791 | A Study of KN046 in Subjects With Locally Advanced or Metastatic Triple-negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT02834975 | Phase II: Pembrolizumab/Carboplatin/Taxol in Epithelial Ovary Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00454649 | Investigational Agent AG-013736 In Combinations With Standard Of Care Treatments For Patient's With Advanced Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT02483104 | Open-label Phase 1b Study of ARQ 092 in Combination With Anastrozole | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00828009 | BLP25 Liposome Vaccine and Bevacizumab After Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT02379416 | Combination Nilotinib and Paclitaxel in Adults With Relapsed Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT05257993 | Study to Assess the Safety, Tolerability of JPI-547 in Combination With Modified FOLFIRINOX or Gemcitabine-nab-paclitaxel in Patients With Locally Advanced and Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00672295 | PH I SRC Kinase, Dasatinib Combo Paclitaxel & Carboplatin in Pts w Ovarian, Peritoneal, & Tubal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01431794 | Gemcitabine + Nab-paclitaxel With LDE-225 (Hedgehog Inhibitor) as Neoadjuvant Therapy for Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00388076 | A Study Of SU011248 Plus Paclitaxel Versus Bevacizumab Plus Paclitaxel In Patients With Advanced Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02630264 | E10A for the Treatment of Squamous Cell Carcinoma of the Head and Neck | upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT00583349 | Phase I & II Trial of Intravesicular Abraxane for Treatment-refractory Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01868984 | Paclitaxel for the Treatment of Upper-Extremity Arteriovenous Access Fistula Stenosis | stricture | Paclitaxel (NPC208553) | |
| NCT03918278 | A Study of MK-0482 as Monotherapy and in Combination With Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-0482-001) | neoplasm | Paclitaxel (NPC208553) | |
| NCT00662311 | Vorinostat, Paclitaxel, and Radiation Therapy in Treating Patients Unable to Tolerate Cisplatin With Stage III Non-Small Lung Cancer That Cannot Be Removed By Surgery | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00449657 | Phase II Trial of Pulsed Taxol With Concurrent Thoracic Radiotherapy, & Adjuvant Chemo in Stage III NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01454102 | Study of Nivolumab (BMS-936558) in Combination With Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab Maintenance, Erlotinib, Ipilimumab or as Monotherapy in Subjects With Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC) (CheckMate 012) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00006027 | Comparison of Radiation Therapy With or Without Combination Chemotherapy Following Surgery in Treating Patients With Stage I or Stage II Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01024101 | Early Phase II Study of Weekly Paclitaxel (BMS-181339) in Patient With Advanced or Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02312804 | Ph Ib/BGJ398/Cervix and Other Solid Tumors | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT00002632 | Paclitaxel in Treating Patients With Metastatic or Recurrent Salivary Gland Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00918203 | A Study of Paclitaxel/Carboplatin With or Without Olaratumab (IMC-3G3) in Previously Untreated Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00217607 | Paclitaxel in Treating Patients With Locally Advanced or Metastatic Soft Tissue Angiosarcoma or Lymphangiosarcoma That Cannot Be Removed By Surgery | sarcoma | Paclitaxel (NPC208553) | |
| NCT00691379 | Weekly Paclitaxel/Carboplatin/Bevacizumab as First Line Therapy for Triple Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01088815 | Hedgehog Inhibitors for Metastatic Adenocarcinoma of the Pancreas | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02520154 | Dose-Escalation Study of Intraperitoneal (IP) Cisplatin, IV/IP Paclitaxel, IV Bevacizumab, and Oral Olaparib for Newly Diagnosed Ovarian, Primary Peritoneal, and Fallopian Tube Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01364012 | A Study of Bevacizumab Versus Placebo in Combination With Carboplatin/Paclitaxel in Participants With Advanced or Recurrent Non-Squamous Non-Small Cell Lung Cancer Who Have Not Received Previous Chemotherapy | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03167177 | QUILT-3.046: NANT Melanoma Vaccine: Combination Immunotherapy in Subjects With Melanoma Who Have Progressed On or After Chemotherapy and PD-1/PD-L1 Therapy | melanoma | Paclitaxel (NPC208553) | |
| NCT04931342 | Chiauranib Plus Weekly Paclitaxel in Patients With Platinum-refractory or Platinum-resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04781413 | Nab-PTX Plus S-1 and Sintilimab as Adjuvant Therapy in Patients With Stage IIIC Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00269828 | A Survival Study for Women With Advanced Lung Cancer Who Have Not Previously Received Chemotherapy. | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00005078 | Tirapazamine, Carboplatin, and Paclitaxel in Treating Patients With Advanced Malignant Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00731861 | Phase I, Pharmacokinetic, Pharmacodynamic Trial of PTK787 and Paclitaxel in Combination for Advanced Solid Tumors | metastasis;carcinoma | Paclitaxel (NPC208553) | |
| NCT02798536 | Mesothelin-Targeted Immunotoxin LMB-100 in People With Malignant Mesothelioma | mesothelioma | Paclitaxel (NPC208553) | |
| NCT02187991 | Study to Compare Alisertib With Paclitaxel vs. Paclitaxel Alone in Metastatic or Locally Recurrent Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04561817 | To Evaluate the Safety and Efficacy of Ipatasertib (GDC-0068) in Combination With Paclitaxel in Platinum-resistant Recurrent Epithelial Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT03003546 | Nab-paclitaxel/Rituximab-coated Nanoparticle AR160 in Treating Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma, LS1681 Trial | chronic lymphocytic leukemia | Paclitaxel (NPC208553) | |
| NCT00319839 | Study of Albumin Bound-Paclitaxel for Treatment of Recurrent or Metastatic Head and Neck Cancer With Cetuximab | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02440425 | A Study of CRLX101(NLG207) in Combination With Weekly Paclitaxel in Patients With Recurrent or Persistent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00001272 | A Phase I Study of Taxol, Cisplatin, Cyclophosphamide and Granulocyte Colony-Stimulating Factor (G-CSF) in Previously Nontreated Ovarian Cancer Patients | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT02630199 | A Study of BBI503 in Combination With Selected Anti-Cancer Therapeutics in Adult Patients With Advanced Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT02725268 | A Study of Sapanisertib, Combination of Sapanisertib With MLN1117, Paclitaxel and Combination of Sapanisertib With Paclitaxel in Women With Endometrial Cancer | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT01107665 | Pazopanib and Paclitaxel as First-Line Treatment for Subjects With Unresectable Stage III and Stage IV Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00003128 | Ifosfamide With or Without Paclitaxel in Treating Patients With Advanced, Refractory, or Recurrent Cancer of the Uterus | sarcoma | Paclitaxel (NPC208553) | |
| NCT02016209 | Neoadjuvant Chemotherapy of Nanoparticle Albumin-bound Paclitaxel in Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00034164 | Safety and Efficacy of S-8184 in Second Line Treatment of Relapsed Stage IIIB or IV Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04865289 | Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) Versus Chemotherapy for Endometrial Carcinoma (ENGOT-en9 / MK-7902-001) - China Extension Study | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT02762981 | Study to Evaluate Relacorilant (CORT125134) in Combination With Nab-paclitaxel in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04089150 | MFOLFIRINOX And Stereotactic Radiotherapy (SBRT) for Pancreatic Cancer With High Risk and Locally Advanced Disease | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01496742 | A Study of Onartuzumab (MetMAb) in Combination With Bevacizumab (Avastin) Plus Platinum And Paclitaxel or With Pemetrexed Plus Platinum in Patients With Non-Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004253 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Stage II or Stage III Non-small Cell Lung Cancer That Cannot Be Removed By Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT03348904 | Nivolumab and Epacadostat With Platinum Doublet Chemotherapy Versus Platinum Doublet Chemotherapy in Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05357846 | PD-1 Inhibitor Combined With Neoadjuvant Chemoradiotherapy Plus Surgery for Locally Advanced ESCC | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT04895358 | Capivasertib+Paclitaxel as First Line Treatment for Patients With Locally Advanced or Metastatic TNBC | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02272699 | Neoadjuvant Treatment of Nimotuzumab With Chemotherapy or Radiotherapy in Resectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00544232 | Preoperative Epirubicin Paclitaxel Aranesp Study (PREPARE) | breast cancer | Paclitaxel (NPC208553) | |
| NCT02677597 | Cisplatin Combined With S-1 or Paclitaxel as First-line Treatment for Metastatic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04868708 | A Study of AK104( an Anti-PD-1 and Anti-CTLA-4 Bispecific Antibody) in Recurrent or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03574324 | TPF Induction Chemotherapy vs PF Adjuvant Chemotherapy Combined With Concurrent Chemoradiotherapy in the Treatment of Locally Advanced NPC | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT03704077 | An Investigational Immuno-therapy Study of Relatlimab Plus Nivolumab Compared to Various Standard-of-Care Therapies in Previously Treated Participants With Recurrent, Advanced or Metastatic Gastric Cancer or Gastroesophageal Junction Adenocarcinoma | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01827111 | Phase II Study of Abraxane Plus Ipilimumab in Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00003379 | Radiation Therapy Plus Paclitaxel and Cisplatin in Treating Patients With Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00070564 | S0221 Adjuvant Doxorubicin, Cyclophosphamide, and Paclitaxel in Treating Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00191854 | Gemcitabine Combinations in Metastatic Breast Cancer (MBC), 1st Line | breast cancer | Paclitaxel (NPC208553) | |
| NCT02300935 | Study of Trametinib and Nab-paclitaxel in Patients With Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00016913 | Chemotherapy, Hormone Therapy, and Radiation Therapy in Treating Patients With Locally Advanced Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT05400915 | Two-Part, Phase Ib/II, Open Label, Single-Arm, Multi-center Study to Evaluate the Safety and Efficacy of Varlitinib in Combination With Weekly Paclitaxel in EGFR/HER2 Co-expressing Advanced or Metastatic Gastric Cancer Patients | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00003089 | Chemotherapy, Amifostine, and Radiation Therapy in Treating Patients With Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02705196 | LOAd703 Oncolytic Virus Therapy for Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00152477 | A Study of Paclitaxel/Carboplatin With or Without CDP791 in Patients With Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02004093 | EWOC-1 Trial: Carboplatin +/- Paclitaxel in Vulnerable Elderly Patients With Stage III-IV Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00112294 | Study of Taxane/Carboplatin +/- Cetuximab as First-Line Treatment for Patients With Advanced/Metastatic Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00193297 | Topotecan Plus Paclitaxel and Carboplatin in the Initial Treatment of Advanced Ovarian and Primary Peritoneal Carcinoma | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03219762 | Study of Nab-paclitaxel in Sensitive and Refractory Relapsed SCLC | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00041119 | Four Versus Six Cycles of Cyclophosphamide/Doxorubicin or Paclitaxel in Adjuvant Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00486668 | A Study of AC Followed by a Combination of Paclitaxel Plus Trastuzumab or Lapatinib or Both Given Before Surgery to Patients With Operable HER2 Positive Invasive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02632071 | ACY-1215 + Nab-paclitaxel in Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00448305 | Neoadjuvant Study With Chemotherapy, Lapatinib And Trastuzumab In Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04308837 | A Phase II Trial of Neoadjuvant Laparoscopic Hyperthermic Intraperitoneal Chemotherapy (HIPEC) With Chemoradiation | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04729387 | Trial on NIraparib-TSR-042 (Dostarlimab) vs Physician's Choice CHEmotherapy in Recurrent, Ovarian, Fallopian Tube or Primary Peritoneal Cancer Patients Not Candidate for Platinum Retreatment | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01326767 | Central European Society for Anticancer Research (CESAR) Study of Paclitaxel Therapeutic Drug Monitoring | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01822613 | Study of Safety & Efficacy of the Combination of LJM716 & BYL719 in Patients With Previously Treated Esophageal Squamous Cell Carcinoma (ESCC) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00343083 | Evaluation of Cetuximab (ERBITUX) and Concurrent Carboplatin, Paclitaxel & Radiotherapy in the Management of Patients With Advanced Locoregional Squamous Cell Carcinomas of the Head and Neck (GCC 0442) | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT03387111 | QUILT-3.090: NANT Squamous Cell Carcinoma (SCC) Vaccine: Subjects With SCC Who Have Progressed | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03498521 | Study of Paclitaxel in Combination With BOS172722 in Patients With Advanced Nonhaematologic Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00737243 | Treatment Based on Molecular Profiling Diagnosis Carcinoma of Unknown Primary Site | carcinoma | Paclitaxel (NPC208553) | |
| NCT00289263 | Maintenance Chemotherapy in Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01649947 | Modulation of Autophagy in Patients With Advanced/Recurrent Non-small Cell Lung Cancer - Phase II | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02109445 | Study Of PF-03084014 In Combination With Gemcitabine And Nab-Paclitaxel In Patients With Metastatic Pancreatic Adenocarcinoma Not Previously Treated With Anticancer Therapies | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00508326 | Paclitaxel Administered by HAI to Patients With Advanced Cancer and Dominant Liver Involvement | cancer | Paclitaxel (NPC208553) | |
| NCT03579784 | Biomarker-oriented Study of Durvalumab (MEDI4736) in Combination With Olaparib and Paclitaxel in Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01649336 | A Phase IIclinical Trial of Carboplatin and Paclitaxel or Carboplatin and Gemcitabine in Platinum-sensitive, Recurrent Ovarian, Fallopian Tube, and Primary Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00198354 | Stage I/II NSCLC Perioperative Chemotherapy | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02474173 | Onalespib and Paclitaxel in Treating Patients With Advanced Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02033993 | Nab-Paclitaxel to Paclitaxel in Advanced Urothelial Cancer Progressing on or After Platinum Containing Regimen. | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT04213898 | SHR-1210 Combined With Albumin-bound Paclitaxel and Epirubicin Neoadjuvant for Triple Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03892018 | The Effect of Food on the Pharmacokinetics of Paclitaxel Administered Orally as Oraxol | neoplasm | Paclitaxel (NPC208553) | |
| NCT01822496 | Erlotinib Hydrochloride or Crizotinib and Chemoradiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02270814 | Cisplatin, Nab-Paclitaxel, and Cetuximab (CACTUX) in Patients With Incurable Head and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04982237 | A Study of AK104 Plus Platinum-containing Chemotherapy±Bevacizumab as First-line Treatment for Persistent, Recurrent, or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03725436 | ALRN-6924 and Paclitaxel in Treating Patients With Advanced, Metastatic, or Unresectable Solid Tumors | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01858883 | Safety Study of Itacitinib (INCB039110) in Combination With Gemcitabine and Nab-Paclitaxel in Subjects With Advanced Solid Tumors | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03693677 | First Line Metastatic Pancreatic Cancer : 5FU/LV+Nal-IRI, Gemcitabine+Nab-paclitaxel or a Sequential Regimen of 2 Months 5FU/LV+Nal-IRI | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04329949 | Study of Relacorilant in Combination With Nab-Paclitaxel in Patients With Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03056833 | Ribociclib (Ribociclib (LEE-011)) With Platinum-based Chemotherapy in Recurrent Platinum Sensitive Ovarian Cancer | peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT03697239 | High Dose Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) in Patients Who Have Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05319730 | A Study to Evaluate Pembrolizumab (MK-3475) in Participants With Advanced Esophageal Cancer Previously Exposed to Programmed Cell Death 1 Protein (PD-1)/ Programmed Cell Death Ligand 1 (PD-L1) Treatment | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03181100 | Atezolizumab With Chemotherapy in Treating Patients With Anaplastic or Poorly Differentiated Thyroid Cancer | thyroid carcinoma | Paclitaxel (NPC208553) | |
| NCT00393068 | Chemotherapy, Radiation Therapy and Immunotherapy Prior to Surgery in Operable Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04221828 | Trial of NanoPac Focal Therapy for Prostate Cancer | prostate adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00001498 | A Pilot Trial of Sequential Chemotherapy With Antimetabolite Induction, High-Dose Alkylating Agent Consolidation With Peripheral Blood Progenitor Cell Support, and Intensification With Paclitaxel and Doxorubicin for Patients With High-Risk Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03818282 | Trial Comparing Niraparib-bevacizumab-Dostarlimab and Niraparib-bevacizumab to Standard of Care in Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00702572 | Carboplatin, Paclitaxel, Bevacizumab and Vorinostat for Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02405910 | Ph2 Nab-paclitaxel With Gemcitabine to Determine Efficacy in Advanced Non-squamous NSCLC. | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00785291 | Paclitaxel, Nab-paclitaxel, or Ixabepilone With or Without Bevacizumab in Treating Patients With Stage IIIC or Stage IV Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03606967 | Testing the Addition of an Individualized Vaccine to Nab-Paclitaxel, Durvalumab and Tremelimumab and Chemotherapy in Patients With Metastatic Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00093756 | Bortezomib, Paclitaxel, Carboplatin and Radiation Therapy for Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00034190 | Safety and Efficacy of S-8184 in Second Line Treatment of Stage III or IV Colorectal Adenocarcinoma | colorectal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00479674 | Phase II Study With Abraxane, Bevacizumab and Carboplatin in Triple Negative Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00278122 | Paclitaxel and GM-CSF in Treating Patients With Stage III or Stage IV Melanoma That Cannot Be Removed By Surgery | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT02513563 | AZD1775 Plus Carboplatin-Paclitaxel in Squamous Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02581501 | Prospective Phase I Study of GAX for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00077220 | Paclitaxel, Carboplatin, and Radiation Therapy With or Without Adjuvant Paclitaxel and Carboplatin in Treating Patients With Stage II or Stage III Unresectable Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00193596 | Gemcitabine/Irinotecan/ZD1839 vs Paclitaxel/Carboplatin/Etoposide/ZD1839 in Carcinoma of Unknown Primary Site | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003299 | Cisplatin Plus Etoposide With or Without Paclitaxel in Treating Patients With Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02892123 | Vevorisertib (ARQ 751) as a Single Agent or in Combination With Other Anti-Cancer Agents, in Solid Tumors With PIK3CA / AKT / PTEN Mutations (MK-4440-001) | cancer | Paclitaxel (NPC208553) | |
| NCT01166542 | Efficacy Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin in Platinum-Refractory Head and Neck Cancers | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04590625 | Study With Afuresertib and Paclitaxel in Platinum Resistant Ovarian | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00431080 | Randomized Phase III Trial Comparing Sequential Administration of FE75C Followed by Docetaxel Versus Paclitaxel as Adjuvant Chemotherapy in Axillary Lymph Node (+) Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00793897 | Multiple Dose Study In Cancer Patients: Safety and Tolerability of BMS-754807 in Combination With Paclitaxel and Carboplatin in Patients With Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03117933 | Ribociclib (Ribociclib (LEE-011)) With Platinum-based Chemotherapy in Recurrent Platinum Sensitive Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00071188 | ZD6474 Alone or in Combination With Paclitaxel and Carboplatin in Subjects With Previously Untreated Locally Advanced or Metastatic Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00453167 | Weekly Paclitaxel Plus Gemcitabine as Second-line in Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03415802 | Efficacy and Safety of Nab-Paclitaxel Plus S-1 in the First-line Treatment of Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02322281 | TIGER-3: Open Label, Multicenter Study of Rociletinib (CO-1686) Mono Therapy Versus Single-agent Cytotoxic Chemotherapy in Patients With Mutant EGFR NSCLC Who Have Failed at Least One Previous EGFR-Directed TKI and Platinum-doublet Chemotherapy | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02827201 | FIrst Line Treatment of Metastatic Pancreatic Cancer: Sequential Nab-paclitaxel + Gemcitabine/FOLFIRI.3 VS Nab-paclitaxel + Gemcitabine | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00707707 | Phase I/II Study of AZD2281 Given in Combination With Paclitaxel in Metastatic Triple Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003111 | Combination Chemotherapy Followed by Surgery in Treating Patients With Stage IIIA Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00004094 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Previously Untreated Advanced Cancer of the Mouth, Pharynx, or Larynx | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02138812 | A Phase 1, Dose Escalation Study to Assess the Safety and Tolerability of ASP9853 With Either Docetaxel or Paclitaxel in Patients With Advanced Non-hematologic Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT01497470 | A Clinical Study in Cancer Patients to Investigate the Potential Impact of Custirsen, on the Blood Levels of the Chemotherapeutic Drug, Paclitaxel, When Given Together as Part of a Treatment Regimen | cancer | Paclitaxel (NPC208553) | |
| NCT04267549 | Conversion Therapy of Sintilimab in Combination With Apatinib and Chemotherapy in Unresectable Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00002519 | Paclitaxel Plus Radiation Therapy in Treating Patients With Untreated Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04527991 | Study of Sacituzumab Govitecan-hziy (IMMU-132) Versus Treatment of Physician's Choice in Participants With Metastatic or Locally Advanced Unresectable Urothelial Cancer | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT05190445 | Cinrebafusp Alfa in Combination With Ramucirumab and Paclitaxel in HER2-High Gastric or GEJ Adenocarcinoma and in Combination With Tucatinib in HER2-Low Gastric or GEJ Andenocarinoma | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01075464 | A Study of the Safety and Pharmacology of MEGF0444A in Combination With Bevacizumab With or Without Paclitaxel in Patients With Locally Advanced or Metastatic Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT04767295 | A Study of Camrelizumab Combined With Chemotherapy as Neoadjuvant Therapy in Adcanced Esophageal Squamous Cell Carcinoma (ESCC) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01980472 | Chemotherapy Plus Bevacizumab in Elderly Non-small Cell Lung Cancer Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00566540 | Ph 2 Intensification Regimen for Previously Untreated, Resectable, Advanced Squamous Cell Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01852292 | Study of Efficacy and Safety of Buparlisib (BKM120) Plus Paclitaxel Versus Placebo Plus Paclitaxel in Recurrent or Metastatic Head and Neck Cancer Previously Pre-treated With a Platinum Therapy | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02157870 | Neoadjuvant Chemotherapy in Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03083470 | Study of SOR007 Ointment for Actinic Keratosis | actinic keratosis | Paclitaxel (NPC208553) | |
| NCT04460066 | A Study of Anti-PD-L1 Antibody in Neoadjuvant Chemotherapy of Esophageal Squamous Cell Carcinoma. | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00979212 | Chemotherapy and Radiation Therapy With or Without Panitumumab in Treating Patients With Stage IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03179904 | FASN Inhibitor TVB-2640 and Trastuzumab in Combination With Paclitaxel or Endocrine Therapy for the Treatment of HER2 Positive Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT05367206 | Neoadjuvant Chemotherapy Followed by Chemoradiation Versus Chemoradiation for Stage IIIC Cervical Cancer Patients: A Randomized Phase III Trial | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00466986 | Abraxane Plus Carboplatin for Recurrent Platinum-Sensitive Ovarian Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00970580 | A Study of BIIB022 in Combination With Paclitaxel and Carboplatin in Subjects With Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02395705 | Neoadjuvant Chemotherapy Versus Surgery Alone for Esophageal Squamous Cell Carcinoma | neoplasm of esophagus | Paclitaxel (NPC208553) | |
| NCT04447092 | Pembrolizumab Plus Chemotherapy in 1st Line Treatment of Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03396445 | Study to Compare Neoadjuvant Combination of Trastuzumab and Pertuzumab With Concurrent Taxane Chemotherapy or Endocrine Therapy and Quality of Life Assessment Under Adjuvant Therapy in Operable HER2+/HR+ Breast Cancer Patients | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03636308 | Nab-paclitaxel Plus S-1(AS) Versus Nab-paclitaxel Plus Gemcitabine(AG) in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05047991 | Study of Irinotecan Liposome Injection-containing Regimens Versus Nab-paclitaxel Plus Gemcitabine in Patients With Previously Untreated, Metastatic Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00246571 | Weekly Taxol Plus Xeloda® vs Taxotere q3wk Plus Xeloda® in the Treatment of Metastatic BC | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01008150 | Phase II Randomized Trial Evaluating Neoadjuvant Therapy With Neratinib and/or Trastuzumab Followed by Postoperative Trastuzumab in Women With Locally Advanced HER2-positive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00807573 | Paclitaxel, Bevacizumab and Pemetrexed in Patients With Untreated, Advanced Non-Small Cell Lung Cancer Using Web-Based Data Collection, Patient Self-Reporting of Adverse Effects and Automated Response Assessment | lung cancer | Paclitaxel (NPC208553) | |
| NCT04634877 | Study of Pembrolizumab (MK-3475) in Combination With Adjuvant Chemotherapy With or Without Radiotherapy in Participants With Newly Diagnosed Endometrial Cancer After Surgery With Curative Intent (MK-3475-B21 / KEYNOTE-B21 / ENGOT-en11 / GOG-3053) | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT01946074 | A Study of ABT-165 in Subjects With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003589 | Combination Chemotherapy in Treating Patients With Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01696032 | Pertuzumab in Platinum-Resistant Low Human Epidermal Growth Factor Receptor 3 (HER3) Messenger Ribonucleic Acid (mRNA) Epithelial Ovarian Cancer (PENELOPE) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00034177 | Safety and Efficacy of S-8184 in Treatment of Locally Advanced, Metastatic, or Recurrent TCC of the Urothelium | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT00660842 | Study Comparing Weekly Versus Every 3 Week Chemotherapy in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03036488 | Safety and Efficacy Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy as Neoadjuvant Treatment for Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-173/KEYNOTE-173) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01031212 | ASA404 in Combination With Carboplatin/Paclitaxel/Cetuximab in Treating Patients With Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03563170 | QUILT-3.072: NANT Hepatocellular Carcinoma (HCC) Vaccine | hepatocellular carcinoma | Paclitaxel (NPC208553) | |
| NCT04135781 | Nab-paclitaxel Combined With S-1 as Adjuvant Chemotherapy for Stage Ⅲ Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00511459 | Phase 2 Study of AMG 386 Plus Paclitaxel With or Without Bevacizumab as First Line Therapy in Her2-Negative Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003281 | Topotecan and Paclitaxel in Treating Patients With Recurrent or Refractory Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02040454 | Paclitaxel for the Treatment of Distal Radial Artery Arteriovenous Access Fistula Stenosis (PaciFIST-2) | stricture | Paclitaxel (NPC208553) | |
| NCT00003449 | Combination Chemotherapy in Treating Patients With Platinum-Resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00193206 | Neo-adjuvant Gemcitabine, Epirubicin, ABI-007 (GEA) in Locally Advanced or Inflammatory Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00513383 | Panitumumab Chemoradiotherapy Chemotherapy for Squamous Cancer of the Head and Neck | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00270790 | EVALUATION OF AMIFOSTINE FOR MUCOSAL AND HEMOPOETIC PROTECTION AND CARBOPLATIN, TAXOL, RADIOTHERAPY IN THE MANAGEMENT OF PATIENTS WITH HEAD AND NECK CANCER.(GCC 0202) | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01822756 | An Open-Label Study of Ruxolitinib Given With Chemotherapy in Patients With Advanced Solid Tumors | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03306121 | TPF+CCRT vs.CCRT+PF in High Risk Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT01303926 | Quality of Life Comparison in Advanced Non-squamous Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00462397 | Paclitaxel and Carboplatin Followed by Cisplatin and Radiation Therapy in Treating Patients With Stage IB, Stage II, Stage III, or Stage IVA Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00392392 | Preoperative Bevacizumab and Trastuzumab With ABI-007 and Carboplatin in HER2+ Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04286711 | Clinical Study of Albumin-paclitaxel Combined With Apatinib and Camrelizumab in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04526470 | Alpelisib and Paclitaxel in PIK3CA-altered Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00290537 | Phase II Study of ZD6474 in Advanced NSCLC | lung cancer | Paclitaxel (NPC208553) | |
| NCT00666692 | A Phase 1b Study With Volociximab in Combination With Carboplatin, Paclitaxel, and Bevacizumab in First-line, Advanced Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02182232 | A Dose Escalation Study of BIBF 1120 Together With Paclitaxel and Carboplatin in Patients With Advanced Stage Non-small-cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00626561 | Bevacizumab and Paclitaxel for Neuroendocrine Tumors of the Cervix | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04060459 | Paclitaxel-binding Albumin and Cisplatin as Neoadjuvant Chemotherapy in Patients With Muscle-Invasive Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT02883062 | Carboplatin and Paclitaxel With or Without Atezolizumab Before Surgery in Treating Patients With Newly Diagnosed, Stage II-III Triple-Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04723875 | Postoperative Adjuvant Chemotherapy in Early-stage Cervical Cancer That Not Meet Criteria of Adjuvant Therapeutic According to NCCN Guideline | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00002717 | Paclitaxel and Cisplatin in Treating Patients With Stage III or Stage IV Ovarian Cancer or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02138383 | Enzalutamide in Combination With Gemcitabine and Nab-Paclitaxel for the Treatment of Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00178256 | Pulsed Paclitaxel And Daily Thoracic Radiotherapy For Inoperable (Stage I/II) Or Unresectable Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00196872 | A Study to Compare ETC vs. EC-TX and Ibandronate vs. Observation in Patients With Node-positive Primary Breast Cancer (GAIN) | breast cancer | Paclitaxel (NPC208553) | |
| NCT00004092 | Combination Chemotherapy in Treating Patients With High-Risk Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01010945 | Erlotinib, Gemcitabine and Nab-Paclitaxel in Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00005838 | Combination Chemotherapy Plus Radiation Therapy With or Without AE-941 in Treating Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Removed By Surgery | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01303497 | Efficacity of Weekly Paclitaxel in Association or Not With Bevacizumab in Metastatic or Locally Advanced Angiosarcomas | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT03486314 | A Study to Evaluate the Effects of Rifampin on Pharmacokinetics (PK) of Pevonedistat in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04764227 | Phase II Study of Postoperative Concurrent Chemoradiotherapy for Esophageal Squamous Cell Carcinoma (ESO- Shanghai 17) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02640755 | Study of AZD6738, DNA Damage Repair/Novel Anti-cancer Agent, in Combination With Paclitaxel, in Refractory Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT00307255 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Gemcitabine in Treating Patients With Advanced Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04001569 | AZD8186 and Paclitaxel in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03912402 | Efficacy and Safety of BCD-100 (Anti-PD-1) in Combination With Platinum-Based Chemotherapy and Bevacizumab in Patients With Recurrent, Persistent or Metastatic Cervical Cancer (CAESURA) | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02023710 | Bevacizumab Combined With Carboplatin Plus Paclitaxel Chemotherapy to Treat Metastatic Mucosal Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT04672928 | A Phase Ib/III Clinical Study to Evaluate the Efficacy and Safety of IBI318 in Combination With Paclitaxel Versus Placebo in Combination With Paclitaxel in Patients With Small Cell Lung Cancer Who Have Failed First-line or Above Chemotherapies | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00738699 | First Line Tx Stage III Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00020449 | Liposomal Doxorubicin and Interleukin-12 in Treating Patients With AIDS-Related Kaposi's Sarcoma | sarcoma | Paclitaxel (NPC208553) | |
| NCT03417921 | A Study of ABTL0812 in Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00017407 | Carboplatin and Paclitaxel With or Without ISIS 3521 in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05179239 | A Study of SHR-1701 Plus Platinum-containing Chemotherapy With or Without BP102 (Bevacizumab) as First-line Treatment in Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02317991 | Study of Nab-Paclitaxel and Ramucirumab as Second-line Treatment for Patients With Metastatic Gastroesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003159 | Surgery With or Without Preoperative Chemotherapy in Treating Patients With Resectable Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00542451 | Adjuvant Paclitaxel and Trastuzumab for Node-Negative HER2-Positive Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00028990 | Paclitaxel With or Without Bevacizumab in Treating Patients With Locally Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00358163 | Trial of PTK787/ZK 222584 Plus Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT00851877 | Nab-Paclitaxel, Cisplatin, and Cetuximab With Concurrent Radiation Therapy for Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01146795 | Trial of Best Supportive Care and Either Cisplatin or Paclitaxel to Treat Patients With Primary Ovarian Cancer, Primary Peritoneal Cancer or Fallopian Tube Cancer and Inoperable Malignant Bowel Obstruction | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00796991 | Drug-Drug Interaction - 3 Arm - Carboplatin/Paclitaxel, Dacarbazine | melanoma | Paclitaxel (NPC208553) | |
| NCT04296175 | Carboplatin Intensified Chemotherapy for TRIple NEgative Breast Cancer(CITRINE) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT04528680 | Ultrasound-based Blood-brain Barrier Opening and Albumin-bound Paclitaxel and Carboplatin for Recurrent Glioblastoma | gliosarcoma | Paclitaxel (NPC208553) | |
| NCT04770272 | Study to Compare a Mono Atezolizumab Window Followed by a Atezolizumab - CTX Therapy With Atezolizumab - CTX Therapy | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00536939 | Trial of Paclitaxel, Bevacizumab, and Enzastaurin Versus Paclitaxel, Bevacizumab and Placebo for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04481204 | New and Emerging Therapies for the Treatment of Resectable, Borderline Resectable, or Locally Advanced Pancreatic Cancer, PIONEER-Panc Study | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04602117 | ISPY-P1.01:Evaluating the Safety of Weekly Paclitaxel With Trastuzumab Duocarmazine (SYD985) in Patients With Metastatic Cancer | carcinoid tumor;endometrium neoplasm;gastric cancer;ovarian carcinoma;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT04269200 | Durvalumab With or Without Olaparib as Maintenance Therapy After First-Line Treatment of Advanced and Recurrent Endometrial Cancer | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT00231582 | High-dose Chemotherapy With Autologous Stem Cell Transplantation in Poor Prognosis Germ-cell Tumors: TAXIF II | testicular neoplasm | Paclitaxel (NPC208553) | |
| NCT00436566 | Doxorubicin and Cyclophosphamide Followed By Trastuzumab, Paclitaxel, and Lapatinib in Treating Patients With Early-Stage HER2-Positive Breast Cancer That Has Been Removed By Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT00002854 | High-Dose Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Advanced Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT02199418 | Addition of Cisplatin to Neoadjuvant Therapy for T Locally Advanced Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00507429 | Study of Combretastatin and Paclitaxel/Carboplatin in the Treatment of Anaplastic Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT02383212 | Phase I Study of Oral BAY 1217389 in Combination With Intravenous Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT02340117 | Study of Combined SGT-53 Plus Gemcitabine/Nab-Paclitaxel for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02412371 | A Study Evaluating the Efficacy and Tolerability of Veliparib in Combination With Paclitaxel/Carboplatin-Based Chemoradiotherapy Followed by Veliparib and Paclitaxel/Carboplatin Consolidation in Adults With Stage III Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02437812 | Study of Paclitaxel, Carboplatin and Oral Metformin in the Treatment of Advanced Stage Ovarian Carcinoma | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00518284 | Prevention of Restenosis Following Revascularization | peripheral vascular disease | Paclitaxel (NPC208553) | |
| NCT01248403 | A Randomized, Double Blind Study Evaluating Paclitaxel With and Without RAD001 in Patients With Gastric Carcinoma After Prior Chemotherapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01836679 | Chidamide in Combination With Carboplatin and Paclitaxel in Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03169777 | QUILT-3.050: NANT Colorectal Cancer (CRC) Vaccine: Combination Immunotherapy in Subjects With Recurrent or Metastatic CRC | colorectal carcinoma | Paclitaxel (NPC208553) | |
| NCT00680758 | Cisplatin, Paclitaxel, and Everolimus in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00480831 | A Study of PRO95780 in Patients With Previously Untreated, Advanced-Stage Non-Small Cell Lung Cancer (APM4074g) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004055 | Topotecan, Paclitaxel, and Filgrastim in Treating Patients With Previously Untreated Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00556088 | LBH589, Paclitaxel, Carboplatin +/- Bevacizumab for Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00614484 | Chemotherapy and Proton Radiation for the Treatment of Locally Advanced Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003133 | Combination Chemotherapy Following Surgery in Treating Patients With Advanced Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01746771 | A Phase I-II Study of HM781-36B Combined With Paclitaxel and Trastuzumab in HER-2 Positive Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00113399 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Recurrent Head and Neck Cancer That Cannot Be Removed By Surgery | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00052312 | Doxorubicin and Cisplatin With or Without Paclitaxel in Treating Patients With Locally Advanced, Metastatic, and/or Relapsed Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02654119 | Cyclophosphamide, Paclitaxel, and Trastuzumab in Treating Patients With Stage I-II HER2/Neu Positive Breast Cancer After Surgery | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02834975 | Adavosertib Plus Chemotherapy in Platinum-Resistant Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03410784 | A Study of VB-111 With Paclitaxel vs Paclitaxel for Treatment of Recurrent Platinum-Resistant Ovarian Cancer (OVAL) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04592211 | Pembrolizumab, Olaparib, Recurrent/Advanced Gastric and Gastro-esophageal Junction(GEJ) Cancer With HRR Mutation and MSS | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04516616 | Pd-1 Antibody Combined Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02574663 | TGR-1202 Alone and in Combination With Either Nab-paclitaxel + Gemcitabine or With FOLFOX in Patients With Select Relapsed or Refractory Solid Tumors | rectum cancer;colorectal carcinoma;gastrointestinal stromal tumor;esophageal cancer;gastric cancer;pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00491855 | Oxaliplatin and Paclitaxel Plus Bevacizumab in Advanced Peritoneal Carcinomatosis | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00054548 | Combination Chemotherapy Plus Oblimersen in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04138212 | Neoadjuvant Chemotherapy Versus Neoadjuvant Chemoradiotherapy for Resectable Locally Advanced Esophageal Cancer (HCHTOG1903) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03935256 | Adjuvant Sequential & Concurrent CarboTaxol + Radiotherapy for High Risk Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT03520790 | Paricalcitol Plus Gemcitabine and Nab-paclitaxel in Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00576225 | CT-2103/Carboplatin vs Paclitaxel/Carboplatin for NSCLC in Women With Estradiol > 25 pg/mL | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02036164 | Adjuvant Chemotherapy for Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04884906 | Camrelizumab Combined With Radiotherapy and Chemotherapy for the Treatment of Recurrent or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01617928 | A Study of Veliparib in Combination With Carboplatin and Paclitaxel in Japanese Subjects With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00028743 | Combination Chemotherapy Regimens in Ovarian Epithelial Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02916511 | Study of Extensive Clinical Target Volumes in Postoperative Radiotherapy Concurrent With Chemotherapy for Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04718415 | Neoadjuvant Sintilimab in Combination With Carboplatin and Nab-paclitaxel in Resectable Oral Cavity or Oropharyngeal Squamous Cell Carcinoma | oral squamous cell carcinoma;oropharynx squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00786110 | Sorafenib Plus Paclitaxel in Adreno-Cortical-Cancer Patients | adrenal cortex carcinoma | Paclitaxel (NPC208553) | |
| NCT02050009 | Nab-Paclitaxel and Bevacizumab in Treating Patients With Unresectable Stage IV Melanoma or Gynecological Cancers | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00800202 | A Study of Avastin (Bevacizumab) in Patients With Non-Squamous Non-Small Cell Lung Cancer With Asymptomatic Untreated Brain Metastasis | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03309150 | ATRi Transition Rollover Study | neoplasm | Paclitaxel (NPC208553) | |
| NCT00661193 | S0709: Erlotinib With or Without Carboplatin and Paclitaxel in Stage IIIB or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00138151 | Isotretinoin, Interferon Alpha-2b, and Paclitaxel in Stage IV, Recurrent, or Persistent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00937560 | ZD4054 (Zibotentan) or Placebo Plus Chemotherapy in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00691054 | Abraxane Therapy in Patients With Pancreatic Cancer Who Failed First-Line Gemcitabine Therapy | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00004013 | Paclitaxel With or Without Trastuzumab Following Peripheral Stem Cell Transplantation in Treating Patients With Refractory Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04289792 | Split-course SBRT for Borderline Resectable and Locally Advanced Pancreatic Cancer | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00777673 | Preoperative Chemotherapy in Triple Negative Invasive Breast Cancer That Can be Removed by Surgery. | breast cancer | Paclitaxel (NPC208553) | |
| NCT04647344 | A Study of AK104 in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003214 | Chemosensitivity Testing to Assign Treatment for Patients With Stage III or Stage IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02172846 | Hypofractionated Proton Beam Radiation Therapy, Paclitaxel, and Carboplatin in Treating Patients With Stage II-III Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04572100 | Pilot Study of Chemotherapy for HPV-Associated Oropharyngeal Cancer | oropharynx squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02468557 | Study of Single Agent Idelalisib Followed by Idelalisib in Combination With Chemotherapy in Adults With Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00028860 | Combination Chemotherapy Following Surgery in Treating Patients With Urinary Tract Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT04339738 | Testing the Addition of Nivolumab to Chemotherapy in Treatment of Soft Tissue Sarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT02389985 | Paclitaxel/Pazopanib for Platinum Resistant/Refractory Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01281176 | High-Dose or Low-Dose Vorinostat in Combination With Carboplatin or Paclitaxel in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00662597 | ASA404 or Placebo in Combination With Paclitaxel and Carboplatin as First-Line Treatment for Stage IIIb/IV Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00466960 | Sargramostim and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer That Did Not Respond to Previous Chemotherapy | ovarian serous cystadenocarcinoma;endometrioid carcinoma;fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT05158062 | Pembrolizumab and Bevacizumab With Chemotherapy Followed by Pembrolizumab, Bevacizumab and Olaparib in Recurrent Ovarian Cancer | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00008021 | Monoclonal Antibody Therapy, Chemotherapy, and Peripheral Stem Cell Transplantation in Treating Patients With Refractory Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00730353 | Sutent + Taxol for Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03322969 | Receiving Modified Chemotherapy Followed With Radical Resection After Neoadjuvant Chemotherapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00735696 | A Study of Ramucirumab (IMC-1121B) With Paclitaxel and Carboplatin in Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02075021 | Phase I/II Trial of the Combination of Lenalidomide (Revlimid) and Nab-paclitaxel (Abraxane) in the Treatment of Relapsed/Refractory Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT02051751 | A Study to Evaluate the Potential Benefit of the Addition of BYL719 to Paclitaxel in the Treatment of Breast Cancer and Head-and-neck Cancer | upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT02644863 | Autologous Tumor Tissue Antigen-sensitized DC-CIK Cells Combined With Chemotherapy for Esophageal Cancer | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT04859465 | Albumin-bound Paclitaxel Combined With Liposomal Doxorubicin in the Treatment of Advanced or Unresectable Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT00287937 | Vorinostat, Paclitaxel, and Carboplatin in Treating Patients With Advanced or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00517621 | Use of FACT-GOG/NTX Questionnaire in Peripheral Neurotoxicity & Validation of a French Version of This Questionnaire | fallopian tube cancer;ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00300885 | A Randomized Controlled Trial Comparing Safety and Efficacy of Carboplatin and Paclitaxel Plus or Minus Sorafenib (BAY 43-9006) in Chemonaive Patients With Stage IIIB-IV Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04850235 | Nab-paclitaxel Based TPX Neoadjuvant Chemotherapy for NPC Patients: a Dose-escalation Study | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT01756170 | Postoperative Chemoradiation v.s Radiotherapy for Lymph Node Negative Cervical Cancer Patients | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00003440 | Paclitaxel With or Without Trastuzumab in Treating Patients With or Without HER-2/Neu Breast Cancer That is Inoperable, Recurrent, or Metastatic | breast cancer | Paclitaxel (NPC208553) | |
| NCT04426955 | Study of Camrelizumab (SHR-1210) in Combination With Concurrent Chemoradiotherapy in Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01616303 | A Controlled Study of the Effectiveness of Oregovomab (Antibody) Plus Chemotherapy in Advanced Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT02403895 | AZD2014 and Weekly Paclitaxel in Squamous NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03322566 | A Study of Pembrolizumab Plus Epacadostat With Platinum-based Chemotherapy Versus Pembrolizumab Plus Platinum-based Chemotherapy Plus Placebo in Metastatic Non-Small Cell Lung Cancer (KEYNOTE-715-06/ECHO-306-06) | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003235 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer That Can Not Be Surgically Removed | lung cancer | Paclitaxel (NPC208553) | |
| NCT00209612 | Phase I/II Study of Paclitaxel Plus CPT-11 in Pts. With 2nd Line Chemotherapy of Inoperable or Recurrent GC. | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01220128 | Lapatinib or Trastuzumab Given Prior to Surgery With Chemotherapy in Patients With Early Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00005644 | Combination Chemotherapy in Treating Patients With Advanced Cancer of the Urothelium and Decreased Kidney Function | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT02134067 | Dose-escalating, Safety, Tolerability and PK Study of TAS-119 in Combination With Paclitaxel in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01275677 | Chemotherapy With or Without Trastuzumab After Surgery in Treating Women With Invasive Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00666991 | Pharmacokinetic, Safety and Efficacy Study of Nanoparticle Paclitaxel in Patients With Peritoneal Cancers | peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00006469 | Combination Chemotherapy and Radiation Therapy Followed By Surgery in Treating Patients With Stage IIB or Stage IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00991796 | CS-1008 With Carboplatin/Paclitaxel in Chemotherapy naïve Subjects With Metastatic or Unresectable Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003251 | Amifostine Plus Chemotherapy and Radiation Therapy in Treating Patients With Advanced, Unresectable Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00006484 | Combination Chemotherapy With or Without Tirapazamine in Treating Patients With Stage IIIB or Stage IV Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03600090 | Phase I Study of EOC202 Plus Paclitaxel in Chinese Patients With Metastatic Breast Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00077129 | Paclitaxel and Carboplatin in Treating Patients With Locally Advanced or Metastatic Renal Cell Cancer | kidney cancer | Paclitaxel (NPC208553) | |
| NCT00077246 | ABI-007 in Treating Patients With Chemotherapy-Naïve Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03693612 | GSK3359609 Plus Tremelimumab for the Treatment of Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00028938 | Chemotherapy and Radiation Therapy With or Without Epoetin Alfa in Treating Patients With Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00316199 | Efficacy Study of Gemcitabine-Paclitaxel to Treat Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03165994 | APX005M With Concurrent Chemoradiation for Resectable Esophageal and Gastroesophageal Junction Cancers | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00004265 | Paclitaxel in Treating Patients With Recurrent or Refractory Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03981796 | A Study to Evaluate Dostarlimab Plus Carboplatin-paclitaxel Versus Placebo Plus Carboplatin-paclitaxel in Participants With Recurrent or Primary Advanced Endometrial Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT02603679 | Neoadjuvant Response-guided Treatment of Luminal B-type Tumors and Luminal A-type Tumors With Node Metastases | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00585052 | A Phase II Study of Interaction of Lovastatin and Paclitaxel For Patients With Refractory or Relapsed Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00144053 | A Phase Ⅲ Randomized Study of Mitomycin/Vindesine/Cisplatin Versus Irinotecan/Carboplatin Versus Paclitaxel/Carboplatin With Concurrent Thoracic Radiotherapy for Unresectable Stage Ⅲ Non-Small-Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03884556 | TTX-030 Single Agent and in Combination With Immunotherapy or Chemotherapy for Patients With Advanced Cancers | lymphoma | Paclitaxel (NPC208553) | |
| NCT01402271 | Study of AMG 386 in Combination With Paclitaxel and Carboplatin in Subjects With Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT02861690 | Liposomal Paclitaxel Combined Nedaplatin in Treatment of Advanced or Recurrent Esophageal Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT03421353 | AZD9150 Plus Durvalumab Alone or in Combination With Chemotherapy in Patients With Advanced, Solid Tumours and in Patients With Non-Small-Cell Lung Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00251095 | Study Of SU011248 Versus Chemotherapy For Patients With Previously Treated Triple Receptor Negative Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03387098 | QUILT-3.070:Pancreatic Cancer Vaccine: Subjects With Pancreatic Cancer Who Have Progressed on or After Standard-of-care Therapy | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02487277 | PEGPH20, Gemicitabine and Nab-Paclitaxel for Pancreatic Ductal Adenocarcinoma | adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05298020 | Envafolimab Combined With Endostar and Chemotherapy for First-line Treatment of Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01534585 | Safety and Efficacy Study of Icotinib With Intensity-modulated Radiotherapy in Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00254891 | Trial of Paclitaxel/Carboplatin + PF-3512676 vs Paclitaxel/Carboplatin Alone in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00093795 | Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Positive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03201861 | Addition of Cisplatin to Adjuvant Chemotherapy for Early Stage Breast Cancer in High-Risk Women | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03287271 | Clinical Trial of Combined Fostamatinib and Paclitaxel in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00087217 | 17-N-Allylamino-17-Demethoxygeldanamycin and Paclitaxel in Treating Patients With Metastatic or Unresectable Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT00653952 | CAELYX Versus Paclitaxel HCl in Patients With Epithelial Ovarian Carcinoma Following Failure of First-Line, Platinum-Based Chemotherapy | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT01872403 | Neoadjuvant Chemotherapy of Nanoparticle Albumin-bound Paclitaxel/Carboplatin vs. Paclitaxel /Carboplatin in Stage Ⅱ B and IIIA Squamous Cell Carcinoma of the Lung | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02767557 | Study of Nab-Paclitaxel and Gemcitabine With or Without Tocilizumab in Pancreatic Cancer Patients | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00493025 | Paclitaxel, Cisplatin, Gefitinib, and Radiation Therapy Followed by Surgery and Gefitinib in Treating Patients With Locally Advanced Cancer of the Esophagus or Gastroesophageal Junction That Can Be Removed By Surgery | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01993810 | Comparing Photon Therapy To Proton Therapy To Treat Patients With Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00024375 | DHA-Paclitaxel in Treating Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02382263 | Nab-paclitaxel in Combination With Gemcitabine in Fragile Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00052351 | Vaccine Therapy Plus Sargramostim and Chemotherapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02054884 | F16IL2 Plus Paclitaxel in Metastatic Merkel Cell Carcinoma | Merkel cell skin cancer | Paclitaxel (NPC208553) | |
| NCT00003972 | Combination Chemotherapy and Peripheral Stem Cell Transplantation in Treating Patients With Stage II or Stage IIIA Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03901118 | Efficacy and Safety of Paclitaxel for Injection (Albumin-bound) for First-line Chemotherapy of Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00429299 | Pazopanib (VOTRIENT) Plus Paclitaxel (TAXOL), Pazopanib Plus Paclitaxel (TAXOL) Plus Carboplatin (PARAPLATIN), and Pazopanib Plus Paclitaxel (TAXOL) Plus Lapatinib (TYKERB) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00003812 | Chemotherapy Plus Radiation Therapy in Treating Patients With Limited-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00442260 | Doxorubicin HCL Liposome Injection (DOXIL) in Combination With Abraxane in Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03641183 | Folfirinox or Gemcitabine-Nab Paclitaxel Followed by Stereotactic Body Radiotherapy for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00049257 | Paclitaxel and Carboplatin in Treating Patients With Metastatic Prostate Cancer That Has Not Responded to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT02250599 | Efficacy and Safety of TC+AVASTIN Versus TC in Patients With Metastatic Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT02607332 | Paclitaxel in Patients With Metastatic or Advanced Gastrointestinal Stromal Tumors (GIST) After Failure to Imatinib and Sunitinib | gastrointestinal stromal tumor | Paclitaxel (NPC208553) | |
| NCT00919984 | Efficacy Study of Additional Intraperitoneal Chemotherapy to Treat Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00002917 | Paclitaxel in Treating Patients With Early-Stage Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00093145 | Study of Albumin-bound Paclitaxel (Abraxane) in Combination With Carboplatin and Herceptin in Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01352689 | CKD-828(80/2.5mg) Pharmacokinetic Study_2nd | hypertension | Paclitaxel (NPC208553) | |
| NCT04258644 | Camrelizumab in Combination With Apatinib Mesylate, Paclitaxel-albumin and S-1 for Translational Treatment of Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00001442 | A Pilot Study of Paclitaxel With Radiation Therapy for Locally Advanced Head and Neck Cancer | squamous cell carcinoma;upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT01138046 | A Phase II, Randomized, Open-label Study of Lapatinib Plus Chemotherapy Versus Trastuzumab Plus Chemotherapy in HER2-positive and p95HER2-positive Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03825328 | A Trial of NS/GEMOX Chemotherapy in Patients With Untreated Pancreatic Cancer ( HZ-NS/GEMOX-PC ) | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00201435 | Wkly Taxol x 12 | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT05070598 | Camrelizumab Combined With Chemotherapy in First-line Treatment of HER2-positive Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02212015 | Evaluation of Votrient in Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT04808687 | Neoadjuvant Nab-Paclitaxel and S-1 in Resectable Pancreatic Cancer | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT02229058 | Albumin Bound Paclitaxel Plus S-1 as the First Line Chemotherapy in Advanced or Recurrent Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02979392 | Phase I Study of TENPA in Advanced Solid Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT04974944 | First-line Treatment With Camrelizumab + Apatinib Versus Chemotherapy + Bevacizumab in Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00006108 | Capecitabine, Paclitaxel, and Trastuzumab in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00002814 | Combination Chemotherapy for Patients With Brain Cancer | Central Nervous System Neoplasm | Paclitaxel (NPC208553) | |
| NCT01293630 | A Dose-escalation Study of Ombrabulin in Combination With Paclitaxel and Carboplatin in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03598270 | Platinum-based Chemotherapy With Atezolizumab and Niraparib in Patients With Recurrent Ovarian Cancer | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT02429622 | Simultaneous Integrated Boost Radiotherapy and Concurrent Chemotherapy for Locally Advanced Esophageal Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT00003117 | Paclitaxel With or Without Carboplatin in Treating Patients With Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02784288 | Phase II Treatment Stratification Trial Using Neck Dissection-Driven Selection to Improve Quality of Life for Low Risk Patients With HPV+ Oropharyngeal Squamous Cell Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03125902 | A Study of Atezolizumab and Paclitaxel Versus Placebo and Paclitaxel in Participants With Previously Untreated Locally Advanced or Metastatic Triple Negative Breast Cancer (TNBC) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03126708 | Cetuximab in Combination With Paclitaxel Plus Cisplatin Versus Paclitaxel Plus Cisplatin Alone for the First-line Treatment of Metastatic Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02442362 | TOF Versus SOX in Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00046423 | A Trial of ABI-007 in Patients With Advanced Non-Hematologic Malignancies | metastasis;neoplasm | Paclitaxel (NPC208553) | |
| NCT04604132 | Derazantinib Alone or in Combination With Paclitaxel, Ramucirumab or Atezolizumab in Gastric Adenocarcinoma | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00006276 | Micellar Paclitaxel to Treat Severe Psoriasis | psoriasis | Paclitaxel (NPC208553) | |
| NCT04249167 | Cryoablation, Atezolizumab/Nab-paclitaxel for Locally Advanced or Metastatic Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02041533 | An Open-Label, Randomized, Phase 3 Trial of Nivolumab Versus Investigator's Choice Chemotherapy as First-Line Therapy for Stage IV or Recurrent PD-L1+ Non-Small Cell Lung Cancer (CheckMate 026) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00198367 | Three Modalities of Treatment in Operable and Resectable Stage IIIA (T1-3, N2) Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02259621 | Neoadjuvant Nivolumab, or Nivolumab in Combination With Ipilimumab, in Resectable NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00959387 | Induction Chemotherapy for Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02595554 | Neoadjuvant Chemotherapy and Radical Surgery in Stage IIB Cervical Cancer | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT00506779 | Gleevec/Taxol for Patients With Uterine Papillary Serous Carcinoma | uterine cancer | Paclitaxel (NPC208553) | |
| NCT03850769 | Neoadjuvant Nab-Paclitaxel and S-1 in Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00520013 | Avastin +/- Erlotinib Consolidation Chemotherapy After Carboplatin, Paclitaxel, and Avastin (CTA) Induction Therapy for Advanced Ovarian, Fallopian Tube, Primary Peritoneal Cancer & Papillary Serous or Clear Cell Mullerian Tumors | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003614 | Paclitaxel Plus Estramustine in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00095914 | Paclitaxel and ABI-007 in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00550771 | Lapatinib +/- Trastuzumab In Addition To Standard Neoadjuvant Breast Cancer Therapy. | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00960297 | Preoperative Chemotherapy and Bevacizumab in Patients With Stage IB (>4 cm), II, or Select Stage III NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00154804 | CCRT With Twice Weekly Paclitaxel and Cisplatin Followed by Surgery for Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03737643 | An uMbrella Study of BIomarker-driven Targeted Therapy In Patients With Platinum-resistant Recurrent OvariaN Cancer(AMBITION) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00600340 | 2-arm Trial of Paclitaxel Plus Bevacizumab vs. Capecitabine Plus Bevacizumab | breast cancer | Paclitaxel (NPC208553) | |
| NCT00652119 | Intraperitoneal Paclitaxel and Carboplatin With IV Avastin Therapy in Patients With Carcinomas of Mullerian Origin | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT05092373 | Tumor Treating Fields Therapy in Combination With Chemotherapy for the Treatment of Advanced Solid Tumors Involving the Abdomen or Thorax | renal cell carcinoma;hepatocellular carcinoma;endometrial carcinoma;Fallopian Tube Carcinoma;ovarian carcinoma;breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00513786 | Evaluation of Carboplatin/Paclitaxel/Bevacizumab in the Treatment of Advanced Stage Endometrial Carcinoma | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00002894 | Platinum-based Chemotherapy With or Without Paclitaxel in Treating Patients With Relapsed Ovarian Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00369070 | A Phase 2 Trial of AMG 706 or Bevacizumab in Combination With Chemo for Advanced NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04683315 | PurIST Classification-Guided Adaptive Neoadjuvant Chemotherapy by RNA Expression Profiling of EUS Aspiration Samples | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03512834 | Paclitaxel-Avelumab for Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT00340184 | A Safety and Efficacy of CCRT With Paclitaxel/Carboplatin as Adjuvant Therapy to Post-operative Cervical Cancer Patients | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00762034 | A Study of Pemetrexed, Carboplatin and Bevacizumab in Participants With Nonsquamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00724386 | Concomitant Chemoradiotherapy With Weekly Paclitaxel and Vinorelbine and Granulocyte Colony Stimulating Factor (GCSF) Support in Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02001272 | LDE225 and Paclitaxel in Solid Tumors | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05427383 | KN026 in Combination With Chemotherapy in the Second Line Treatment of HER-2 Positive Advanced or Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00445458 | A Phase 1/2 Study of HKI-272 (Neratinib) in Combination With Paclitaxel (Taxol) in Subjects With Solid Tumors and Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04831320 | Nab-Paclitaxel in Combination With Nivolumab to Treat Recurrent or Metastatic Head and Neck Squamous-Cell Carcinoma That Progressed on a PD-1 or PD-L1 Inhibitor | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003880 | Paclitaxel Plus Carboplatin With or Without SCH-58500 in Treating Patients With Newly Diagnosed Stage III Ovarian or Stage III Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT05241249 | Stimulation With Bethanechol in Combination With Gemcitabine and Nab-paclitaxel in Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00889382 | Paclitaxel-Loaded Polymeric Micelle and Carboplatin as First-Line Therapy in Treating Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02412722 | Study of REGN2810 (Anti-PD-1) in Patients With Advanced Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT01893801 | Nab-Pac+Cis+Gem in Pts w Previously Untreated Metastatic PDA | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01966003 | Efficacy and Safety Study of ABP 215 Compared With Bevacizumab in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04341883 | Study of Anti-PD-1 Combined With Albumin-Bound Paclitaxel in Patients With Recurrent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00532285 | Primary Paclitaxel Plus Gemcitabine in Patients With Stage II and III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03635489 | Carboplatin-Paclitaxel-Bevacizumab vs Carbo-Pacli-Beva-Rucaparib vs Carbo-Pacli-Ruca, Selected According to HRD Status, in Patients With Advanced Ovarian, Primary Peritoneal and Fallopian Tube Cancer, Preceded by a Phase I Dose Escalation Study on Ruca-Beva Combination | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01912625 | Trametinib, Combination Chemotherapy, and Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Removed by Surgery | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04634539 | Trial of First-line L-glutamine With Gemcitabine and Nab-paclitaxel in Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00230451 | Pre-operative Chemotherapy and Radiation Therapy for Esophageal Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00392704 | Treatment of Head & Neck Cancer With Chemotherapy and Radiation | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00003624 | Amifostine in Treating Women With Ovarian, Peritoneal, Cervical, Fallopian Tube, Uterine, or Endometrial Cancer | cervical cancer;endometrial cancer;fallopian tube cancer;ovarian cancer;peritoneum cancer;sarcoma | Paclitaxel (NPC208553) | |
| NCT00006004 | Comparison of Two Combination Chemotherapy Regimens in Treating Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01204749 | Saracatinib and Paclitaxel in Platinum-resistant Ovarian Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02383251 | Study of Metformin With Carboplatin/Paclitaxel Chemotherapy in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01953536 | Effect of Trastuzumab on Disease Free Survival in Early Stage HER2-Negative Breast Cancer Patients With ERBB2 Expressing Disseminated Tumor Cells | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01301716 | A Study of the Safety and Pharmacology of GDC-0980 in Combination With Either Paclitaxel and Carboplatin (With or Without Bevacizumab) or Pemetrexed and Cisplatin in Patients With Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT05294900 | Trial of Neoadjuvant Therapy With Paclitaxel and Carboplatin in Operable Locally Advanced Head and Neck Cancer Patients | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04506138 | Camrelizumab in Combination With Neoadjuvant Chemotherapy for Resectable Thoracic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02931890 | Multicentric Randomised Trial for Resectable Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT05251038 | Study of Sotorasib Combined With Chemotherapy for Second Line Treatment of Pancreas Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01192165 | Safety and Tolerability Study of GSK1120212, a MEK Inhibitor, in Combination With Docetaxel, Erlotinib, Pemetrexed, Pemetrexed + Carboplatin, Pemetrexed + Cisplatin, or Nab-Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT02730416 | Combination Chemotherapy With Nintedanib / Placebo in Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02107378 | Efficacy of DCVAC/OvCa Plus Standard of Care in Relapsed Platinum Resistant Epithelial Ovarian Carcinoma | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00005028 | Paclitaxel and Bryostatin 1 in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT02027428 | Safety and Efficacy Study of Abraxane as Maintenance Treatment After Abraxane Plus Carboplatin in 1st Line Stage IIIB / IV Squamous Cell Non-small Cell Lung Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05171530 | Lenvatinib With Taxane Drugs Treatment for Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01009983 | Paclitaxel, Carboplatin, and Panitumumab in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01593228 | Treatment Extension Study for Patients Who Have Previously Participated and Have Benefited From Iniparib in a Clinical Trial | neoplasm | Paclitaxel (NPC208553) | |
| NCT00002772 | S9623, Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Women With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00770809 | Paclitaxel and Trastuzumab With or Without Lapatinib in Treating Patients With Stage II or Stage III Breast Cancer That Can Be Removed by Surgery | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT05322499 | Phase II Clinical Study of Camrelizumab Combined With Chemotherapy or Anlotinib in Advanced Esophageal Squamous Cell Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003385 | Combination Chemotherapy in Treating Patients With Untreated Ovarian, Peritoneal, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01248949 | A Study to Evaluate the Safety and Antitumor Activity in Subjects With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00231075 | Evaluation of the Safety and Efficacy of Paraplatin in Combination With Taxol in Patients of 70 Years and Older With Ovary Cancer F.I.G.O. Stages III-IV | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03281369 | A Study of Multiple Immunotherapy-Based Treatment Combinations in Patients With Locally Advanced Unresectable or Metastatic Gastric or Gastroesophageal Junction Cancer (G/GEJ) or Esophageal Cancer (Morpheus-Gastric and Esophageal Cancer) | esophageal carcinoma;gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04498689 | Efficacy and Safety of Camrelizumab Combined With Nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00129389 | FAC Versus FAC Plus Weekly Paclitaxel as Adjuvant Treatment of Node Negative High Risk Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT02312661 | Lower Dose Decitabine (DAC)-Primed TC (Carboplatin-Paclitaxel) Regimen in Ovary Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02103062 | Phase 2 Study With Abraxane (Nab®Paclitaxel) in Metastatic Colorectal Cancer | colorectal neoplasm | Paclitaxel (NPC208553) | |
| NCT04209686 | Paclitaxel, Pembrolizumab and Olaparib in Previously Treated Advanced Gastric Adenocarcinoma | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00031954 | Combination Chemotherapy in Treating Patients With Ovarian Epithelial, Fallopian Tube, or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02215447 | A Feasibility Study Of NAB-Paclitaxel In Combination With Carboplatin As First Line Treatment Of Gastrointestinal Neuroendocrine Carcinomas | neuroendocrine carcinoma | Paclitaxel (NPC208553) | |
| NCT00009802 | Calcitriol Plus Paclitaxel in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02483247 | A Safety, Tolerability, and Pharmacokinetics Study of MLN0128 as a Single Agent and in Combination With Paclitaxel in Adults With Advanced Nonhematologic Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00236899 | Phase III Study of Two Different Schedules (Weekly and Tri-weekly) of Combination of Gemcitabine and Two Taxanes in MBC | breast cancer | Paclitaxel (NPC208553) | |
| NCT03308552 | Radiotherapy With or Without Concurrent Chemotherapy for Limited Lymphatic Metastasis of Esophageal Cancer - 3JECROG P-02 | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT02715804 | A Study of PEGylated Recombinant Human Hyaluronidase in Combination With Nab-Paclitaxel Plus Gemcitabine Compared With Placebo Plus Nab-Paclitaxel and Gemcitabine in Participants With Hyaluronan-High Stage IV Previously Untreated Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00781612 | A Safety Extension Study of Trastuzumab Emtansine in Participants Previously Treated With Trastuzumab Emtansine Alone or in Combination With Other Anti-Cancer Therapy in One of the Parent Studies | metastasis | Paclitaxel (NPC208553) | |
| NCT01938846 | BI 860585 Dose Escalation Single Agent and in Combination With Exemestane or With Paclitaxel in Patients With Various Advanced and/or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02525653 | Albumin-Bound Paclitaxel and Gemcitabine in Patients With Untreated Stage IV or Recurrent Squamous Cell Lung Cancers | lung cancer | Paclitaxel (NPC208553) | |
| NCT02583477 | Phase Ib/II Study of MEDI4736 Evaluated in Different Combinations in Metastatic Pancreatic Ductal Carcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02682693 | Denosumab as an add-on Neoadjuvant Treatment (GeparX) | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01091428 | Alisertib (MLN8237) in Participants With Ovarian, Fallopian Tube or Peritoneal Cancer Preceded by Phase 1 Study of MLN8237 Plus Paclitaxel Treatment of Ovary or Breast Cancer | ovarian carcinoma;breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00883116 | A Study of Ixabepilone as Second-line Therapy for Locally Advanced, Recurrent, or Metastatic Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02297217 | Chemoradiotherapy for Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00006229 | Paclitaxel + Carboplatin With/ut BMS-275291 in Advanced or Metastatic Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00483782 | Carboplatin and Paclitaxel With or Without Bevacizumab in Treating Patients With Newly Diagnosed Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cavity Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00370552 | A Trial of 2 Schedules of Ixabepilone Plus Bevacizumab and Paclitaxel Plus Bevacizumab for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00861627 | Phase 2 Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin for Non-Small Cell Lung Cancer With KRAS or EGFR Activation | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02766582 | Avelumab in Previously Untreated Patients With Epithelial Ovarian Cancer (JAVELIN OVARIAN 100) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01006252 | A Study of Tasisulam-sodium Versus Paclitaxel as Treatment for Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00373217 | Vaccine Therapy, Paclitaxel, and Carboplatin in Treating Patients Who Are Undergoing Surgery for Stage III or Stage IV Ovarian Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04581265 | Nab-PTX, Ifosfamide and Cisplatin in the Treatment of Pediatric Extracranial Germ Cell Tumor. | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT03030287 | A Phase 1b Study of OMP-305B83 Plus Paclitaxel in Subjects With Ovarian, Peritoneal or Fallopian Tube Cancer | Fallopian Tube Carcinoma;ovarian carcinoma;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT02501902 | Dose-Escalation Study Of Palbociclib + Nab-Paclitaxel In mPDAC | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01838538 | Abraxane/Bevacizumab | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00915369 | A Clinical Trial to Study the Effects of Nanoparticle Based Paclitaxel Drug, Which Does Not Contain the Solvent Cremophor, in Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01929941 | An Open-Label Study of a Novel JAK-inhibitor, INCB047986, Given in Patients With Advanced Malignancies | Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT00005051 | Combination Chemotherapy in Treating Patients With Advanced Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01633970 | A Study of Atezolizumab Administered in Combination With Bevacizumab and/or With Chemotherapy in Participants With Locally Advanced or Metastatic Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT00951665 | A Study of Trastuzumab Emtansine, Paclitaxel, and Pertuzumab in Patients With HER2-Positive, Locally Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03336216 | A Study of Cabiralizumab Given With Nivolumab With and Without Chemotherapy in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04077255 | EGFR-targeted Therapy for Gastric Cancer | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00356811 | Combined Treatment of Cetuximab and Paclitaxel in Basal Like Breast Carcinoma | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00894569 | Paclitaxel/Carboplatin With or Without Cetuximab in CUP | carcinoma | Paclitaxel (NPC208553) | |
| NCT00272987 | Comparison of Safety and Efficacy of TOCOSOL(R) Paclitaxel Versus Taxol(R) for Treatment of Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00005046 | Paclitaxel in Treating Patients With Recurrent or Persistent Ovarian or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03563144 | QUILT-3.088: NANT Pancreatic Cancer Vaccine | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01283204 | Trial of 4-regimen (SP, FL/Tax, FL/Doc, FOLFOX) in Patients With Recurrent or Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04883281 | A Prospective Study of Daily Adaptive Radiotherapy to Better Organ-at-Risk Doses in Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04257448 | Safety and Tolerance of Epigenetic and Immunomodulating Drugs Combined With Chemotherapeutics in Patients Suffering From Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05002686 | Safety and Efficacy of Sintilimab in Combination With Chemoradiothrapy Followed by D2 Surgical Resection in Patients With Advanced Gastric Cancer With Retroperitoneal Lymph Node Metastasis | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01525602 | Safety Study of PLX3397 and Paclitaxel in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00609791 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients of Different Ages With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04761601 | First in Human Study of UCT-01-097 in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04862585 | Safely Stopping Pre-medications in Patients With Breast Cancer Who Are Receiving Paclitaxel | breast carcinoma in situ | Paclitaxel (NPC208553) | |
| NCT00004089 | Chemotherapy Plus Radiation Therapy in Treating Patients With Previously Untreated Thyroid Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00002587 | Paclitaxel Plus Topotecan in Treating Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00259675 | Alternating Cycles of Carboplatin/Gemcitabine and Carboplatin/Taxol for Advanced Stage NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05420636 | Iadademstat in Combination With Paclitaxel in Relapsed/Refractory SCLC and Extrapulmonary High Grade NET | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04888403 | Exploring the Safety and Effectiveness of Toripalimab Combined With Neoadjuvant Radiotherapy and Chemotherapy in the Locally Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00654836 | Carboplatin, Paclitaxel, and Bevacizumab in Treating Patients With Locally Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00912639 | A Clinical Trial of Paclitaxel Loaded Polymeric Micelle in Patients With Taxane-Pretreated Recurrent Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04589234 | Saltikva for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00033553 | Combination Chemotherapy and Computer-Planned Radiation Therapy in Treating Patients With Unresectable Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04815408 | Batiraxcept (AVB-S6-500)/Placebo in Combination With Paclitaxel in Patients With Platinum-Resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00343291 | A Study of Cetuximab and Bevacizumab in Combination With Paclitaxel and Carboplatin in Stage IIIb/IV NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02741856 | Study of Chemoradiotherapy in Oesophageal Cancer Including PET Response and Dose Escalation | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02477826 | An Investigational Immuno-therapy Trial of Nivolumab, or Nivolumab Plus Ipilimumab, or Nivolumab Plus Platinum-doublet Chemotherapy, Compared to Platinum Doublet Chemotherapy in Patients With Stage IV Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03762564 | Study With Paclitaxel +/- Ramucirumab in Patients With Squamous-cell Carcinoma of the Esophagus After Prior Therapy | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01225302 | A Study of Linifanib (ABT-869) in Combination With Carboplatin/Paclitaxel in Japanese Subjects With Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00993655 | Two Different Schedules of Carboplatin, Paclitaxel, Gemcitabine, and Surgery in Treating Patients With Newly Diagnosed Stage IIIC or Stage IV Primary Epithelial Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer | fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02569242 | Study of Nivolumab in Unresectable Advanced or Recurrent Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003377 | Radiation Therapy, Paclitaxel, and Cisplatin in Treating Patients With Cancer of the Cervix | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01055028 | Paclitaxel + Bevacizumab (Avastin) for the Treatment of Metastatic or Unresectable Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT01493505 | A Study of MM-121 With Paclitaxel in Platinum Resistant/ Refractory Advanced Ovarian Cancers | fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT05095467 | HIPEC-AS in Patients With Peritoneal Metastasis of the Stomach or Esophagogastric Junction | stomach neoplasm | Paclitaxel (NPC208553) | |
| NCT02038621 | The Maintenance Therapy of Capecitabine of Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03272477 | Study of Cirmtuzumab and Paclitaxel for Metastatic or Locally Advanced, Unresectable Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04753879 | Multi-agent Low Dose Chemotherapy GAX-CI Followed by Olaparib and Pembro in Metastatic Pancreatic Ductal Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00687817 | Study of Bavituximab Plus Paclitaxel and Carboplatin in Patients With Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00111787 | Study Of Lapatinib In Combination With Paclitaxel In The Treatment Of Newly Diagnosed Inflammatory Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03694002 | Carboplatin and Paclitaxel With or Without Ramucirumab in Treating Patients With Locally Advanced, Recurrent, or Metastatic Thymic Cancer That Cannot Be Removed by Surgery | Thymic Carcinoma | Paclitaxel (NPC208553) | |
| NCT01244789 | Chemotherapy or Observation in Stage I-II Intermediate or High Risk Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02597036 | A Study of LY3127804 With Ramucirumab in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT05383196 | Onvansertib + Paclitaxel In TNBC | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01253681 | Study of Intraperitoneal Carboplatin With IV Paclitaxel and Bevacizumab in Untreated Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT02350517 | Endostar Combined With Paclitaxel and Nedaplatin in Treating Patients With Recurrent or Metastatic Esophageal Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00228319 | Treatment of Newly Diagnosed Ovarian Cancer With Antioxidants | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02413320 | Neoadjuvant Study of Two Platinum Regimens in Triple Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00301028 | Cetuximab, Carboplatin, and Paclitaxel Followed by Radiation Therapy, With or Without Cisplatin, in Treating Patients With Metastatic Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00110084 | ABI-007 (Nab-Paclitaxel) and Gemcitabine in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05493995 | A Study of Penpulimab and Anlotinib Plus Nab-paclitaxel and Gemcitabine for Metastatic Pancreatic Cancer. | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00003612 | Combination Chemotherapy and Trastuzumab in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00111904 | Paclitaxel in Treating Patients With Unresectable Locally Advanced or Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00337532 | A Phase II Trial Neoadjuvant Paclitaxel and Cisplatin in Patients With Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04278287 | Chemoradiotherapy in Unresectable Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03940196 | Chiauranib in Combination With Chemotherapy in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00006472 | Combination Chemotherapy Plus Radiation Therapy Followed By Surgery in Treating Patients With Stage I, Stage II, or Stage III Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04261465 | Adoptive T Cell Therapy in Patients With Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04138719 | Nab-paclitaxel Plus Carboplatin Versus Nab-paclitaxel Plus Epirubicin in the Neoadjuvant Therapy for Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00686322 | Concurrent Chemoradiotherapy (CCRT) With Paclitaxel Plus Cisplatin in LA Non-small-cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04856137 | A Phase I/II Study of Diffuse Large B-cell Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00970996 | Cisplatin, Temozolomide, Abraxane, With Interleukin-2 and Interferon for Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT02303977 | Phase II Study of Abraxane and Gemicitabine in Patients With Advanced Adenocarcinoma Non-Small Cell Lung Cancer Progressing After First-Line Platinum-Based Chemotherapy | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01219777 | TRINOVA-1: A Study of AMG 386 or Placebo, in Combination With Weekly Paclitaxel Chemotherapy, as Treatment for Ovarian Cancer, Primary Peritoneal Cancer and Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00005819 | Combination Chemotherapy in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03057600 | Study of CB-839 in Combination w/ Paclitaxel in Participants of African Ancestry and Non-African Ancestry With Advanced Triple Negative Breast Cancer (TNBC) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00934895 | Phase I/II Study of Weekly Abraxane and RAD001 in Women With Locally Adv. or Metastatic Breast Ca | breast cancer | Paclitaxel (NPC208553) | |
| NCT01730586 | Abraxane in CIMP-High Colorectal and Small Bowel Adenocarcinomas | colorectal carcinoma | Paclitaxel (NPC208553) | |
| NCT02048943 | Dovitinib Lactate, Gemcitabine Hydrochloride, and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Solid Tumors or Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02121990 | Metformin Hydrochloride, Carboplatin, and Paclitaxel in Treating Patients With Recurrent Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01258192 | Neoadjuvant Chemotherapy With Nab-paclitaxel Plus Cisplatin for Stage Ⅱ-Ⅲ Esophageal Cancer Patients | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03197935 | A Study to Investigate Atezolizumab and Chemotherapy Compared With Placebo and Chemotherapy in the Neoadjuvant Setting in Participants With Early Stage Triple Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT02978495 | Neoadjuvant Carboplatin in Triple Negative Breast Cancer | Hereditary breast and ovarian cancer syndrome | Paclitaxel (NPC208553) | |
| NCT00798252 | Ascending Multiple-Dose Study of Brivanib Alaninate in Combination With Chemotherapeutic Agents in Subjects With Advanced Cancers | cancer | Paclitaxel (NPC208553) | |
| NCT00944047 | Evaluate Trastuzumab Plus Standard Chemotherapy Given Before Surgery in Breast Cancer Patients With Low HER 2 Expression | breast cancer | Paclitaxel (NPC208553) | |
| NCT00618657 | Carboplatin+Nab-paclitaxel, Plus Trastuzumab (HER2+) or Bevacizumab (HER2-) in the Neoadjuvant Setting | breast cancer | Paclitaxel (NPC208553) | |
| NCT02283372 | Nab-Paclitaxel Plus Gemcitabine With Concurrent MR-Guided IMRT in Patients With Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02713386 | Ruxolitinib Phosphate, Paclitaxel, and Carboplatin in Treating Patients With Stage III-IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | endometrioid carcinoma | Paclitaxel (NPC208553) | |
| NCT02476955 | Open-label Phase 1b Study of ARQ 092 in Combination With Anastrozole | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00010257 | Carboplatin Combined With Paclitaxel in Treating Patients With Advanced Thymoma | Thymic Carcinoma;Thymoma | Paclitaxel (NPC208553) | |
| NCT04632992 | A Study to Determine the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of ABBV-368 Plus Tilsotolimod and Other Therapy Combinations in Participants With Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma | cancer | Paclitaxel (NPC208553) | |
| NCT00003087 | Combination Chemotherapy, Radiation Therapy and Surgery in Treating Patients With Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00954512 | Study of Robatumumab (SCH 717454, MK-7454) in Combination With Different Treatment Regimens in Participants With Advanced Solid Tumors (P04722, MK-7454-004) | neoplasm | Paclitaxel (NPC208553) | |
| NCT05390710 | PhI to Solid Tumors and PhII to Locally Advanced or mTNBC | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT02240212 | A Phase I Trial of the Combination of AZD2014 and Weekly Paclitaxel. | cancer | Paclitaxel (NPC208553) | |
| NCT04460352 | Chemoradiotherapy Followed by Planned Surgery or by Surveillance and Surgery Only When Needed for Oesophageal Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04581343 | A Phase 1B Study of Canakinumab, Spartalizumab, Nab-paclitaxel, and Gemcitabine in Metastatic PC Patients | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04159155 | A Study of Various Treatments in Serous or p53 Abnormal Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01938833 | Romidepsin and Abraxane in Treating Patients With Metastatic Inflammatory Breast Cancer | inflammatory breast carcinoma;male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04015661 | Induction Chemotherapy Followed by Concurrent Chemoradiotherapy in Patients With LA-NPC | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00753038 | Phase 2 Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin in Patients With Head and Neck Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02308553 | Efficacy and Safety of Nintedanib Combined With Paclitaxel Chemotherapy for Patients With BRAF wt Metastatic Melanoma | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT00003896 | S9912 Combination Chemo in Stage III Ovarian Cancer, | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02319889 | Feasibility Study of SBRT Plus Chemotherapy for Non-Small Cell Lung Carcinoma | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01268059 | A Study of Carboplatin and Paclitaxel With or Without MEDI-575 in Untreated, Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00317200 | A Study of Paclitaxel Plus Bevacizumab in Patients With Chemosensitive Relapsed Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02121990 | Dose Escalation Study of OMP-54F28 in Combination With Paclitaxel and Carboplatin in Patients With Recurrent Platinum-Sensitive Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02107937 | Phase II Study DCVAC/OvCa Added to First Line Carboplatin and Paclitaxel Newly Diagnosed Epithelial Ovarian Carcinoma | malignant epithelial tumor of ovary | Paclitaxel (NPC208553) | |
| NCT05227664 | A Study of AK117/AK112 in Metastatic Triple-Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT01647828 | A Phase 1b/2 Study of OMP-59R5 (Tarextumab) in Combination With Nab-Paclitaxel and Gemcitabine in Subjects With Previously Untreated Stage IV Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00844649 | Phase III Study of ABI-007(Albumin-bound Paclitaxel) Plus Gemcitabine Versus Gemcitabine in Metastatic Adenocarcinoma of the Pancreas | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05247619 | A Clinical Study to Explore the Efficacy and Safety of Tislelizumab in Combination With Bevacizumab and Chemotherapy in Patients With Persistent, Recurrent, or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00003943 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Metastatic Cancer. | lung cancer;carcinoma | Paclitaxel (NPC208553) | |
| NCT00003130 | Paclitaxel in Treating Women With Recurrent Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01412229 | Induction Chemotherapy for Locally Advanced Squamous Cell Carcinoma of the Head and Neck | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00075712 | Timing of Surgery and Chemotherapy in Treating Patients With Newly Diagnosed Advanced Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cavity Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03586869 | QUILT-3.080: NANT Pancreatic Cancer Vaccine | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01470417 | Gemcitabine With Abraxane and Other Investigational Therapies in Neoadjuvant Treatment of Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01566435 | Induction Chemotherapy With ACF Followed by Chemoradiation Therapy for Adv. Head & Neck Cancer | upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT02976909 | Efficacy and Safety of TPF Induction Chemotherapy for Borderline-resectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00515073 | Papillary Serous Carcinoma of the Endometrium | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00252798 | ZD1839 (Iressa™) and Concurrent Chemo-Radiation in Patients With Locally Advanced Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00486954 | Lapatinib in Combination With Weekly Paclitaxel in Patients With ErbB2 Amplified Advanced Gastric Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT04882462 | Sintilimab and Nimotuzumab Combined With Chemotherapy for the Treatment of R/M HNSCC. | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02392507 | A Study of Nab-Paclitaxel and Carboplatin Plus Necitumumab (LY3012211) in Participants With Stage IV Squamous NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00310011 | Gemcitabine, Paclitaxel, and Cisplatin in Treating Patients With Advanced Cancer of the Urothelium | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00629278 | Combination Chemotherapy and Trastuzumab in Treating Women With Stage I, Stage II, or Stage III HER2-Positive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00176241 | Phase I Trial of Biweekly Gemcitabine & Paclitaxel & Low-Dose Radiation for Metastatic or Recurrent Head & Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01183559 | A Trial of ZD6474, Paclitaxel, Carboplatin, 5-Fluorouracil, and Radiation Therapy Followed by Surgery | esophageal carcinoma;stomach neoplasm | Paclitaxel (NPC208553) | |
| NCT00158379 | Taxol Carboplatin and Erythropoetin | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003313 | Amifostine in Treating Patients With Stage II or Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003992 | Chemotherapy Plus Monoclonal Antibody Therapy in Treating Women With Stage II or Stage IIIA Breast Cancer That Overexpresses HER2 | breast cancer | Paclitaxel (NPC208553) | |
| NCT00002819 | Chemotherapy With or Without Peripheral Stem Cell Transplantation in Treating Patients With Persistent Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03329248 | QUILT-3.060: NANT Pancreatic Cancer Vaccine: Molecularly Informed Integrated Immunotherapy in Subjects With Pancreatic Cancer Who Have Progressed on or After Standard-of-care Therapy | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01471132 | Sequential HIPEC of Oxaliplatin and Paclitaxel for Gastric Cancer Patients With Peritoneum Metastasis | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00001426 | A Multi-Institutional Phase II Study of Cyclophosphamide, Paclitaxel, Cisplatin With G-CSF for Patients With Newly Diagnosed Advanced Stage Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00005065 | Chemotherapy, Radiation Therapy, and Surgery in Treating Patients With Stage IIIA Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01802749 | Phase I/Ib Study of Paclitaxel in Combination With VS-6063 in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00368992 | S0536: Cetuximab, Paclitaxel, Carboplatin, and Bevacizumab in Treating Patients With Advanced Non-Small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02630823 | MK-3475 Immunotherapy in Endometrial Carcinoma | endometrial carcinoma | Paclitaxel (NPC208553) | |
| NCT02930902 | Pembrolizumab and Paricalcitol With or Without Chemotherapy in Patients With Pancreatic Cancer That Can Be Removed by Surgery | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00004054 | Hormone Therapy Plus Radiation Therapy With or Without Combination Chemotherapy in Treating Patients With Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT01205217 | Phase I/II Study of Lapatinib in Combination With Paclitaxel as 1L Chemotherapy for ErbB2-positive MBC | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01271725 | Evaluation of an Anti-cancer Immunotherapy Combined With Standard Neoadjuvant Treatment in Patients With WT1-positive Primary Invasive Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00603941 | A Phase 1/2 Study of CS7017, an Oral PPARγ Agonist, in Combination With Paclitaxel | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT02152137 | Inolitazone Dihydrochloride and Paclitaxel in Treating Patients With Advanced Anaplastic Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT03585062 | Trial of Neoadjuvant Chemotherapy With S1 Plus Paclitaxel-albumin on Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01612351 | Multimodality Risk Adapted Tx Including Induction Chemo for SCCHN Amenable to Transoral Surgery | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04390763 | Study of Efficacy and Safety of NIS793 (With and Without Spartalizumab) in Combination With SOC Chemotherapy in First-line Metastatic Pancreatic Ductal Adenocarcinoma (mPDAC) | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04879368 | RegoNivo vs Standard of Care Chemotherapy in AGOC | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01351350 | Dose Escalation Study of MLN0128 in Combination With Paclitaxel, With/Without Trastuzumab, in Subjects With Advanced Solid Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT02615730 | PI3Kβ Selective Inhibitor With Paclitaxel, Advanced Gastric Adenocarcinoma | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02608229 | BVD-523 Plus Nab-paclitaxel and Gemcitabine in Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00993655 | AURELIA: A Study of Avastin (Bevacizumab) Added to Chemotherapy in Patients With Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02622074 | Phase II Study With Trastuzumab + Paclitaxel in Locally Advanced HER2+ Tumors or Epirubicin + Taxotere in HER2- Tumors | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00006110 | Multimodality Treatment for Women With Stage II, Stage III, or Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02717091 | Neoadjuvant FOLFIRINOX or Nab-paclitaxel With Gemcitabine for Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01572038 | LUX-Breast 2 | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00716534 | Study of Carboplatin/Paclitaxel in Combination With ABT-869 in Subjects With Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00589238 | Paclitaxel, Doxorubicin, and Cyclophosphamide With Or Without Carboplatin in Treating Women With Locally Advanced Breast Cancer That Can Be Removed by Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT01701349 | Fosbretabulin or Placebo in Combination With Carboplatin/Paclitaxel in Anaplastic Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT04858009 | Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Pancreatic Cancer and Peritoneal Metastasis | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00198432 | Chemoradiotherapy of NSCLC Stage IIIB | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00499525 | Phase 2b Study of Taxol Plus Sorafenib or Placebo in Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01506973 | A Phase I/II/Pharmacodynamic Study of Hydroxychloroquine in Combination With Gemcitabine/Abraxane to Inhibit Autophagy in Pancreatic Cancer | adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00262847 | Carboplatin and Paclitaxel With or Without Bevacizumab in Treating Patients With Stage III or Stage IV Ovarian Epithelial, Primary Peritoneal, or Fallopian Tube Cancer | endometrioid carcinoma | Paclitaxel (NPC208553) | |
| NCT00043108 | Combination Chemotherapy, Surgery, and Radiation Therapy in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00437749 | A Study of CBT-1 and Paclitaxel With Carboplatin in Patients With Advanced Inoperable Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02451956 | Study of AZD5363 in Combination With Paclitaxel, in Advanced Gastric Adenocarcinoma Patients Harboring PIK3CA Mutation and/or PIK3CA Amplification as a Second-line Chemotherapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00607438 | A Phase II Study Of Abraxane and Nexavar in the First-Line Treatment of Locally Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01688700 | Study of Nimotuzumab in Combination With Neoadjuvant Chemotherapy for Resectable Esophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01820858 | The Efficacy and Safety of the Postoperative Adjuvant Treatment in Patients With High-risk Stage I Endometrial Carcinoma | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT00070278 | Neoadjuvant Epirubicin, Cyclophosphamide, and Paclitaxel With or Without Gemcitabine in Treating Women Who Are Undergoing Surgery for Early Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03400306 | A Study Evaluating the Bioavailability and Food Effect of Veliparib Tablets Followed by an Extension in Subjects With Ovarian Cancer | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT05145569 | A Clinical Study of TQB2450 Injection Combined With Anlotinib Hydrochloride Capsules Versus Paclitaxel as Weekly Treatment of Relapsed Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01757288 | Phase I/II NabPaclitaxel, Paclitaxel&Carboplatin w RTX Followed by Consolidation in Patients w Favorable Prognosis Inoperable Stage IIIA/B NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01332656 | Study of AMG 386 in Combination With Paclitaxel and Carboplatin in Subjects With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00350025 | Study of Cetuximab With and Without Weekly Paclitaxel for Patients With Previously Treated Advanced Urothelial Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003691 | Combination Chemotherapy With or Without G-CSF in Treating Patients With Stage III, Stage IV, or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT04211012 | A Study on Toripalimab Plus Nab-Paclitaxel With or Without Cisplatin as First-line Treatment of Urothelial Carcinoma | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT01488552 | Gemcitabine+Nab-paclitaxel and FOLFIRINOX and Molecular Profiling for Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04527900 | The UPPROACH (Upfront Intensity Modulated Proton Beam Therapy) Approach | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02453282 | Phase III Open Label First Line Therapy Study of MEDI 4736 (Durvalumab) With or Without Tremelimumab Versus SOC in Non Small-Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003696 | Combination Chemotherapy in Treating Patients Who Have Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05410847 | Nab-paclitaxel Plus Camrelizumab and S-1 Conversion Therapy for Gastric Cancer With Positive Exfoliative Cancer Cells | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01187199 | Phase I Trial of Bevacizumab and Temsirolimus in Combination With 1) Carboplatin, 2) Paclitaxel, 3) Sorafenib for the Treatment of Advanced Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT01711697 | An Alternative Radiation Fractionation Strategy for Locally Advanced Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00426556 | Efficacy and Safety of Everolimus in Combination Therapy, in Patients With HER2-overexpressing Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03367871 | Combination Pembrolizumab, Chemotherapy and Bevacizumab in Patients With Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00495170 | Concurrent Proton and Chemotherapy in Locally Advanced Stage IIIA/B Non-Small Cell Lung Cancer (NSCLC) | lung cancer | Paclitaxel (NPC208553) | |
| NCT04937673 | The Neoadjuvant Treatment of Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02975141 | Afatinib and Gemcitabine/Nab-paclitaxel in Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00936702 | Carboplatin, Paclitaxel, and Everolimus in Treating Patients With Previously Untreated Cancer of Unknown Primary | carcinoma | Paclitaxel (NPC208553) | |
| NCT00295893 | Combination Chemotherapy With or Without Trastuzumab in Treating Patients With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00773695 | A Study of Bevacizumab (Avastin) in Combination With Neoadjuvant Treatment Regimens in Participants With Primary Human Epidermal Growth Factor Receptor 2 (HER2) Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04821284 | Sonoporation and Chemotherapy for the Treatment of Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04472429 | Carboplatin-paclitaxel With Retifanlimab or Placebo in Participants With Locally Advanced or Metastatic Squamous Cell Anal Carcinoma (POD1UM-303/InterAACT 2). | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02779855 | Talimogene Laherparepvec in Combination With Neoadjuvant Chemotherapy in Triple Negative Breast Cancer | breast ductal carcinoma in situ | Paclitaxel (NPC208553) | |
| NCT00768131 | A Study to Determine Whether EGFR Status by FISH Can Predict Results in Non Small Cell Lung Cancer (NSCLC) Patients Treated With Cetuximab, Carboplatin and Paclitaxel | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00005048 | Estramustine and Paclitaxel in Treating Patients With Prostate Cancer That Has Not Responded to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT01641783 | Efficacy and Safety Study of ABI-007 Plus Capecitabine as First-line Chemotherapy for Advanced Gastric Cancer Patients | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05272696 | Pembrolizumab and Induction Chemotherapy in Locally Advanced HNSCC | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03515798 | Study of Immunotherapy in Combination With Chemotherapy in HER2-negative Inflammatory Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00574587 | Trial for Locally Advanced Breast Cancer Using Vorinostat Plus Chemotherapy | breast cancer | Paclitaxel (NPC208553) | |
| NCT04707118 | Ntraperitoneal Thermal Perfusion Combined With Chemotherapy Versus Chemotherapy | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01386385 | Veliparib With or Without Radiation Therapy, Carboplatin, and Paclitaxel in Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Removed by Surgery | squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00278148 | Erlotinib, Paclitaxel, and Carboplatin Combined With Radiation Therapy for Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04282070 | SHR-1701 in Patients With Recurrent/Metastatic Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00014599 | Paclitaxel in Treating Patients With Locally Advanced, Metastatic, or Recurrent Cancer of the Vulva | vulva cancer | Paclitaxel (NPC208553) | |
| NCT02732938 | Ph1b/2 Study of PF-04136309 in Combination With Gem/Nab-P in First-line Metastatic Pancreatic Patients | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02364999 | A Comparative Study Of PF-06439535 Plus Paclitaxel-Carboplatin And Bevacizumab Plus Paclitaxel-Carboplatin Patients With Advanced Non-Squamous NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02328105 | LCI-LUN-ABR-001: Carbo With Nab-Paclitaxel in Patients With Advanced NSCL Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04188860 | Immunotherapy for Recurrent Cervical Cancer Refractory to Platinum-based Chemotherapy | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT03742102 | 9-ING-41 in Patients With Advanced Cancers | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01621243 | M402 in Combination With Nab-Paclitaxel and Gemcitabine in Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02399137 | A Phase 2 Study of MM-141 Plus Nab-paclitaxel and Gemcitabine in Front-line Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003127 | S9720 Combination Chemotherapy in Treating Patients With Metastatic, Recurrent, or Refractory Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00668616 | Adjuvant Treatment of Breast Cancer With 1-3 Aflicted Lymph Nodes | breast cancer | Paclitaxel (NPC208553) | |
| NCT01632306 | A Study of LY2090314 and Chemotherapy in Participants With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01769391 | A Study of Necitumumab and Chemotherapy in Participants With Stage IV Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05214976 | A Trial of Camrelizumab for Injection in Combination With Famitinib Malate Capsule and Paclitaxel For Injection(Albumin Bound) in Advanced Solid Tumors of Patients | neoplasm | Paclitaxel (NPC208553) | |
| NCT00038545 | A Phase II Study of Paclitaxel and Topotecan With Filgrastim-SD/01 Support For Relapsed and Refractory Aggressive Non-Hodgkin's Lymphoma | non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT00016341 | Combination Chemotherapy Compared With Hormone Therapy in Treating Patients With Recurrent, Stage III, or Stage IV Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00003350 | Paclitaxel Compared With Doxorubicin in Treating Patients With Advanced AIDS-Related Kaposi's Sarcoma | Kaposi's sarcoma | Paclitaxel (NPC208553) | |
| NCT01763671 | Paclitaxel-bevacizumab in Advanced Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01688791 | Weekly Paclitaxel, 5FU+Irinotecan or Carboplatin+Paclitaxel in Subjects With Advanced / Metastatic Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT00003193 | Paclitaxel and Radiation Therapy Plus Chemoprotection With Amifostine in Treating Patients With Stage III or Stage IV Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02311907 | Glutathione in Preventing Peripheral Neuropathy Caused by Paclitaxel and Carboplatin in Patients With Ovarian Cancer, Fallopian Tube Cancer, and/or Primary Peritoneal Cancer | neuropathy;pain | Paclitaxel (NPC208553) | |
| NCT00112489 | Paclitaxel and Carboplatin in Treating Patients With Persistent or Recurrent Stage III or Stage IV Uterine Cancer | sarcoma | Paclitaxel (NPC208553) | |
| NCT01653912 | Phase Ib Study of Olaparib Plus Weekly Carboplatin and Paclitaxel in Relapsed Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00025688 | Paclitaxel With or Without Carboplatin in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05067530 | Study Assessing the Efficacy and Safety of Alpelisib + Nab-paclitaxel in Subjects With Advanced TNBC Who Carry Either a PIK3CA Mutation or Have PTEN Loss | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01366131 | Study Evaluating the Safety and Efficacy of MEGF0444A in Combination With Carboplatin, Paclitaxel and Bevacizumab in Patients With Advanced or Recurrent Non-Squamous Non-Small Cell Lung Cancer Who Have Not Received Prior Chemotherapy for Advanced Disease (NILE) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00113438 | Safety and Effectiveness of Combretastatin A-4 Phosphate Combined With Chemotherapy in Advanced Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT05319639 | Phase I Study of the Combination of Irinotecan and POF (POFI) and Tislelizumab | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01225523 | Perioperative Vs. Preoperative Chemotherapy With Surgery in the Squamous Carcinoma of Esophagus | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03154294 | Evaluation of the Safety and Tolerability of TAK-228 With TAK-117 and Paclitaxel in Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00280150 | Combination Chemotherapy, Bev, RT, and Erlotinib in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03829722 | Radiotherapy, Carboplatin/Paclitaxel and Nivolumab for High Risk HPV-related Head and Neck Cancer | oropharynx squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02903004 | Trial on Trabectedin (ET-743) vs Clinician's Choice Chemotherapy in Recurrent Ovarian, Primary Peritoneal or Fallopian Tube Cancers of BRCA Mutated or BRCAness Phenotype Patients | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT01100931 | A Phase I/II Study of Paclitaxel, Carboplatin and YM155 (Survivin Suppressor) in Subjects With Solid Tumors (Phase I) and Advanced Non-Small Cell Lung Carcinoma (Phase II) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004057 | Paclitaxel Plus L-778,123 in Treating Patients With Recurrent or Refractory Solid Tumors or Lymphomas | lymphoma | Paclitaxel (NPC208553) | |
| NCT03517176 | CEND-1 in Combination With Nabpaclitaxel and Gemcitabine in Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05455918 | Paclitaxel/Ifosfamide/Cisplatin Chemotherapy for High Risk Pediatric Germ Cell Tumor | childhood germ cell tumor | Paclitaxel (NPC208553) | |
| NCT00533585 | BAY 43-9006 in Previously Untreated Patients With Non-Small Cell Lung Cancer (NSCLC) | lung cancer | Paclitaxel (NPC208553) | |
| NCT02639650 | Study of Paclitaxel Plus Cisplatin as the First-line Chemotherapy in High Risk Gestational Trophoblastic Tumor | gestational trophoblastic neoplasm | Paclitaxel (NPC208553) | |
| NCT03056833 | A Study of Atezolizumab Versus Placebo in Combination With Paclitaxel, Carboplatin, and Bevacizumab in Participants With Newly-Diagnosed Stage III or Stage IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00002679 | Adjuvant High-Dose, Sequential Chemotherapy in Treating Patients With Resected Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00691912 | Therapy of Metastatic Breast Cancer With Paclitaxel and Liposomal Doxorubicin | breast cancer | Paclitaxel (NPC208553) | |
| NCT00584857 | A Phase II Study of Therapy With Paclitaxel, Carboplatin and Megesterol Acetate for the Management of Uterine Cancer | uterine cancer | Paclitaxel (NPC208553) | |
| NCT00011986 | Combination Chemotherapy in Treating Patients With Stage III or Stage IV Ovarian Epithelial Cancer or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00629499 | Nanoparticle Albumin-Bound (Nab) Paclitaxel/Cyclophosphamide in Early-Stage Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02625623 | Avelumab in Third-Line Gastric Cancer (JAVELIN Gastric 300) | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00089297 | Cetuximab, Chemotherapy, and Radiation Therapy for Operable Stage III or IV Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02570711 | Phase 2, Nab Paclitaxel/Gemcitabine Alone and in Combination With ACP-196 in Subjects With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00748163 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Sunitinib as First-Line Therapy in Treating Patients With Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00397761 | Capecitabine and Paclitaxel (Albumin-Stabilized Nanoparticle Formulation) in Treating Women Undergoing Surgery for Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03081143 | Ramucirumab Plus FOLFIRI Versus Ramucirumab Plus Paclitaxel in Patients With Advanced or Metastatic Gastric Cancer, Who Failed One Prior Line of Palliative Chemotherapy | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT01818063 | Carboplatin and Combination Chemotherapy With or Without Veliparib in Treating Patients With Stage IIB-IIIC Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00003732 | Combination Chemotherapy in Treating Patients With Stage II, Stage III, or Stage IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00002922 | Paclitaxel in Treating Patients With Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00583830 | A Study of Mapatumumab in Combination With Paclitaxel and Carboplatin in Subjects With Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01171170 | Paclitaxel-Carboplatin-Bevacizumab +/- Nitroglycerin in Metastatic Non-Squamous-Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00022230 | Combination Chemotherapy Plus Biological Therapy in Treating Patients With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00005032 | Bcl-2 Antisense Oligodeoxynucleotide G3139 and Paclitaxel in Treating Patients With Recurrent Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02072317 | Paclitaxel Plus Raltitrexed Plug Compare With Taxol Second-line Treatment for Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03539328 | Study on Decitabine Plus Carboplatin Versus Physician's Choice Chemotherapy in Recurrent, Platinum-resistant Ovarian Cancer. | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04046575 | Radiation Dose Intensification With Accelerated Hypofractionated Intensity Modulated Radiation Therapy and Concurrent Carboplatin and Paclitaxel for Inoperable Esophageal Cancer | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT00655876 | Paclitaxel, Cisplatin, and Radiation Therapy With or Without Cetuximab in Treating Patients With Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04223024 | Concurrent Chemoradiotherapy With Nimotuzumab for High Risk Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT05449366 | Intraperitoneal Paclitaxel for Patients With Primary Malignant Peritoneal Mesothelioma | malignant peritoneal mesothelioma | Paclitaxel (NPC208553) | |
| NCT01570582 | TRINOVA-3: A Study of AMG 386 or AMG 386 Placebo in Combination With Paclitaxel and Carboplatin to Treat Ovarian Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03263741 | Exploratory Study on the Paclitaxel + S-1 + Oxaliplatin (PSOX) for Locally Advanced or Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01374620 | Weekly Paclitaxel and Cyclophosphamide in Metronomic Administration : Dose Escalation Study of Weekly Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT03748134 | Sintilimab or Placebo With Chemotherapy in Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02822157 | Circulating Tumor DNA Guiding (Olaparib) Lynparza® Treatment in Ovarian Cancer | malignant epithelial tumor of ovary | Paclitaxel (NPC208553) | |
| NCT03975270 | Sintilimab in Combination With Nab-paclitaxel in Patients With HNSCC (Head and Neck Squamous Cell Carcinoma ) | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00002939 | Irinotecan and Paclitaxel in Treating Patients With Metastatic or Recurrent Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT04729608 | Alpelisib Plus Olaparib in Platinum-resistant/Refractory, High-grade Serous Ovarian Cancer, With no Germline BRCA Mutation Detected | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00110019 | Carboplatin and Paclitaxel With or Without Sorafenib Tosylate in Treating Patients With Stage III or Stage IV Melanoma That Cannot Be Removed by Surgery | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT03517449 | Lenvatinib in Combination With Pembrolizumab Versus Treatment of Physician's Choice in Participants With Advanced Endometrial Cancer (MK-3475-775/E7080-G000-309 Per Merck Standard Convention [KEYNOTE-775]) | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT01763645 | A Safety and Efficacy Study of BCD-021 With Paclitaxel and Carboplatin Compared to Avastin With Paclitaxel and Carboplatin in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00088114 | STA-4783 and Paclitaxel for Treatment of Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04594005 | CDK4/6 Tumor, Abemaciclib, Paclitaxel | neoplasm | Paclitaxel (NPC208553) | |
| NCT00989651 | Carboplatin, Paclitaxel, Bevacizumab, and Veliparib in Treating Patients With Newly Diagnosed Stage II-IV Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cancer | endometrioid carcinoma;undifferentiated carcinoma | Paclitaxel (NPC208553) | |
| NCT00050960 | Evaluation of Efficacy, Safety and Tolerability of Targretin Capsules in Patients With Advanced or Metastatic Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00816634 | Efficacy Comparison Study of Combination Regimens to Treat Advanced Esophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003284 | High-Dose Combination Chemotherapy Followed by Peripheral Stem Cell Transplantation in Treating Patients With Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00954642 | A Study of MNRP1685A in Combination With Bevacizumab With or Without Paclitaxel in Patients With Locally Advanced or Metastatic Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT03002103 | A Trial Evaluating the Efficacy and Safety of EndoTAG®-1 in Combination With Paclitaxel and Gemcitabine Compared With Paclitaxel and Gemcitabine as First-line Therapy in Patients With Visceral Metastatic Triple-negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00002662 | Paclitaxel or Docetaxel in Treating Women With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02133612 | Adjuvant Chemotherapy With Paclitaxel and Cisplatin in Lymph Node-Positive Thoracic Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02250326 | Safety and Efficacy Study of Nab®-Paclitaxel With CC-486 or Nab®-Paclitaxel With Durvalumab, and Nab®-Paclitaxel Monotherapy as Second/Third-line Treatment for Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00616967 | Carboplatin and Nab-Paclitaxel With or Without Vorinostat in Treating Women With Newly Diagnosed Operable Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00005635 | Trastuzumab Plus Paclitaxel in Treating Women With Metastatic Breast Cancer That Overexpresses HER2 | breast cancer | Paclitaxel (NPC208553) | |
| NCT00009776 | Monoclonal Antibody Therapy, Paclitaxel, and Cyclosporine in Treating Patients With Recurrent or Refractory Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT02718417 | Phase I Low Dose WART in Combination With Weekly Paclitaxel for Platinum Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04672005 | Modified FOLFIRINOX Alternated With Biweekly Gemcitabine Plus Nab-Paclitaxel Untreated Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00772798 | Paclitaxel and Carboplatin Combination as 1st Line Treatment in Ovarian Carcinomas | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00320541 | A Trial of Paclitaxel and Bevacizumab vs. Gemcitabine, Paclitaxel, and Bevacizumab in Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03714555 | Disulfiram-Copper Gluconate in Met Pancreas Cancer w Rising CA19-9 on Abraxane-Gemzar, FOLFIRINOX or Gemcitabine | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03410030 | Trial of Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00337376 | A Study of the mTOR Inhibitor Rapamycin (Rapamune, Sirolimus) in Combination With Abraxane in Advanced Solid Cancers | cancer | Paclitaxel (NPC208553) | |
| NCT00322452 | First Line IRESSA™ Versus Carboplatin/Paclitaxel in Asia | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03328234 | Radiotherapy With or Without Concurrent Chemotherapy for Extensive Lymphatic Metastasis of Esophageal Cancer - 3JECROG P-03 | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00465907 | Study of Weekly Paclitaxel, Carboplatin and Irinotecan to Treat Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03292822 | Effects of (Licochalcone A) and Paclitaxel on Human Oral Squamous Cell Carcinoma Cell Line | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01515306 | A Study of Ramucirumab (IMC-1121B) and Paclitaxel in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00635050 | Therapy for Locally Advanced Breast Cancer Using Doxil, Paclitaxel, and Cyclophosphamide With Avastin | breast cancer | Paclitaxel (NPC208553) | |
| NCT05346146 | A Prospective, Single-arm, Exploratory Clinical Study of Sintilimab Injection in Combination With Paclitaxel (Albumin-bound) and Gemcitabine in Translational Therapy in Patients With Unresectable Locally Advanced Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01980810 | First Line Chemotherapy for Advanced Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00686114 | Concurrent Chemoradiotherapy Containing Paclitaxel&Cisplatin With/Without Tarceva in Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003230 | Paclitaxel in Treating Patients With Refractory or Recurrent Acute Leukemia or Chronic Myelogenous Leukemia | leukemia | Paclitaxel (NPC208553) | |
| NCT00042835 | Erlotinib and Radiation Therapy Plus Combination Chemotherapy in Treating Patients With Inoperable Stage III Non-Small Cell Lung Cancer | lung adenocarcinoma;squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03279237 | A Pilot Study of FOLFIRINOX in Combination With Neoadjuvant Radiation for Gastric and GE Junction Cancers | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00838656 | Intraperitoneal Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Cancer of the Peritoneal Cavity | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02723955 | Dose Escalation and Expansion Study of GSK3359609 in Participants With Selected Advanced Solid Tumors (INDUCE-1) | neoplasm | Paclitaxel (NPC208553) | |
| NCT03912415 | Efficacy and Safety of BCD-100 (Anti-PD-1) in Combination With Platinum-Based Chemotherapy With and Without Bevacizumab as First-Line Treatment of Subjects With Advanced Cervical Cancer (FERMATA) | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02252796 | Phase I Hypofractionated Stereotactic Boost (Radiotherapy) for Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02155088 | BYL719 in Combination With Gemcitabine and (Nab)-Paclitaxel in Locally Advanced and Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00483301 | A Phase II Trial OF Carboplatin, ABI-007 (Abraxane) And Sorafenib (BAY 43-9006) in Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00971867 | Rollover Study of Weekly Paclitaxel (BMS-181339) in Patients With Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00481078 | Vorinostat, Carboplatin, and Paclitaxel in Treating Patients With Advanced or Metastatic Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01136031 | Paclitaxel and Irinotecan in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00039039 | Combination Chemotherapy Followed by Radiation Therapy With or Without Paclitaxel in Treating Patients With Unresectable Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00584909 | A Phase II Study of Paclitaxel and Carboplatin in Patients With an Elevated-Risk Cancer of the Uterus | uterine cancer | Paclitaxel (NPC208553) | |
| NCT02181634 | Phase II Trial of Nab-Paclitaxel and Gemcitabine for First-Line Treatment of Patients With Cholangiocarcinoma | cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT00176254 | Paclitaxel, Carboplatin and Radiotherapy as Induction Therapy in Locally Advanced Head and Neck Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00915005 | Trial of Image-Guided Adaptive Conformal Photon vs Proton Therapy, With Concurrent Chemotherapy, for Locally Advanced Non-Small Cell Lung Carcinoma: Treatment Related Pneumonitis and Locoregional Recurrence | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00626405 | Bevacizumab and Temozolomide or Bevacizumab and Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients With Stage IV Malignant Melanoma That Cannot Be Removed by Surgery | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT00846027 | A Study of Avastin (Bevacizumab) in Combination With Taxane-based Chemotherapy as First Line Treatment in Patients With HER-2 Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00360360 | Paclitaxel/Carboplatin Plus Bevacizumab/Erlotinib in the First Line Treatment of Carcinoma of Unknown Primary Site | neoplasm | Paclitaxel (NPC208553) | |
| NCT00966914 | Phase 3 Study of Tavocept Versus Placebo in Patients With Newly Diagnosed or Relapsed Advanced Primary Adenocarcinoma of the Lung Treated With Docetaxel or Paclitaxel Plus Cisplatin | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02711137 | Open-Label Safety and Tolerability Study of INCB057643 in Subjects With Advanced Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT02744898 | A Phase 2 Feasibility Study of Abraxane and Carboplatin in Epithelial Neoplasms of the Uterus | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT04149015 | Neoadjuvant POF in the Treatment of Locally Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00004011 | S9900: Surgery With or Without Combination Chemotherapy in Treating Patients With Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00508625 | A Study of AMG 951 [rhApo2L/TRAIL] in Subjects With Previously Untreated Non-Small Cell Lung Cancer (NSCLC) Treated With Chemotherapy +/- Bevacizumab | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05213312 | Study of Neoadjuvant Nivolumab or Placebo Plus Chemotherapy Followed by Surgery and Adjuvant Treatment in Subjects With Resectable ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04000295 | An Open-label, Phase I/II Study of the Pan-immunotherapy in Patients With Relapsed/Refractory Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00146562 | Pegfilgrastim and Darbepoetin Alfa in Support of Adjuvant Chemotherapy for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00005849 | Bryostatin 1 Plus Paclitaxel in Treating Patients With Stage IIIB, Stage IV, or Recurrent Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00004077 | Paclitaxel, Ifosfamide, and Cisplatin in Treating Patients With Metastatic Testicular Cancer | testicular neoplasm | Paclitaxel (NPC208553) | |
| NCT02879513 | Trial of Adjuvant Chemotherapy in Breast Cancer Patients With Pathological Partial Response and Complete Response to Neoadjuvant Chemotherapy | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04807140 | Neoadjuvant Toripalimab or Toripalimab in Combination With Carboplatin and Nab-paclitaxel in Untreated HNSCC (HNSCC-002) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04821765 | Study of PD-1 Antibody Combined With Chemoradiotherapy in Oligometastatic Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01873326 | Paclitaxel, Ifosfamide and Cisplatin (TIP) Versus Bleomycin, Etoposide and Cisplatin (BEP) for Patients With Previously Untreated Intermediate- and Poor-risk Germ Cell Tumors | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT00520975 | Bevacizumab in Treating Patients With Metastatic Breast Cancer That Overexpresses HER-2/NEU | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00045630 | S0219, Combination Chemotherapy Followed By Observation or Surgery in Patients With Stage II or Stage III Cancer of the Urothelium | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT03278626 | Immune Checkpoint Therapy With Nivolumab Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00752115 | Combination Chemotherapy With Sildenafil Plus Carboplatin and Paclitaxel in Patients With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00005581 | Combination Chemotherapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04943653 | Intraperitoneal Paclitaxel With XELOX in Gastric Cancer With Peritoneal Metastasis | stomach neoplasm | Paclitaxel (NPC208553) | |
| NCT00815308 | Erbitux Combined With Chemo-radiotherapy in Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02672475 | Galunisertib and Paclitaxel in Treating Patients With Metastatic Androgen Receptor Negative (AR-) Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03013218 | A Study of Evorpacept (ALX148) in Patients With Advanced Solid Tumors and Lymphoma (ASPEN-01) | non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT00979082 | Glutathione in Preventing Peripheral Neuropathy Caused by Paclitaxel and Carboplatin in Patients With Ovarian Cancer, Fallopian Tube Cancer, and/or Primary Peritoneal Cancer | peripheral neuropathy | Paclitaxel (NPC208553) | |
| NCT01034189 | Cetuximab/Paclitaxel/Cisplatin Concurrent Chemoradiotherapy Followed by Esophagectomy for Loco-regional Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02327169 | A Study of RO6927005 Either As Monotherapy (Part A) or in Combination With Gemcitabine and Nab-Paclitaxel (Part B) to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Clinical Activity in Patients With Mesothelin-positive Metastatic and/or Locally Advanced Malignant Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT01847677 | Neoadjuvant Therapy in Advanced Ovarian Cancer With Avastin | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT03942068 | Effect of Tumor Treating Fields (TTFields, 200 kHz) Concomitant With Weekly Paclitaxel for the Treatment of Recurrent Ovarian Cancer (ENGOT-ov50 / GOG-3029 / INNOVATE-3) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00050167 | Evaluation of Differing Taxanes/Taxane Combinations on the Outcome of Patients With Operable Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003294 | Chemotherapy Given With Amifostine and Filgrastim in Treating Patients With Recurrent or Metastatic Cancer | leukemia;lymphoma | Paclitaxel (NPC208553) | |
| NCT02607982 | CCRT for Esophageal Cancer. | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00005612 | Combination Chemotherapy Followed by Peripheral Stem Cell Transplantation in Treating Patients With Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00373256 | Combination Of Lapatinib With Carboplatin, Paclitaxel, and With or Without Trastuzumab In Metastatic Breast Cancer. | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00002837 | High-Dose Combination Chemotherapy and Peripheral Stem Cell Transplantation in Treating Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02112552 | Paclitaxel and Intraperitoneal Carboplatin Followed by Radiation Therapy in Treating Patients With Stage IIIC-IV Uterine Cancer | uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT02092363 | GANNET53: Ganetespib in Metastatic, p53-mutant, Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00004916 | Ifosfamide, Teniposide, and Paclitaxel in Treating Patients With Relapsed Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00006377 | Doxorubicin, Paclitaxel, and Carboplatin in Treating Patients With Primary Stage III, Stage IV, or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01281150 | Veliparib in Combination With Carboplatin and Paclitaxel in Treating Patients With Locally Advanced or Metastatic Solid Tumors | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01666418 | Pilot Study Investigating the Metabolic Activity and Transcriptional Profiling in Vivo in Tumor Biopsies in Melanoma Patients During Treatment With Pazopanib Alone and in Combination With Paclitaxel | melanoma | Paclitaxel (NPC208553) | |
| NCT00003035 | Doxorubicin and Paclitaxel in Treating Women With Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02697838 | Clinical Trial of Apatinib Reverses Chemotherapy-Resistance of Patients With Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00083057 | Gefitinib, Paclitaxel, and Radiation Therapy in Treating Patients With Advanced or Recurrent Squamous Cell Carcinoma of the Head and Neck | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00954174 | Paclitaxel and Carboplatin or Ifosfamide in Treating Patients With Newly Diagnosed, Persistent or Recurrent Uterine, Ovarian, Fallopian Tube, or Peritoneal Cavity Cancer | Fallopian Tube Carcinoma;Uterine Carcinosarcoma | Paclitaxel (NPC208553) | |
| NCT00331422 | Carboplatin, Paclitaxel, and Surgery in Treating Patients With Advanced Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cavity Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02109341 | Phase I/II Study to Evaluate Nab-paclitaxel in Substitution of CPT11 or Oxaliplatin in FOLFIRINOX Schedule as First Line Treatment in Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00503906 | Abraxane, Avastin, and Gemcitabine as First-Line Therapy for Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01755845 | Trial of Postoperative Chemoradiotherapy With or Without Consolidation Chemotherapy for Cervical Cancer Patients | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01235897 | MK-2206, Paclitaxel and Trastuzumab in Treating Patients With HER2-overexpressing Solid Tumor Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00479856 | Lapatinib In Combination With Chemotherapy In Subjects With Relapsed Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04617067 | Paricalcitol Trial: Phase II, Open Label Clinical Trial of Paricalcitol in Combination With Gemcitabine/ Nab-Paclitaxel Therapy in Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01721746 | A Study to Compare BMS-936558 to the Physician's Choice of Either Dacarbazine or Carboplatin and Paclitaxel in Advanced Melanoma Patients That Have Progressed Following Anti-CTLA-4 Therapy (CheckMate 037) | metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT05025033 | Chemotherapy Combined With Apatinib and PD-1 Antibody | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04004234 | A Phase I/II Study of the Pan-immunotherapy in Patients With Local Advanced/Metastatic BTC | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT04195828 | Camrelizumab Combined With Apatinib Mesylate Tablets, Nab-paclitaxel and S-1 in the Treatment of Locally Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01083537 | Intraperitoneal vs Intravenous Chemotherapy Following Neoadjuvant Chemotherapy in Ovarian Cancer | fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02125513 | Neoadjuvant Chemotherapy in Epithelial Ovarian Cancer | Fallopian Tube Carcinoma;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT02573493 | Nab-Paclitaxel and Cisplatin or Nab-paclitaxel as Induction Therapy for Locally Advanced Squamous Cell Carcinoma of the Head and Neck (HNSCC) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04046887 | Study of Lonsurf in Combination With Gemcitabine and Nab-Paclitaxel in Patients With Advanced (PDAC) | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00055601 | Combination Chemotherapy and Radiation Therapy With/Without Surgery In Patients With Stage II/III Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01333085 | Everolimus, Carboplatin, and Paclitaxel in Locally Advanced Head and Neck Cancer That Cannot Be Removed by Surgery | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00929162 | A Study Evaluating Intermittent and Continuous OSI-906 and Weekly Paclitaxel in Patients With Recurrent Epithelial Ovarian Cancer (and Other Solid Tumors) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02414685 | First Line TIP in Poor Prognosis TGCTs. | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT00960960 | A Study of PI3-Kinase Inhibitor GDC-0941 in Combination With Paclitaxel, With and Without Bevacizumab or Trastuzumab, and With Letrozole, in Participants With Locally Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01527864 | Paclitaxel-Carboplatin Alone or With M2ES for Non-Small-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01650376 | Phase Ib Study of Olaparib Plus Weekly Carboplatin and Paclitaxel in Relapsed Ovarian Cancer | uterine cancer | Paclitaxel (NPC208553) | |
| NCT01285466 | A Trial of Oral BEZ235 and BKM120 in Combination With Paclitaxel With or Without Trastuzumab | neoplasm | Paclitaxel (NPC208553) | |
| NCT05328336 | Perioperative Tislelizumab Combined With Nab-Paclitaxel for Muscle-invasive Urothelial Bladder Cancer: A Multicenter Study | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00001569 | Phase I Trial of Continuous Hyperthermic Peritoneal Perfusion (CHPP) With Cisplatin Plus Early Postoperative Intraperitoneal Paclitaxel and 5-FU for Peritoneal Carcinomatosis | peritoneal neoplasm;carcinoma | Paclitaxel (NPC208553) | |
| NCT02684227 | Enzalutamide, Carboplatin, and Paclitaxel in Treating Patients With Stage III-IV or Recurrent Endometrioid Endometrial Cancer | uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT00054392 | Combination Chemotherapy in Treating Patients With Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02163291 | Study on Neoadjuvant Chemotherapy for Advanced Gastric Cancer | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT02593175 | Women's MoonShot: Neoadjuvant Treatment With PaCT for Patients With Locally Advanced TNBC | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03252808 | Phase I Study of TBI-1401(HF10) Plus Chemotherapy in Patients With Unresectable Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01083537 | Study to Compare the Efficacy and Safety of Olaparib When Given in Combination With Carboplatin and Paclitaxel, Compared With Carboplatin and Paclitaxel in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00322400 | Phase1b to Evaluate Safety of AMG706 in Combination With Paclitaxel or Docetaxel for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00004934 | Paclitaxel and Carboplatin With or Without Epirubicin in Treating Patients With Stage IIB, Stage III, or Stage IV Invasive Ovarian Epithelial, Fallopian Tube, or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00915603 | Trial of Paclitaxel/Bevacizumab +/- Everolimus for Patients With HER2-Negative Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003386 | Vaccine Therapy in Treating Patients With Stage III or Stage IV Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01594099 | Concurrent Chemoradiotherapy for Cervical Cancer in Elderly Women | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00005847 | Chemotherapy With or Without Biological Therapy in Treating Patients With Metastatic Prostate Cancer That Has Not Responded to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT04164238 | Neoadjuvant Anti-PD-1 Antibody (Toripalimab) or Combined With Chemotherapy in HNSCC Patients | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03197571 | QUILT-3.048: NANT Urothelial Cancer Vaccine: Combination Immunotherapy in Subjects With Urothelial Cancer Who Have Progressed on or After Chemotherapy and PD-1/PD-L1 Therapy | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT04876313 | An Open Label, Single-arm, Phase 2 Study of Neoadjuvant Nivolumab and Nab-paclitaxel Before Radical Cystectomy for Patients With Muscle-invasive Bladder Cancer (NURE-Combo) | urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT03524898 | NAPAGE: NAb-PAclitaxel and GEmcitabine in Advanced Soft Tissue Sarcoma | soft tissue sarcoma | Paclitaxel (NPC208553) | |
| NCT00107341 | Bortezomib, Paclitaxel, and Carboplatin in Treating Patients With Unresectable, Metastatic Cancer of the Esophagus or Gastroesophageal Junction | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04238988 | Carboplatin-Paclitaxel-Pembrolizumab in Neoadjuvant Treatment of Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00254592 | Neoadjuvant Treatment of Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04723030 | The Transformation of Locally Advanced Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00084448 | Paclitaxel and Celecoxib in Treating Patients With Recurrent or Persistent Platinum-Resistant Ovarian Epithelial or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00838656 | First Line Tx Stage III Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT03635567 | Efficacy and Safety Study of First-line Treatment With Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy in Women With Persistent, Recurrent, or Metastatic Cervical Cancer (MK-3475-826/KEYNOTE-826) | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03361319 | Combination Nab-paclitaxel (N-P) and Nintedanib or N-P and Placebo in Relapsed NSCLC Adenocarcinoma | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04241276 | Phase IIb Randomised Trial of ATRA in a Novel Drug Combination for Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03554044 | T-VEC With Chemotherapy or Endocrine Therapy in Treating Participants With HER2- Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02315196 | Pegylated Liposomal Doxorubicin Hydrochloride and Carboplatin Followed by Surgery and Paclitaxel in Treating Patients With Triple Negative Stage II-III Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03815461 | Nab-paclitaxel Combined With S-1 as Induction Therapy for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01649336 | Weekly Paclitaxel With or Without Pazopanib in Platinum Resistant or Refractory Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00006454 | Paclitaxel Plus Carboplatin With or Without Topotecan in Treating Patients With Stage IIB, Stage III, or Stage IV Ovarian Epithelial Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02276560 | Cisplatin and Nab-paclitaxel for (N2) Defined NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00828841 | Phase 2b Study of Cetuximab With Platinum-Based Chemo as First Line Treatment of Recurrent or Advanced NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01253681 | Study of AMG 386 in Combination With Paclitaxel and Carboplatin in Subjects With Ovarian Cancer | carcinoma | Paclitaxel (NPC208553) | |
| NCT00042809 | Erlotinib, Trastuzumab, and Paclitaxel in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02262897 | The Efficacy and Safety of Nab-paclitaxel in Pretreated Patients With Extensive Disease of Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04742036 | Capivasertib China PK Study | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003064 | Combination Chemotherapy and Peripheral Stem Cell Transplantation in Treating Patients With Recurrent or Persistent Epithelial Ovarian Cancer, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00479817 | Phase 2 AMG 386 in Comb. Paclitaxel for Subjects With Advanced Recurrent Epithelial Ovarian or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05192798 | Albumin-Bound Paclitaxel Combined With Antiangiogenic Agents in First-line Treatment of Relapsed or Metastatic TNBC | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00539331 | Phase I/II Study of AZD2171 in Combination With Paclitaxel/Carboplatin in Japanese Non-Small Cell Lung Cancer Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00567554 | Bevacizumab, Everolimus (RAD001), and Lapatinib as Neoadjuvant Chemotherapy Regimes for Primary Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01821859 | Bevacizumab Beyond Progression in Platinum Sensitive Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00023907 | Paclitaxel in Treating Patients With Ovarian Epithelial Cancer or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00470301 | Tipifarnib and Combination Chemotherapy in Treating Patients With Stage II or Stage III Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00005646 | Paclitaxel in Treating Patients With Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00004859 | Carboplatin, Paclitaxel, and Radiation Therapy With or Without Thalidomide in Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00084565 | Paclitaxel, Topotecan, and Estramustine in Treating Patients With Metastatic Hormone-Refractory Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00334802 | Combination Chemotherapy of Gemcitabine and Paclitaxel for Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04973904 | Toripalimab Combined With Chemotherapy and Bevacizumab as First-Line Treatment in Patients With Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00052468 | Carboplatin/Paclitaxel +/-Gemcitabine in Treating Patients With Ovarian Epithelial or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00068757 | Lonafarnib, Trastuzumab, and Paclitaxel in Treating Patients With HER2/Neu-Overexpressing Stage IIIB, Stage IIIC, or Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02193633 | Phase I Dose Escalation of Oral BAY1161909 in Combination With Intravenous Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT02124317 | Nab-paclitaxel Plus S-1 in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04958785 | Study of Magrolimab Combination Therapy in Patients With Non-Surgically Removable Locally Advanced or Metastatic Triple-Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00291850 | Phase II Trial of Dose-dense Paclitaxel and Cisplatin as Neo-adjuvant Chemotherapy for Operable Stage II and IIA NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02835586 | STREAMER : STent Restenosis And MEdicaments Release | ischemia | Paclitaxel (NPC208553) | |
| NCT00856492 | S0800, Nab-Paclitaxel, Doxorubicin, Cyclophosphamide, and Pegfilgrastim With or Without Bevacizumab in Treating Women With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04314895 | Trial of NanoPac Intratumoral Injection in Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00005649 | Paclitaxel and Capecitabine in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00055133 | A Study Using Intravenous Paxceed™ to Treat Patients With Rheumatoid Arthritis | rheumatoid arthritis | Paclitaxel (NPC208553) | |
| NCT05218889 | Surufatinib Plus Camrelizumab and AS in First Line Treatment of Advanced Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00016900 | PNU-93914 in Treating Patients With Locally Advanced or Metastatic Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00004921 | High-Dose Chemotherapy Compared With Standard Chemotherapy in Treating Patients With Stage III or Stage IV Ovarian Epithelial Cancer That Has Been Removed During Surgery | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00806286 | Study of Carboplatin/Paclitaxel With or Without Investigational Drug (CS-7017) in Subjects With Metastatic Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003092 | Paclitaxel in Treating Older Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00124943 | Use of Nanoparticle Paclitaxel (ABI-007) for the Prevention of In-Stent Restenosis | Coronary Restenosis | Paclitaxel (NPC208553) | |
| NCT04338399 | The BURAN Study of Buparlisib in Patients With Recurrent or Metastatic HNSCC | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00861705 | Paclitaxel With or Without Carboplatin and/or Bevacizumab Followed by Doxorubicin and Cyclophosphamide in Treating Patients With Breast Cancer That Can Be Removed by Surgery | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01649336 | TRINOVA-3: A Study of AMG 386 or AMG 386 Placebo in Combination With Paclitaxel and Carboplatin to Treat Ovarian Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02506803 | Phase I Study of Neoadjuvant Chemotherapy for Patients With Borderline Resectable Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00687687 | Evaluation of Paclitaxel (Taxol, NSC | Uterine Carcinosarcoma | Paclitaxel (NPC208553) | |
| NCT05255471 | Antitumor Activity of Neoadjuvant Chemotherapy With or Without BINTRAFUSP ALFA in Patients With Metastatic Advanced Stage Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01964534 | Efficacy of ABI-007 Plus Gemcitabine or sLV5FU2 as First-line Therapy in Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01583426 | Nanoparticle-based Paclitaxel vs Solvent-based Paclitaxel as Part of Neoadjuvant Chemotherapy for Early Breast Cancer (GeparSepto) | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03416153 | Individualized Adaptive De-escalated Radiotherapy for HPV-related Oropharynx Cancer | oropharynx cancer | Paclitaxel (NPC208553) | |
| NCT04806945 | A Phase III Study to Evaluate Efficacy and Safety of First-Line Treatment With HLX10 + Chemotherapy in Patients With Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00353717 | Study In Women And Men With Metastatic Breast Cancer That Have Overexpression Of ErbB2 | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01039844 | Study of Weekly LOC-paclitaxel Injection for Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT02948075 | Pembrolizumab, Paclitaxel, and Carboplatin in Patients With Advanced Stage Epithelial Ovarian Cancer (EOC). | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02891083 | Adjuvant Therapies or Surgery Alone for High Risk pN0 Esophageal Cancer | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT03601897 | A Phase 1b/2 Study of Rebastinib (DCC-2036) in Combination With Paclitaxel in Patients With Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00502203 | Paclitaxel and Carboplatin in Women With Malignant Mixed Mullerian Tumors (MMMT) of the Uterus | mixed neoplasm | Paclitaxel (NPC208553) | |
| NCT00874107 | Efficacy/Safety of Imprime PGG® Injection With Bevacizumab and Paclitaxel/Carboplatin in Patients With Untreated Advanced Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04510064 | PD-1 Antibody Combined With Modified FLOT Regimen in the Treatment of Unresectable Locally Advanced or Limited Metastatic Gastric Cancer | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00825201 | First Line Tx Stage III Ovarian Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT05344742 | A Study of Mitoxantrone Hydrochloride Liposome Injection Combination Therapy in Chinese Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01620190 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Previously Treated Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05342636 | A Study of Combination Therapies With Pembrolizumab (MK-3475) in Participants With Advanced Esophageal Cancer (MK-3475-06A) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00011921 | Chemotherapy Followed by Peripheral Stem Cell or Bone Marrow Transplant Compared With Chemotherapy Alone in Treating Patients With Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02033538 | Neoadjuvant Chemotherapy of Nanoparticle Albumin-bound Paclitaxel in Squamous Cell Carcinoma of Esophagus | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00014222 | Combination Chemotherapy With or Without Colony-stimulating Factors in Treating Women With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00002711 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02876302 | Study Of Ruxolitinib (INCB018424) With Preoperative Chemotherapy For Triple Negative Inflammatory Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00503750 | Phase II Neoadjuvant Trial of Trastuzumab in Combination With Dose-Dense ABI-007 (Abraxane™) | breast cancer | Paclitaxel (NPC208553) | |
| NCT00004137 | S9914: Combination Chemotherapy Plus Filgrastim in Untreated Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02272790 | Metformin Hydrochloride, Carboplatin, and Paclitaxel in Treating Patients With Recurrent Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00033410 | Chemotherapy, Tirapazamine, and Radiation Therapy in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00456846 | First Line Therapy for Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00881101 | Clinical Study of Liposomal Paclitaxel in Chinese Patients | neoplasm | Paclitaxel (NPC208553) | |
| NCT00723957 | A Randomized Phase 2 Study of Ixabepilone Plus Carboplatin and Paclitaxel Plus Carboplatin in Advanced Nonsmall-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00016276 | Combination Chemotherapy, Surgery, and Radiation Therapy With or Without Dexrazoxane and Trastuzumab in Treating Women With Stage III or Stage IV Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00476827 | A Phase II Safety and Tolerability Study of Bevacizumab When Added to Single-agent Chemotherapy to Treat Patient With Breast Cancer Metastatic to Brain | breast cancer | Paclitaxel (NPC208553) | |
| NCT00035152 | Study Comparing Weekly Taxol and Carboplatin vs Standard Taxol and Carboplatin Regimen for Stage IIIB or IV Non-Small-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00113516 | A Study Of SU011248 As Therapy In Patients With Locally Advanced Or Metastatic Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01357161 | Study of Ombrabulin in Patients With Platinum-Sensitive Recurrent Ovarian Cancer Treated With Carboplatin/Paclitaxel | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01641939 | A Study of Trastuzumab Emtansine Versus Taxane in Participants With Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04908787 | PD-1 Antibody Combined Neoadjuvant Chemotherapy for Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02210559 | A Study of Gemcitabine Plus Nab-paclitaxel With or Without FG-3019 in Participants With Locally Advanced, Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003158 | S9712: Radiation Therapy and Combination Chemotherapy in Treating Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00513695 | Sunitinib Malate, Paclitaxel, Doxorubicin Hydrochloride, and Cyclophosphamide Before Surgery in Treating Patients With Stage IIB-IIIC Breast Cancer | inflammatory breast carcinoma;male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04180384 | A Safety Study of Oraxol (HM30181 + Oral Paclitaxel) in Cancer Patients | neoplasm | Paclitaxel (NPC208553) | |
| NCT00046527 | Study of ABI-007 and Taxol in Patients With Metastatic Breast Cancer | metastasis;breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03751761 | GC 2nd Line Durvalumab(MEDI4736)/Tremelimumab Plus Paclitaxel Study | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03314740 | Best Approach in Recurrent-Ovarian-Cancer-with Cediranib-Olaparib | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT01246193 | CKD-828(80/2.5mg) Pharmacokinetic Study | hypertension | Paclitaxel (NPC208553) | |
| NCT02073968 | PET-Adjusted Intensity Modulated Radiation Therapy and Combination Chemotherapy in Treating Patients With Stage II-IV Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05189483 | Albumin-bound Paclitaxel Plus Camrelizumab for Advanced Soft Tissue Sarcoma. | soft tissue sarcoma | Paclitaxel (NPC208553) | |
| NCT00006118 | Cisplatin, Paclitaxel, and Gemcitabine in Treating Patients With Progressive Unresectable Regional or Metastatic Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00578149 | Bevacizumab and Carboplatin/Paclitaxel and Radiation in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02012192 | A Study of MEK162 and Paclitaxel in Patients With Epithelial Ovarian, Fallopian Tube or Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01693276 | Gemcitabine/Abraxane Chemotherapy and Dose Escalated Radiotherapy for Locally Advanced, Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00368875 | Phase I-II Study of Vorinostat, Paclitaxel, and Bevacizumab in Metastatic Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02651727 | Ph 1 Study of VS-4718, a FAK Inhibitor, in Combination With Nab-paclitaxel and Gemcitabine in Advanced Cancer Subjects | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00787852 | A Study of Dasatinib With Concurrent Chemoradiation for Stage III Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03579771 | Gemcitabine, Cisplatin, and Nab-Paclitaxel Before Surgery in Patients With High-Risk Liver Bile Duct Cancer | intrahepatic cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT00111007 | A Treatment Combination for Patients With Unresectable Stage III or Stage IV Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT05145218 | Biomarker-driven Targeted Therapy in Patients With Recurrent Platinum-resistant Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00398086 | Gemcitabine Plus Albumin-bound Paclitaxel In Patients With Advanced Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003413 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Stage III or Stage IV Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01167712 | Paclitaxel and Carboplatin With or Without Bevacizumab in Treating Patients With Stage II, Stage III, or Stage IV Ovarian Epithelial Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer | endometrioid carcinoma;Fallopian Tube Carcinoma | Paclitaxel (NPC208553) | |
| NCT00004097 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Advanced and/or Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04258657 | Neoadjuvant Chomotherapy With Paclitaxel-albumin and S-1 for Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00505492 | Malignant Mixed Mesodermal Tumor (MMMT) - Early Stage With Postoperative XRT/Chemotherapy | uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT01821859 | A Study of MEK162 and Paclitaxel in Patients With Epithelial Ovarian, Fallopian Tube or Peritoneal Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03487016 | First-line Therapy in Metastatic PDAC | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00005087 | Paclitaxel, Cisplatin, and Filgrastim Combined With Radiation Therapy in Treating Patients With Locally Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01862081 | A Dose-escalation Study to Assess the Safety, Tolerability, and Pharmacokinetics of GDC-0032 in Combination With Docetaxel or With Paclitaxel in Patients With HER2-negative Locally Recurrent or Metastatic Breast Cancer or Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00667251 | Chemotherapy and Lapatinib or Trastuzumab in Treating Women With HER2/Neu-Positive Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03167164 | QUILT-3.045: NANT Merkel Cell Carcinoma (MCC) Vaccine: Combination Immunotherapy in Subjects With MCC Who Have Progressed on or After PD-L1 Therapy | Merkel cell skin cancer | Paclitaxel (NPC208553) | |
| NCT00174356 | PH 1 Evaluation Of Oral CI-1033 In Combination With Paclitaxel/ Carboplatin As 1st Line Chemotherapy In NSCLC Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05422794 | Testing the Addition of Anti-Cancer Drug, ZEN003694 (ZEN-3694) and PD-1 Inhibitor (Pembrolizumab), to Standard Chemotherapy (Nab-Paclitaxel) Treatment in Patients With Advanced Triple-Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02106546 | Study Comparing Veliparib Plus Carboplatin and Paclitaxel Versus Placebo Plus Carboplatin and Paclitaxel in Previously Untreated Advanced or Metastatic Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01285609 | Phase 3 Trial in Squamous Non Small Cell Lung Cancer Subjects Comparing Ipilimumab Versus Placebo in Addition to Paclitaxel and Carboplatin | lung cancer | Paclitaxel (NPC208553) | |
| NCT01011075 | Study of Gleevec and Weekly Paclitaxel in Patients Aged 70 or Older With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04278092 | Paclitaxel Plus Cetuximab After First-line Checkpoint Inhibitor Failure | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00080418 | Liposome Entrapped Paclitaxel Easy to Use (LEP-ETU) in Patients With Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003120 | S9701 Paclitaxel in Treating Patients With Advanced Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Remission | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02391662 | Nab-paclitaxel and Gemcitabine, in Elderly Patients Untreated Metastatic Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02289456 | Phase II Safety and Tolerability Trial With Nab-Paclitaxel Plus Carboplatin Followed by Nab-Paclitaxel for First Line Treatment of NSCLC Subjects With ECOG PS 2 | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01457846 | Efficacy and Safety of AZD4547 Versus Paclitaxel in Patients With Advanced Gastric or Gastro-oesophageal Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00866528 | Study of Pazopanib and Paclitaxel in Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00023673 | Radiation Therapy Combined With Paclitaxel and Carboplatin in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03797443 | High Dose Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) in Patients Who Have Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03467178 | Pembrolizumab With Chemotherapy in Front Line Advanced Ovarian, Primary Peritoneal and Fallopian Tube Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00873119 | Belinostat, Carboplatin and Paclitaxel (BelCaP) Compared to Carboplatin and Paclitaxel in Patients With Cancer of Unknown Primary | carcinoma | Paclitaxel (NPC208553) | |
| NCT04579224 | Comparing the New Anti-cancer Drug Eribulin With or Without Chemotherapy Against the Usual Chemotherapy Alone in Metastatic Urothelial Cancer | urethra cancer | Paclitaxel (NPC208553) | |
| NCT01998347 | Paclitaxel Liposome and Cisplatin as First-line Chemotherapy in Patients With Metastatic Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01160601 | Study of Paclitaxel/Carboplatin With or Without Bavituximab in Previously Untreated Non Small-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01276496 | Weekly Doses of Cilengitide and Paclitaxel in Treating Patients With Advanced Solid Tumors That Cannot Be Removed by Surgery | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00161278 | Pilot Study for the Determination of Tumor Response to Chemotherapy in Advanced NSCLC Through Gene Expression Profiling | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03763123 | Study of Chemotherapy With Pembrolizumab (MK-3475) Followed by Maintenance With Olaparib (MK-7339) for the First-Line Treatment of Women With BRCA Non-mutated Advanced Epithelial Ovarian Cancer (EOC) (MK-7339-001/KEYLYNK-001/ENGOT-ov43/GOG-3036) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01455532 | A Dose Escalation Study of Iniparib as a Single Agent and in Combination in Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03957590 | Study of Tislelizumab (BGB-A317) Versus Placebo in Combination With Chemoradiotherapy in Participant With ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02039674 | A Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy or Immunotherapy in Participants With Non-small Cell Lung Cancer (MK-3475-021/KEYNOTE-021) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02446574 | Postoperative Chemoradiation in Patients With Node-positive Esophageal Squamous Cell Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT00005026 | Combination Chemotherapy in Treating Patients With Stage III or Stage IV Ovarian Epithelial or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00096668 | Evaluation of Safety and Efficacy of TOCOSOL(R) Paclitaxel as Initial Treatment for Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00550537 | Proteomic Profiling in Predicting Response in Patients Receiving Erlotinib for Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00553358 | Phase II Trial to Compare the Safety of Two Chemotherapy Plus Trastuzumab Regimens as Adjuvant Therapy for HER2-positive Breast Cancer (Study P05048) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00832494 | Phase II Study of DMXAA (ASA404) in Combination With Chemotherapy in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02858206 | Simultaneous Integrated Boost Radiotherapy and Concurrent Nimotuzumab or Chemotherapy for Esophageal Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT00003042 | Chemotherapy and Stem Cell Transplantation in Treating Patients With Stage IIIB Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00955305 | Paclitaxel, Carboplatin, and Bevacizumab With or Without Cixutumumab in Treating Patients With Stage IV or Recurrent Non-small Cell Lung Cancer | large cell lung carcinoma;lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00511849 | Study Of SU011248 In Combination With Paclitaxel/Carboplatin In Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00334763 | Radiation Therapy, Chemotherapy, and Bevacizumab in Treating Patients With Recurrent, Unresectable or Stage III or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03038100 | A Study of Atezolizumab Versus Placebo in Combination With Paclitaxel, Carboplatin, and Bevacizumab in Participants With Newly-Diagnosed Stage III or Stage IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00645177 | Phase 2 Study of ABT-869 in Combination With Paclitaxel Versus Paclitaxel Alone to Treat Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03783442 | A Study of Tislelizumab (BGB-A317) in Combination With Chemotherapy as First Line Treatment in Participants With Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01057342 | Paclitaxel, Carboplatin, and Dimethylxanthenone Acetic Acid in Treating Patients With Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02142738 | Study of Pembrolizumab (MK-3475) Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non-Small Cell Lung Cancer (MK-3475-024/KEYNOTE-024) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00069901 | Phase II CT-2103/Carboplatin in Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00675259 | Phase II NCT (Neoadjuvant Chemotherapy) w/ Weekly Abraxane in Combination With Carboplatin & Bevacizumab in Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00820170 | Dasatinib In Combination With Weekly Paclitaxel For Patients With Metastatic Breast Carcinoma CA 180 194 | breast cancer | Paclitaxel (NPC208553) | |
| NCT00046514 | ABI-007 in Taxol Resistant Patients With Metastatic Breast Cancer | metastasis;breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04006041 | Combination of Toripalimab and Neoadjuvant Chemoradiotherapy in Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00048893 | Vaccine and Chemotherapy for Previously Untreated Metastatic Breast Cancer | metastasis;breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00028873 | R101933 Combined With Chemotherapy in Treating Patients With Metastatic Breast Cancer That Has Not Responded to Previous Chemotherapy | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003401 | Peripheral Stem Cell Transplantation Plus Combination Chemotherapy in Treating Patients With Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT02050009 | GANNET53: Ganetespib in Metastatic, p53-mutant, Platinum-resistant Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT04835896 | Study of M7824 and Paclitaxel Combination as a Second-line Treatment in Patients With Recurrent/Metastatic Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00208936 | Phase II Study of Pre-Operative Chemotherapy in Patients With Resectable Local-Regional Carcinoma of Esophagus | gastrointestinal disease | Paclitaxel (NPC208553) | |
| NCT00009763 | Monoclonal Antibody Therapy, Cyclosporine, and Paclitaxel in Treating Patients With Recurrent or Refractory Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00057928 | S0227 Cisplatin With Either Paclitaxel or Gemcitabine in Recurrent, Persistent, or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02459457 | A Comparison of Paclitaxel-based Three Regimens Concurrent With Radiotherapy for Patients With Local Advanced Esophageal Cancer | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00006378 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00017303 | Combination Chemotherapy Plus IM-862 in Treating Patients With Resected Stage III Ovarian Cancer or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00022633 | S0028, Gemcitabine and Paclitaxel in Treating Patients With Advanced or Recurrent Cancer of the Urinary Tract | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT04072263 | Apatinib and Etoposide Capsule Versus Weekly Paclitaxel in Patients With Platinum Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00809133 | Trial Exploring Afatinib (BIBW 2992) + Paclitaxel (Part A), Afatinib + Paclitaxel + Bevacizumab (Part B), Afatinib + Carboplatin (Part C) and Afatinib+ Paclitaxel +Carboplatin(Part D) in Patients With Advanced Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003558 | Chemotherapy in Treating Patients With Cancer of Unknown Primary Origin | carcinoma | Paclitaxel (NPC208553) | |
| NCT01783197 | Study of Selumetinib in Patients With Previously Treated or Untreated Advanced/Metastatic NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00832819 | E7080 in Combination With Carboplatin and Paclitaxel in Patients With Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00708812 | Paclitaxel Plus Carboplatin With or Without Endostar in Patients With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02715531 | A Study of the Safety and Efficacy of Atezolizumab Administered in Combination With Bevacizumab and/or Other Treatments in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02366949 | A Study MLN2480 in Combination With MLN0128 or Alisertib, or Paclitaxel, or Cetuximab, or Irinotecan in Adult Participants With Advanced Nonhematologic Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT03668730 | Reduced-dose Radiotherapy for Low-risk Stage III Patients With Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT03806049 | Study of Relacorilant in Combination With Nab-Paclitaxel for Patients With Recurrent Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00063401 | Phase II Study in Patients With Epidermal Growth Factor Receptor (EGFR) + Advanced Stage Ovarian, Primary Peritoneal and Fallopian Tube Cancer | Fallopian Tube Carcinoma;ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00294762 | Erlotinib (Tarceva) as a Single Agent or Intercalated With Combination Chemotherapy in Patients With EGFR Positive NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01548924 | Determination of Dose of Antiangiogenic Multitargeted DOVITINIB (TKI258) Plus Paclitaxel in Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00976911 | Paclitaxel and Cisplatin as First-Line Treatment for Patients With Stage I, Stage II, Stage III, or Stage IV Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04001829 | Docetaxel or Paclitaxel in Reducing Chemotherapy-Induced Peripheral Neuropathy in African American Patients With Stage I-III Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00533936 | Trastuzumab or Observation After Combination Chemotherapy and Trastuzumab in Treating Patients Undergoing Surgery for Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02520154 | Veliparib in Combination With Carboplatin And Weekly Paclitaxel in Japanese Subjects With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01229930 | Carboplatin and Paclitaxel With or Without Cediranib Maleate in Treating Patients With Metastatic or Recurrent Cervical Cancer That Cannot Be Removed by Surgery | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03393884 | A Study of Apatinib Treatment in for Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00194792 | Hormone Therapy and Combination Chemotherapy Before and After Surgery in Treating Patients With Stage I-IIIA Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04143711 | Study of DF1001 in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04794699 | Study of IDE397 in Participants With Solid Tumors Harboring MTAP Deletion | neoplasm | Paclitaxel (NPC208553) | |
| NCT00001499 | Phase II Neoadjuvant Trial of a Continuous Infusion of Paclitaxel Plus Cisplatin Followed by Chest Radiotherapy for Patients With Stage III Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02221999 | Weekly Paclitaxel and Cisplatin to Treat Hormone Receptor Positive and Triple Negative Breast Cancer Patients | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT04864782 | QL1604 Plus Chemotherapy Versus Chemotherapy in Subjects With Stage ⅣB, Recurrent, or Metastatic Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00003004 | Combination Chemotherapy in Treating Patients With Refractory or Recurrent Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00072215 | Cisplatin and Ifosfamide Combined With Either Paclitaxel or Vinblastine in Treating Men With Progressive or Recurrent Metastatic Germ Cell Tumors | testicular neoplasm | Paclitaxel (NPC208553) | |
| NCT03087864 | PDL-1 Targeting in Resectable Oesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01238133 | Gamma-Secretase/Notch Signalling Pathway Inhibitor RO4929097, Paclitaxel, and Carboplatin Before Surgery in Treating Patients With Stage II or Stage III Triple-Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT05189730 | Selected Chemotherapy Combined Immunotherapy Treated High Risk Patient After NCRT in Resected Locally Advanced ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003944 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Stage III or Stage IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03246074 | QUILT-3.051: NANT Ovarian Cancer Vaccine: Combination Immunotherapy in Subjects With Epithelial Ovarian Cancer Who Have Progressed on or After Standard-of-care (SoC) Therapy | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00657878 | Efficacy Study of Chemotherapy to Treat Ovarian Cancer Recurrence by Prolonging the Platinum Free Interval | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01199055 | CS-7017 in Combination With Carboplatin/Paclitaxel in Subjects With Stage IIIb/IV Non-small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05083247 | Preoperative mFOLFIRINOX (or Gem-Nab-P) +/- Isotoxic High-dose SBRT for Borderline Resectable Pancreatic Adenocarcinoma | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT02017015 | Safety and Efficacy Study of Nab-paclitaxel Plus Gemcitabine in Chinese Patients With Metastatic Pancreatic Cancer | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00038402 | Evaluation of the Addition of Herceptin to Standard Chemotherapy in the Neoadjuvant Setting for Operable Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01843829 | A Feasibility Study of Chemo-radiotherapy to Treat Operable Oesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01676259 | A Phase 2 Study of siG12D LODER in Combination With Chemotherapy in Patients With Locally Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05125055 | Neoadjuvant Anti-PD-1 and TP Versus TPF on Pathological Response in OSCC | oral squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01969578 | Androgen Deprivation Therapy in Advanced Salivary Gland Cancer | salivary gland cancer | Paclitaxel (NPC208553) | |
| NCT00553462 | Carboplatin and Paclitaxel Albumin-Stabilized Nanoparticle Formulation Followed by Radiation Therapy and Erlotinib in Treating Patients With Stage III Non-Small Cell Lung Cancer That Cannot Be Removed By Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00585689 | Neoadjuvant ABI-007, Carboplatin and Gemcitabine in Locally Advanced Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00891605 | Safety Study of ABT-263 in Combination With Paclitaxel in Subjects With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00864253 | A Trial of ABI-007 Versus Dacarbazine in Previously Untreated Patients With Metastatic Malignant Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00002826 | Drug Resistance Inhibition in Treating Women With Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04047862 | Study of Ociperlimab (BGB-A1217) in Combination With Tislelizumab in Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00251472 | A Phase II Trial of Abraxane™ Given Weekly as a Single Agent in First-line Treatment of Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00980954 | Chemotherapy and Pelvic Radiation Therapy With or Without Additional Chemotherapy in Treating Patients With High-Risk Early-Stage Cervical Cancer After Radical Hysterectomy | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02461043 | Adjuvant Chemotherapy With Paclitaxel and Cisplatin in Lymph Node-Positive Adjuvant Chemotherapy in the Treatment of the Lymph Node Positive Thoracic Esophageal Squamous Cell Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01288261 | Paclitaxel and Bavituximab in Treating Patients With HER2-Negative Metastatic Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02733250 | Pembrolizumab With Nab-Paclitaxel in Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003376 | Two-Drug Combination Chemotherapy Compared With Four-Drug Combination Chemotherapy in Treating Patients With Advanced Cancer of the Urothelium | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT03430843 | A Study of Tislelizumab (BGB-A317) Versus Chemotherapy as Second Line Treatment in Participants With Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05013697 | TQB2450 Solution for Injection (TQB2450)+Paclitaxel+Cisplatin ± Anlotinib in the Treatment of Esophageal Cancer. | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT01953445 | A Study of Pertuzumab in Combination With Trastuzumab (Herceptin) and a Taxane in First-Line Treatment in Participants With Human Epidermal Growth Factor 2 (HER2)-Positive Advanced Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00003322 | Combination Chemotherapy in Treating Patients With Primary Peritoneal or Stage III Epithelial Ovarian Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04799639 | Efficacy and Toxicity of Paclitaxel, Cisplatin Combined With Sindilimab in NACT for Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03990103 | S1+ Paclitaxel (IV&IP) + Bevacizumab (IP) Versus S1+Oxaliplatin as First-line Treatment in Gastric Cancer With Malignant Ascites | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01310244 | MTD Study PXD-101 in Combination With Paclitaxel + Carboplatin in Chemotherapy-Naive Patients With Stage IV NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00136539 | Neoadjuvant Therapy With Herceptin and Taxol for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02890511 | A Study DHP107, a Novel Oral Paclitaxel Formulation, in Patients With Advanced Solid Tumours or Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT05316376 | MITO 35B: Olaparib Beyond Progression Compared to Platinum Chemotherapy After Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer Patients. | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03705429 | Study of Boserolimab (MK-5890) as Monotherapy and in Combination With Pembrolizumab (MK-3475) in Adults With Advanced Solid Tumors (MK-5890-001) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02012192 | A Study to Evaluate the Effect of the Combination of Pertuzumab With Carboplatin-Based Standard Chemotherapy in Patients With Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04469556 | Pancreatic Adenocarcinoma Signature Stratification for Treatment | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00404235 | Carboplatin and ABI-007 in Treating Patients With Stage IV Melanoma That Cannot Be Removed By Surgery | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT02560038 | Trial of Paclitaxel Plus Gemcitabine and Cisplatin in Bladder Cancer | bladder tumor | Paclitaxel (NPC208553) | |
| NCT00003050 | Radiation Therapy Plus Paclitaxel in Treating Patients With Stage IIB or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02272790 | Adavosertib Plus Chemotherapy in Platinum-Resistant Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | Fallopian Tube Carcinoma;ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00876395 | Everolimus in Combination With Trastuzumab and Paclitaxel in the Treatment of HER2 Positive Locally Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03871036 | Improve Checkpoint-blockade Response in Advanced Urothelial Cancer | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT03723967 | FRAIL-IMMUNE (GORTEC 2018-03) - Combination of Durvalumab With Carboplatin/Paclitaxel | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003054 | Paclitaxel in Treating Patients With Recurrent or Persistent Cancer of the Uterus | sarcoma | Paclitaxel (NPC208553) | |
| NCT04337632 | NUVOLA TRIAL Open-label Multicentre Study | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03286244 | Second-line CM082 Combined With Paclitaxel For Patients With Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00434356 | A Study of Sunitinib in Combination With Bevacizumab and Paclitaxel in Previously Untreated Patients With Metastatic Breast Cancer (SABRE-B) | breast cancer | Paclitaxel (NPC208553) | |
| NCT03086369 | A Study of Nab-Paclitaxel and Gemcitabine With or Without Olaratumab (LY3012207) in Participants With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00255762 | Carboplatin, Paclitaxel, and Bevacizumab in Treating Patients With Stage IV Melanoma That Cannot Be Removed By Surgery | melanoma | Paclitaxel (NPC208553) | |
| NCT00081042 | ABI-007 in Treating Patients With Inoperable Locally Recurrent or Metastatic Melanoma | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT03018080 | Pilot Study of Paclitaxel Plus Pembrolizumab in Metastatic HER2-Negative Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00004865 | Cetuximab Plus Cisplatin in Treating Patients With Metastatic or Recurrent Cancer of the Head and Neck That Has Not Responded to Cisplatin Chemotherapy | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01239732 | Study of Intraperitoneal Carboplatin With IV Paclitaxel and Bevacizumab in Untreated Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01336062 | Trial of ABI-007 Plus S-1 as Second-line Chemotherapy in Advanced Gastric Cancer Patients | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00919880 | Comparison of Neo-adjuvant Weekly Paclitaxel With or Without Carboplatin in Early Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04652076 | GYNecological Cancers Treated With NETrin mAbs in Combination With Chemotherapy and /or Pembrolizumab | cervical carcinoma;endometrial carcinoma | Paclitaxel (NPC208553) | |
| NCT05459129 | A Study Evaluating Efficacy and Safety of Multiple Treatment Combinations in Patients With Locally Advanced Squamous Cell Carcinoma of the Head and Neck (Morpheus-Head and Neck Cancer) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00070629 | CPG 7909 Injection in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04213937 | Nab-paclitaxel Versus Topotecan As Second-Line Treatment for Patients With Extensive Stage Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04374630 | Evaluation of the Sensitivity to Different Chemotherapy Regimens in Platinum-Partial Sensitive Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003327 | Paclitaxel in Treating Patients With Recurrent or Refractory Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02317874 | Testing the Addition of the Anti-Cancer Drug Talazoparib to the Combination of Carboplatin and Paclitaxel for the Treatment of Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT04084158 | A Study of Toripalimab Combined With Concurrent Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma. | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00807859 | Safety Study of AMG 386 to Treat HER2-positive Locally Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01218516 | A Safety and Efficacy Study of Farletuzumab in Participants With Adenocarcinoma of the Lung | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00930930 | Cisplatin and Paclitaxel With or Without Everolimus in Treating Patients With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03678883 | Lapatinib Plus Chemotherapy Versus Trastuzumab Plus Chemotherapy in HER2-positive Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01121406 | BI 6727 (Volasertib) Randomised Trial in Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT05290935 | Immunotherapy for Recurrent Cervical Cancer Refractory to Platinum-based Chemotherapy: Multi-Center Trial | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT03756818 | TAK-659 and Paclitaxel in Treating Patients With Advanced Solid Tumors | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00003899 | Chemotherapy Plus Bone Marrow or Peripheral Stem Cell Transplantation in Treating Patients With Recurrent or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03428425 | A Study of HIPEC Plus Apatinib and S-1 Conversion Therapy for Gastric Cancer With Positive Exfoliative Cancer Cells | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03915444 | Nab-Paclitaxel + Cisplatin + Gemcitabine in Untreated Metastatic Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01963325 | S-1 in Combination With Abraxane in Treating Cholangiocarcinoma | cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT01954355 | Clinical Study in Treatment of Malignant Ascites of Ovarian Cancer With Intraperitoneal Injection Bevacizumab Combined With Intraperitoneal Hyperthermic Perfusion Chemotherapy | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04731038 | Combination Therapy for First Line Treatment of Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01405235 | Study on Paclitaxel Plus Topotecan in Comparison With Topotecan Plus Cisplatin in Recurrent or Persistent Cervical Carcinoma | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04081389 | Chemokine Modulation Therapy and Standard Chemotherapy Before Surgery for the Treatment of Early Stage Triple Negative Breast Cancer | breast carcinoma in situ | Paclitaxel (NPC208553) | |
| NCT00933387 | A Study of Neoadjuvant Bio-C/T Followed by Concurrent Bio-R/T in High-risk Locally Advanced Oral Squamous Cell Carcinoma | mouth neoplasm | Paclitaxel (NPC208553) | |
| NCT00140140 | A Phase I/II Study of ABI-007 (Abraxane®, Nab®-Paclitaxel)and Vinorelbine in Patients With Stage IV (Metastatic) Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03678883 | 9-ING-41 in Patients With Advanced Cancers | Malignant Bone Neoplasm;malignant glioma;kidney cancer;lung neoplasm;metastasis;sarcoma | Paclitaxel (NPC208553) | |
| NCT02432365 | Study of Weekly Paclitaxel and Cisplatin in FIGO IB2 and IIA2 Cervical Cancer Followed by Radical Hysterectomy | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00568451 | Paclitaxel and Carboplatin or Temozolomide in Treating Patients With Stage IV Melanoma | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT00022191 | Cisplatin Plus Gemcitabine With or Without Paclitaxel in Treating Patients With Stage IV Urinary Tract Cancer | urethra cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT05497778 | A Phase 1b Study of Gemcitabine and Nab-paclitaxel in Combination With IM156 in Patients With Advanced Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00314678 | Cisplatin Induction With Paclitaxel Consolidation for Stage III-IV Epithelial Ovarian and Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03537690 | FID-007 in Treating Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00709761 | Neo ALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) Study | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00174434 | Study Of SU011248 In Combination With Paclitaxel In Patients With Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00091377 | Phenoxodiol Combined With Either Cisplatin or Paclitaxel in Patients With Recurrent Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT05170438 | Lenvatinib Plus Paclitaxel for Patients With Advanced Biliary Tract Cancer Who Failed to Gemcitabine-based Treatment | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT02244502 | Safety, Feasibility and Effect of TTFields (200 kHz) Concomitant With Weekly Paclitaxel in Recurrent Ovarian Carcinoma (INNOVATE) | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00055887 | Chemotherapy and Radiation Therapy With or Without Efaproxiral in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01279291 | Study of Anti-HB-EGF Antibody KHK2866 in Subjects With Advanced Solid Tumors and Ovarian Cancer | Fallopian Tube Carcinoma;ovarian neoplasm;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00019812 | Monoclonal Antibody Plus Chemotherapy in Treating Patients With Metastatic Breast Cancer That Overexpresses HER2 | breast cancer | Paclitaxel (NPC208553) | |
| NCT00411138 | Randomized Trial of Radiation Therapy With or Without Chemotherapy for Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02506842 | Second-Line Adjuvant Therapy With Nab-Paclitaxel Plus Gemcitabine Versus Oxaliplatin Plus Folinic Acid and Fluorouracil for Gemcitabine-Refractory Pancreatic Cancer After Curative Resection | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00004979 | Topotecan and Paclitaxel in Treating Patients With Advanced Non-small Cell Lung Cancer or Other Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01779050 | Afatinib in HER2 (Human Epidermal Growth Factor Receptor)-Treatment Failures | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03639246 | A Study of the Efficacy and Safety of Bevacizumab in Chinese Women With Newly Diagnosed, Previously Untreated Stage III or Stage IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01820325 | Safety and Efficacy of Buparlisib (BKM120) in Patients With Untreated Squamous Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03977220 | Nab-paclitaxel Combined With S-1 Treating Diffuse Type of Stage Ⅲ Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00454324 | Study of Abraxane and Carboplatin to Treat Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00563784 | TARCEVA (Erlotinib) in Combination With Chemoradiation in Patients With Stage IIIA/B Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01413750 | Carboplatin and Paclitaxel With or Without Vorinostat in Treating Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00531687 | Trial of Paclitaxel, Gemcitabine and Cisplatin in Patients With Relapsing Germ Cell Cancer | testicular carcinoma | Paclitaxel (NPC208553) | |
| NCT03197584 | Neo-adjuvant Pembrolizumab in Primary Stage IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01868022 | Study to Evaluate GSK3052230 in Combination With Paclitaxel and Carboplatin, or Docetaxel or as Single Agent in Subjects With Solid Malignancies and Deregulated Fibroblast Growth Factor (FGF) Pathway Signaling | neoplasm | Paclitaxel (NPC208553) | |
| NCT03257033 | Intra-arterial Gemcitabine vs. IV Gemcitabine and Nab-Paclitaxel Following Radiotherapy for LAPC | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02754726 | Combination Therapy for Patients With Untreated Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00025480 | Tipifarnib Plus Radiation Therapy After Combination Chemotherapy in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00087802 | Gemcitabine/Oxaliplatin (GEMOX) vs Carboplatin/Paclitaxel (CP) in Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03807999 | Nab-Paclitaxel Plus Gemcitabine Versus Gemcitabine For The First Line Treatment of Pancreas Cancer | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT02426281 | Nab-pacliatxel Plus Gemcitabine in Korean Patients With Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04979585 | Camrelizumab Plus Amlotinib and Chemotherapy in the First-line Treatment of Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT05052931 | Nab-paclitaxel Combined With Oxaliplatin and S-1 for Perioperative Treatment of Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03635489 | Study on MK-3475 Plus Chemotherapy Versus Chemotherapy Alone in Recurrent, Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00006929 | Suramin, Paclitaxel, and Carboplatin in Treating Patients With Stage IIIB or Stage IV Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00268970 | Satraplatin and Paclitaxel in Patients With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00226915 | Trial of Tri-weekly TJ Versus Weekly TJ for Stage II-IV Mullerian Carcinoma | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00006256 | Paclitaxel Plus Radiation Therapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04390399 | Combination Immunotherapy Plus Standard-of-Care Chemotherapy Versus Standard-of-Care Chemotherapy for the Treatment of Locally Advanced or Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02060253 | Ganetespib, Paclitaxel, Trastuzumab and Pertuzumab for Metastatic Human Epidermal Growth Factor Receptor 2 Positive Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03502343 | Efficacy and Safety of Modified Nab-Paclitaxel Plus Gemcitabine Chemotherapy for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02545010 | Pembrolizumab, Carboplatin, and Paclitaxel in Treating Patients With Stage III-IV Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00006012 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Limited-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003742 | Combination Chemotherapy in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00151034 | Trastuzumab (Herceptin), Paclitaxel, Carboplatin and Gemcitabine in Advanced Urothelial Cancer | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT00561119 | Maintenance Versus Observation After 6 Cycles of Gemcitabine Plus Paclitaxel in Pts With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02256436 | A Study of Pembrolizumab (MK-3475) Versus Paclitaxel, Docetaxel, or Vinflunine for Participants With Advanced Urothelial Cancer (MK-3475-045/KEYNOTE-045) | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT04046016 | A Study Using Whole-body PET After Oral Microdose of 18F-labeled Liporaxel in Patients With Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT00066716 | Celecoxib, Paclitaxel, and Carboplatin in Treating Patients With Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003387 | Carboplatin, Paclitaxel, and Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Removed During Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT04692051 | A Phase II Study for Nab-paclitaxel Plus Cisplatin vs Gemcitabine Plus Cispatin as First Line Chemotherapy in Advanced Biliary Tract Cancer | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT02005315 | A Study of Vantictumab (OMP-18R5) in Combination With Nab-Paclitaxel and Gemcitabine in Previously Untreated Stage IV Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00434135 | Alimta and Gemcitabine in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00009997 | Trastuzumab and Chemotherapy Followed by Surgery and Combination Chemotherapy in Treating Women With Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02013453 | A Phase II Trial of Proton Pump Inhibitors With Chemotherapy in Patients With Metastatic Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01009515 | Carboplatin, Paclitaxel, and Temozolomide for Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT04134468 | MRI Effects of Pegvorhyaluronidase Alfa (PEGPH20) in Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00399126 | Phase I Trial of Paclitaxel With Perifosine | neoplasm | Paclitaxel (NPC208553) | |
| NCT00008138 | S0009 Combination Chemo and Surgery in Stage III or Stage IV Ovarian Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02366143 | A Study of Atezolizumab in Combination With Carboplatin Plus (+) Paclitaxel With or Without Bevacizumab Compared With Carboplatin+Paclitaxel+Bevacizumab in Participants With Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02481635 | A Study of Gemcitabine/Nab-paclitaxel and Radiation Therapy Followed by Surgery in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01485848 | A Study of MM-121 With Paclitaxel in Platinum Resistant/ Refractory Advanced Ovarian Cancers | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00881166 | Safety of MP470 in Combination With Standard-of-Care Chemotherapy Regimens to Treat Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT00002949 | Vinorelbine and Paclitaxel Plus Radiation Therapy in Treating Patients With Advanced Cancer Arising in the Pelvis | vaginal cancer;cervical cancer;endometrial cancer;urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00085839 | Erlotinib vs. Standard Chemotherapy in Patients With Advanced Non-small Cell Lung Cancer (NSCLC) and Eastern Cooperative Oncology Group (ECOG)Performance Status (PS) 2 | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05000892 | Neoadjuvant Sintilimab in Combination With Carboplatin and Nab-paclitaxel in Untreated Salivary Gland Malignant Neoplasms | salivary gland cancer | Paclitaxel (NPC208553) | |
| NCT00028587 | PS-341 and Combination Chemotherapy in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01090830 | Safety and Efficacy of Belinostat When Used With Standard of Care Chemotherapy for Untreated Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003877 | Peripheral Stem Cell Transplantation With or Without Stromagen Following Chemotherapy in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00532857 | Phase II Study of Primary Chemotherapy With Paclitaxel, Gemcitabine, and Trastuzumab | breast cancer | Paclitaxel (NPC208553) | |
| NCT04148911 | A Study of Atezolizumab Plus Nab-Paclitaxel in the Treatment of Unresectable Locally Advanced or Metastatic PD-L1-Positive Triple-Negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT05054439 | A Clinical Study of SI-B001 in Combination With Paclitaxel in the Treatment of Recurrent and Metastatic HNSCC | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01249443 | Paclitaxel and Carboplatin in Treating Patients With Metastatic or Recurrent Solid Tumors and HIV Infection | anus cancer;hypopharyngeal carcinoma;verrucous carcinoma;laryngeal squamous cell carcinoma;malignant epithelial tumor of ovary;oropharyngeal carcinoma;esophageal cancer;gastric cancer;HIV infection;non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00407888 | Doxorubicin Hydrochloride, Cyclophosphamide, and Filgrastim Followed By Paclitaxel Albumin-Stabilized Nanoparticle Formulation With or Without Trastuzumab in Treating Patients With Breast Cancer Previously Treated With Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT01752205 | Paclitaxel Plus Radiation With Erlotinib to Treat Esophageal Squamous Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00976677 | Carboplatin, Paclitaxel, and Bevacizumab With or Without Erlotinib Hydrochloride in Treating Non-Smokers With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00882973 | Trial to Determine the Maximum Tolerated Dose of Genexol-PM Plus Gemcitabine and Evaluate Efficacy and Safety of Genexol-PM Regimens in Subjects With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01154920 | Paclitaxel, Carboplatin and Cetuximab (PCC) With Cetuximab, Docetaxel, Cisplatin and Fluorouracil (C-TPF) in Previously Untreated Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02593708 | Phase1 of Neratinib+Trastuzumab, Pertuzumab, Paclitaxel in Patients With Advanced Solid Tumors/HER2+ | neoplasm | Paclitaxel (NPC208553) | |
| NCT01694576 | NPC Staged N2-3M0:Adjuvant Chemotherapy or Just Observation After Concurrent Chemoradiation | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT03435289 | A Study of CPI-613 With Gemcitabine and Nab-paclitaxel for Patients With Advanced or Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00006049 | ZD 1839 Plus Chemotherapy in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01402271 | A Study of MK-1775 in Combination With Paclitaxel and Carboplatin Versus Paclitaxel and Carboplatin Alone for Participants With Platinum-Sensitive Ovarian Tumors With the P53 Gene Mutation (MK-1775-004) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00984464 | Study of REOLYSIN® in Combination With Paclitaxel and Carboplatin in Patients With Metastatic Melanoma | metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT02122770 | Effects of Fluconazole and Itraconazole CYP3A-Mediated Inhibition on the Pharmacokinetics, Safety, and Tolerability of MLN4924 in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01159418 | LBH589 Oral in Combination With Carboplatin and Paclitaxel in Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02422563 | NeoAdjuvant Chemotherapy Followed by Radical Hysterectomy (OP) Versus Primary Chemo-RADiation in Cervical Cancer FIGO Stage IB2 and IIB | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00331630 | Abraxane and Lapatinib in Treating Patients With Stage I, Stage II, or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01770301 | Efficacy and Safety of Bevacizumab (Avastin®) Combined to Weekly Paclitaxel Followed by Bevacizumab (Avastin®) Alone in Patients With Relapsed Ovarian Sex-cord Stromal Tumours (ALIENOR) | sex cord-gonadal stromal tumor | Paclitaxel (NPC208553) | |
| NCT04807673 | Pembrolizumab Plus Neoadjuvant Chemotherapy vs. Neoadjuvant Chemoradiotherapy for Locally Advanced ESCC (KEYSTONE-002) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01654146 | Dose-finding Study in Platinum-Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00642759 | Carboplatin, Abraxane, and Bevacizumab in Previously Untreated Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01778803 | A Study of Bevacizumab (Avastin) in Neoadjuvant Therapy in Participants With International Federation of Gynecology and Obstetrics (FIGO) Stage IIIC/IV Ovarian, Tubal, or Peritoneal Cancer, Initially Unresectable | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02632305 | A Study to See the Effects That a New Combination of the Three Drugs, Nab-paclitaxel, Gemcitabine, and Cisplatin Has on Biliary Tract Cancer | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT01057264 | HAI Abraxane With Gemcitabine and Bevacizumab | cancer | Paclitaxel (NPC208553) | |
| NCT04190745 | Toripalimab Combined With Apatinib Mesylate for the Treatment of Gastric Adenocarcinoma in a Prospective Randomized Multicenter Phase II Clinical Study | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01113476 | Nab-paclitaxel, Gemcitabine, and Bevacizumab in Advanced Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT04943445 | Study of a Pembrolizumab-based Organ Preservation Strategy for Locally Advanced Larynx Cancers | laryngeal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05449483 | Conversion of Tislelizumab Combined With Chemotherapy in Unresectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00011999 | Chemotherapy and Radiation Therapy Following Surgery in Treating Patients With Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01934634 | Phase I Trial of LCL161 and Gemcitabine Plus Nab-Paclitaxel in Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03057366 | A Study of [14 C]-Pevonedistat in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04216472 | Nab-paclitaxel and Alpelisib for the Treatment of Anthracycline Refractory Triple Negative Breast Cancer With PIK3CA or PTEN Alterations | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00006374 | Combination Chemotherapy in Treating Patients With Extensive-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04999969 | Safety, Pharmacokinetics and Clinical Activity of AZD0171 in Combination With Durvalumab and Chemotherapy in Locally Advanced or Metastatic Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT04804696 | Toripalimab With Paclitaxel and Cisplatin as Neoadjuvant Treatment for Esophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00069953 | Combination Chemotherapy Followed By Chemoradiotherapy, With or Without Surgery, in Treating Patients With Resectable Locally Advanced Cancer of the Esophagus or Gastroesophageal Junction | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04938583 | A Study Evaluating the Efficacy and Safety of Biomarker-Driven Therapies in Patients With Persistent or Recurrent Rare Epithelial Ovarian Tumors | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00287989 | Erlotinib, Paclitaxel, and Carboplatin in Treating Patients With Stage III, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02043730 | Abraxane and Gemcitabine Versus Gemcitabine Alone in Locally Advanced Unresectable Pancreatic Cancer. | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00786552 | Pemetrexed + Paclitaxel in Patients With Recurrent/Advanced Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT01646762 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Relapsed or Refractory Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT00002888 | Combination Chemotherapy in Treating Patients With Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01558492 | Carboplatin and Paclitaxel in Patients With Metastatic, Castrate-Resistant Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03503604 | A Study of Anti-VEGF Monoclonal Antibody hPV19 in Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00085358 | Carboplatin and Paclitaxel With or Without Bevacizumab Compared to Docetaxel, Carboplatin, and Paclitaxel in Treating Patients With Stage II, Stage III, or Stage IV Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cavity Carcinoma (Cancer) | ovarian serous cystadenocarcinoma;endometrioid carcinoma;fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04572542 | Camrelizumab Combined With Apatinib Mesylate Tablets and Nab-paclitaxel in the Second-line Treatment of Advanced Gastric Cancer | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00001450 | Phase II Trial of a 96-Hour Continuous Infusion of Paclitaxel Followed by Cisplatin for Patients With Stage III/IV and Relapsed NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04802980 | A Study of HB002.1T Plus Chemotherapy in Subjects With Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT01263145 | MK2206 and Paclitaxel in Treating Patients With Locally Advanced or Metastatic Solid Tumors or Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00003957 | Combination Chemotherapy Plus Peripheral Stem Cell Transplantation in Treating Patients With Relapsed Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00452803 | Pre-operative Chemotherapy Versus Concurrent Chemoradiotherapy in N2 Positive IIIA Non Small Cell Lung Cancer (NSCLC) | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003317 | Combination Chemotherapy With or Without Radiation Therapy in Treating Patients With Stage IIIA Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03345810 | Durvalumab (MEDI4736) in Frail and Elder Patients With Metastatic NSCLC (DURATION) | large cell lung carcinoma;lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03507491 | A Phase II Randomized Study Comparing the Efficacy and Safety of Targeted Therapy or Cancer Immunotherapy Versus Platinum-Based Chemotherapy in Patients With Cancer of Unknown Primary Site | cancer | Paclitaxel (NPC208553) | |
| NCT00390611 | Paclitaxel and Carboplatin With Or Without Sorafenib In The First-Line Treatment Of Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02911350 | Safety Study of Combination of Hormone Therapy, Paclitaxel and Radiation Therapy to Treat Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03805399 | FUSCC Refractory TNBC Umbrella (FUTURE) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT01461915 | Phase 2 Study to Assess the Efficacy & Safety of Gemcitabine + Nab Paclitaxel With or Without Dociparstat in Metastatic Pancreatic Cancer Patients | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03064126 | RANGER™ Paclitaxel Coated Balloon vs Standard Balloon Angioplasty | peripheral arterial disease | Paclitaxel (NPC208553) | |
| NCT00104676 | Combination Chemotherapy in Treating Patients With Stage II or Stage III Germ Cell Tumors | teratoma;testicular neoplasm | Paclitaxel (NPC208553) | |
| NCT03025477 | Safety and Efficacy of Quisinostat, a Histone Deacetylase Inhibitor, in Combination With Chemotherapy | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01839773 | A Phase 3 Study to Compare Efficacy and Safety of DHP107 Versus Taxol® in Patients With Metastatic or Recurrent Gastric Cancer After Failure of First-line Chemotherapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03776812 | Study of Relacorilant in Combination With Nab-Paclitaxel for Patients With Recurrent Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | Fallopian Tube Carcinoma | Paclitaxel (NPC208553) | |
| NCT02769832 | Nab-Paclitaxel With Gemcitabine for Relapsed Small Cell Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01732640 | A Phase I/II Study Afatinib/Carboplatin/Paclitaxel Induction Chemotherapy In HPV-Negative HNSCC. | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00040794 | Combination Chemotherapy, Radiation Therapy, and Gefitinib in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT03423979 | Optilume™ BPH Prostatic Drug Coated Balloon Dilation Catheter | benign prostatic hyperplasia | Paclitaxel (NPC208553) | |
| NCT05481645 | Efficacy and Safety of TQB2450 Combined With Chemotherapy ± Anlotinib for Advanced Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT04342910 | Study to Evaluate the Efficacy and Safety of Camrelizumab and Apatinib in Patients With GC/GEJC | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00001387 | Phase I and Pharmacokinetic Trial of Paclitaxel (Taxol) Given as a 3-Hour Infusion in Pediatric Patients With Refractory Malignancy | neoplasm | Paclitaxel (NPC208553) | |
| NCT01441349 | Irinotecan/Cisplatin With or Without Simvastatin in Chemo-naive Patients With Extensive Disease-small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01491204 | Clinical Trial to Determine the Maximum Tolerated Dose and to Assess the Safety and Pharmacokinetic Profile of Oral Paclitaxel in Patients With Advanced Solid Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00356681 | A Study of AMG 706 or Bevacizumab, in Combination With Paclitaxel Chemotherapy, as Treatment for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01911598 | A Study of MEHD7945A in Combination With Cisplatin and 5-Fluorouracil (5-FU) or Paclitaxel and Carboplatin in Participants With Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M SCCHN) | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04004871 | Induction Chemotherapy With Nab-paclitaxel, Cisplatin and Fluorouracil for Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00729612 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients With Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02125344 | A Phase III Trial Comparing Two Dose-dense, Dose-intensified Approaches (ETC and PM(Cb)) for Neoadjuvant Treatment of Patients With High-risk Early Breast Cancer (GeparOcto) | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02449655 | Trial of AZD5363 Plus Paclitaxel /AZD2014 Plus Paclitaxel in Biomarker Negative (PIK3CA/MEK/RAS/TP53/MET) Gastric Adenocarcinoma Patients as Second-line Chemotherapy | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00607048 | Dose Finding Study Of CP-870,893, An Immune System Stimulating Antibody, In Combination With Paclitaxel And Carboplatin For Patients With Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00334321 | Pelvic IMRT With Tomotherapy in Post-Hysterectomy Endometrial Cancer Patients | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00840450 | Two Different Schedules of Carboplatin, Paclitaxel, Gemcitabine, and Surgery in Treating Patients With Newly Diagnosed Stage IIIC or Stage IV Primary Epithelial Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03574779 | A Study to Evaluate the Efficacy and Safety of Novel Treatment Combinations in Participants With Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00001384 | A Pilot Trial of AC (Adriamycin, Cyclophosphamide) Chemotherapy With G-CSF (Granulocyte Colony-Stimulating Factor) Followed by Infusional Taxol (Paclitaxel) as Adjuvant Treatment for High Risk Stage II and Stage III Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT01650376 | A Study of MEK162 and Paclitaxel in Patients With Epithelial Ovarian, Fallopian Tube or Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03564691 | Study of MK-4830 as Monotherapy and in Combination With Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-4830-001) | neoplasm | Paclitaxel (NPC208553) | |
| NCT01367002 | Evaluation of Carboplatin/Paclitaxel With and Without Trastuzumab (Herceptin) in Uterine Serous Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT04705519 | Nab-paclitaxel Combined With Bevacizumab in the Treatment of Metastatic Neuroendocrine Carcinoma | neuroendocrine carcinoma | Paclitaxel (NPC208553) | |
| NCT00231868 | A Study of Radiation Therapy and Paclitaxel and Carboplatin in Patients With Uterine Papillary Serous Carcinoma | uterine cancer | Paclitaxel (NPC208553) | |
| NCT00094835 | Study to Evaluate Motesanib With or Without Carboplatin/Paclitaxel or Panitumumab in the Treatment of Patients With Advanced Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05283226 | Study to Evaluate the Safety and Efficacy of Oral NRC-2694-A in Combination With Paclitaxel in Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma, Who Progressed on or After Immune Checkpoint Inhibitor Therapy | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01566240 | Induction Chemotherapy Plus Chemoradiation as First Line Treatment for Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04182724 | Camrelizumab, Apatinib and Nab-paclitaxel as Second-line Treatment in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00521781 | Study of Abraxane Plus Hormonal Therapy as Initial Treatment of Unresectable or Metastatic Prostate Cancer | prostate adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00731380 | A Phase I Trial of Abraxane, Cisplatin and 5-Fluorouracil Along With Chemoradiotherapy in Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT04827953 | Study to Evaluate the Safety and Efficacy of Treatment With NLM-001 and Standard Chemotherapy Plus Zalifrelimab in Patients With Advanced Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02279134 | Phase II/III Study Compare Adjuvant Chemoradiotherapy, Radiotherapy and Surgery Alone for Esophageal Carcinoma | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT01024712 | Paclitaxel, Carboplatin, and Gefitinib in Treating Patients With Advanced Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00006257 | SU5416 and Paclitaxel in Treating Patients With Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT03603756 | SHR-1210 in Combination With Apatinib and Chemotherapy in Patients With Advanced Esophageal Squamous Cell Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00022217 | Cisplatin-Epinephrine Injectable Gel Plus Paclitaxel and Carboplatin in Treating Patients With Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00653939 | A Safety and Efficacy Study of Carboplatin, Paclitaxel, Bevacizumab and CA4P in Non-Small Cell Lung Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT02101021 | Gemcitabine and Nab-paclitaxel Combined With Momelotinib in Participants With Previously Untreated Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01967043 | A Study to Evaluate Safety, Tolerability, Pharmacokinetics and Activity of Oraxol in Subjects With Advanced Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT01970722 | Surgery and Chemotherapy With or Without Chemotherapy After Surgery in Treating Patients With Ovarian, Fallopian Tube, Uterine, or Peritoneal Cancer | Fallopian Tube Carcinoma;ovarian carcinoma;uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT04481009 | A Study to Evaluate YH003 in Combination With Toripalimab (Anti-PD-1 mAb) in Subjects With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03699449 | Efficacy and Safety Study of AVB-S6-500 in Patients With Platinum-Resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03719924 | Nal-iri/lv5-fu Versus Paclitaxel as Second Line Therapy in Patients With Metastatic Oesophageal Squamous Cell Carcinoma | squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03801668 | Albumin-bound Paclitaxel Plus S-1 Versus SOX as First-line Treatment in Advanced or Recurrent Gastric Adenocarcinoma | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04661696 | Dose Escalation of Lapatinib With Paclitaxel in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003591 | Radiation Therapy Plus Paclitaxel in Treating Patients With Nonmetastatic, Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00027937 | Combination Chemotherapy, Peripheral Stem Cell Transplantation, and Biological Therapy in Treating Patients With Solid Tumors or Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00276588 | Gemcitabine and Carboplatin Followed by Paclitaxel in Treating Patients With Stage III or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00311636 | Triptorelin in Preventing Early Menopause in Premenopausal Women Who Are Receiving Chemotherapy for Stage I, Stage II, or Stage III Breast Cancer That Has Been Removed By Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT04674956 | A Prospective, Randomized, Double-blinded, Multi-center Clinical Trial to Evaluate the Efficiency and Safety of Anti-PD1 Antibody (Camrelizumab) Combined With Paclitaxel(Albumin Bound) and Gemcitabine as First-line Therapy in Patients With Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003008 | Paclitaxel in Treating Patients With AIDS-Related Kaposi's Sarcoma | sarcoma | Paclitaxel (NPC208553) | |
| NCT04608409 | Apatinib Combined With Abraxane and Carboplatin or Cisplatinum as First-line Treatment for Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03085056 | Trametinib in Combination With Paclitaxel in the Treatment of Anaplastic Thyroid Cancer | thyroid cancer | Paclitaxel (NPC208553) | |
| NCT04085276 | Toripalimab in Combination With Nab-Paclitaxel For Patients With Metastatic or Recurrent Triple-Negative Breast Cancer (TNBC) With or Without Systemic Treatment | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00459121 | Vandetanib, Carboplatin, and Paclitaxel in Treating Patients With Stage I, Stage II, or Stage III Non-Small Cell Lung Cancer That Can Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT01493843 | Safety and Efficacy of Carboplatin/Paclitaxel and Carboplatin/Paclitaxel/Bevacizumab With and Without Pictilisib in Previously Untreated Advanced or Recurrent Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003733 | Sequential Chemotherapy in Treating Patients With Residual Disease Following Surgery for Stage IIB, Stage III, or Stage IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02341456 | Phase Ib Study AZD1775 in Combination With Carboplatin and Paclitaxel in Adult Asian Patients With Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT01487226 | Adjuvant Chemotherapy in Patients With Lymph Node Metastasis After Radical Surgery in Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01063517 | Efficacy Study of Olaparib With Paclitaxel Versus Paclitaxel in Gastric Cancer Patients | gastric cancer | Paclitaxel (NPC208553) | |
| NCT04844385 | Toripalimab Plus Neoadjuvant Chemotherapy Combined With Chemoradiotherapy for Locally Advanced Unresectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00422682 | A Study Evaluating BSI-201 in Combination With Chemotherapeutic Regimens in Subjects With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04063683 | Paclitaxel and DDP Combined With Anlotinib in the First-line Treatment for Patients With Advanced Esophageal Squamous Cell Carcinoma(ESCC). | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00039546 | Combination Chemotherapy in Treating Women With Breast Cancer Who Have Undergone Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT01969955 | Nab-paclitaxel as Second-line Therapy in Locally Advanced or Metastatic Squamous Lung Cancer | squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03101748 | Neratinib and Paclitaxel With or Without Pertuzumab and Trastuzumab Before Combination Chemotherapy in Treating Patients With Metastatic or Locally Advanced Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00025298 | Chemotherapy Plus Radiation Therapy With or Without Amifostine in Treating Patients With Locally Advanced Cancer of the Nasopharynx | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01146795 | Alisertib (MLN8237) in Participants With Ovarian, Fallopian Tube or Peritoneal Cancer Preceded by Phase 1 Study of MLN8237 Plus Paclitaxel Treatment of Ovary or Breast Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01015339 | Paclitaxel Plus Capecitabine With Capecitabine Maintenance Treatment in Metastatic Adenocarcinoma of the Stomach or Esophagogastric Junction | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03398655 | Atezolizumab With Neoadjuvant Chemotherapy for Patients With Newly-Diagnosed Advanced-Stage Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00004126 | Paclitaxel and Oxaliplatin in Treating Patients With Recurrent or Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05262335 | Anlotinib Plus Chemotherapy as First-line Therapy for Gastrointestinal Tumor Patients With Unresectable Liver Metastasis (ALTER-G-001) | neoplasm | Paclitaxel (NPC208553) | |
| NCT02106884 | Quality of Life in Locally Advanced or Metastatic Pancreatic Cancer Treated With Gemcitabine and Nab-paclitaxel | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03588039 | Study of Oraxol and Pembrolizumab in Subjects With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04625543 | Neoadjuvant Immunotherapy to ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01234038 | Safety and Tolerability Study of ISIS EIF4E Rx in Combination With Carboplatin and Paclitaxel | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01591135 | A Phase III Study of Comparing Paclitaxel Plus 5-Fluorouracil Versus Cisplatin Plus 5-Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003378 | Combination Chemotherapy in Treating Patients With Previously Untreated Stage III or Stage IV Ovarian or Primary Peritoneal Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT04054362 | Paricalcitol Addition to Chemotherapy in Patients With Previously Untreated Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01839487 | PEGPH20 Plus Nab-Paclitaxel Plus Gemcitabine Compared With Nab-Paclitaxel Plus Gemcitabine in Participants With Stage IV Untreated Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01653470 | Study to Evaluate Safety & Tolerability of BMS-906024 in Combination With Chemotherapy & to Define DLTs & MTD of BMS-906024 in Combination With One of the Following Chemotherapy Regimens | cancer | Paclitaxel (NPC208553) | |
| NCT00403130 | Phase 2 Study of Gemzar, Taxol & Avastin Combination as 1st Line Treatment for Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003392 | High-Dose Chemotherapy and Peripheral Stem Cell Transplantation in Treating Patients With Recurrent or Refractory Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00038246 | Study of Paclitaxel, Estramustine Phosphate and Thalidomide for Patients With Metastatic Androgen-Independent Prostate Carcinoma | prostate cancer | Paclitaxel (NPC208553) | |
| NCT04778839 | Study of Paclitaxel Micelles for Injection in Chinese Patients With Advanced Solid Tumors. | neoplasm | Paclitaxel (NPC208553) | |
| NCT00453323 | Paclitaxel and Capecitabine in Patients With Metastatic/Recurrence Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00001383 | A Phase I Study of Infusional Paclitaxel With the P-Glycoprotein Antagonist PSC 833 | renal cell carcinoma;lymphoma;ovarian cancer;breast cancer | Paclitaxel (NPC208553) | |
| NCT03595059 | A Study With ABBV-155 Alone and in Combination With Taxane Therapy in Adults With Relapsed and/or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00003587 | S9806: Combination Chemotherapy in Treating Patients With Stage IIIB or Stage IV Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02047513 | Neoadjuvant Plus Adjuvant or Only Adjuvant Nab- Paclitaxel Plus Gemcitabine for Resectable Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00193310 | Preoperative or Postoperative Therapy of Patients With Stages IB, II, IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04826679 | Neoadjuvant Camrelizumab in Combination With Cisplatin and Nab-paclitaxel in Resectable HNSCC | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04150575 | A Clinical Study to Evaluate Efficacy and Safety of HLX10 Combined With Albumin-Bound Paclitaxel in Patients With Advanced Cervical Cancer Who Have Progressive Disease or Intolerable Toxicity After First-Line Standard Chemotherapy | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00088088 | STA-4783 in Combination With Paclitaxel and Carboplatin for the Treatment of Chemotherapy Naive Patients With Stage IIIB/IV Non Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00007904 | Adjuvant Stage 2-3A Breast Cancer With Positive Lymph Nodes | breast cancer | Paclitaxel (NPC208553) | |
| NCT00168883 | Study for Patients With Non Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00005647 | SU5416 and Paclitaxel in Treating Patients With Recurrent, Locally Advanced or Metastatic Cancer of the Head and Neck | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00478361 | Gemcitabine, Paclitaxel, Doxorubicin in Metastatic or Unresectable Bladder Cancer With Decreased Kidney Function | urethra cancer;urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT00945191 | A Study of First Line Treatment With Avastin (Bevacizumab) in Combination With Carboplatin and Weekly Paclitaxel in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00336791 | Randomized Clinical Trial to Evaluate the Predictive Accuracy of a Gene Expression for Stage I-II Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03503786 | Carboplatin, Paclitaxel With or Without Avelumab in Advanced or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00147537 | Combination Study Of CP-751,871 With Paclitaxel And Carboplatin In Advanced Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03519308 | A Pilot Study of Perioperative Nivolumab and Paricalcitol to Target the Micoenvironment in Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00041470 | Navelbine, Taxol, Herceptin and Neupogen in Stage IV Breast Cancer: A Phase I - II Trial | breast cancer | Paclitaxel (NPC208553) | |
| NCT00535119 | Veliparib, Carboplatin, and Paclitaxel in Treating Patients With Advanced Solid Cancer | Hereditary breast and ovarian cancer syndrome | Paclitaxel (NPC208553) | |
| NCT00002972 | Paclitaxel in Treating Patients With Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01579578 | Assess the Efficacy of AZD8931 in Combination With Paclitaxel Versus Paclitaxel Alone in Patients With Gastric Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT03394885 | Study of GEN-1 With NACT for Treatment of Ovarian Cancer (OVATION 2) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00654758 | A Phase 1b Study With Volociximab in Combination With Carboplatin and Paclitaxel in First-line, Advanced Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03740165 | Durvalumab Treatment in Combination With Chemotherapy and Bevacizumab, Followed by Maintenance Durvalumab, Bevacizumab and Olaparib Treatment in Advanced Ovarian Cancer Patients | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02162719 | Safety and Efficacy Study of Vintafolide and Vintafolide Plus Paclitaxel Compared to Paclitaxel Alone in Participants With Triple Negative Breast Cancer (TNBC) (MK-8109-004) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00193362 | Study of Paclitaxel, Carboplatin, and Gemcitabine Versus Gemcitabine and Vinorelbine for Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00108745 | Paclitaxel, Polyglutamate Paclitaxel, or Observation in Treating Patients With Stage III or Stage IV Ovarian Epithelial, Peritoneal Cancer, or Fallopian Tube Cancer | endometrioid carcinoma;undifferentiated carcinoma | Paclitaxel (NPC208553) | |
| NCT05189184 | Study of Camrelizumab in Combination With Apatinib Mesylate Plus Albumin-bound Paclitaxel and Cisplatin as the First Line Treatment of Recurrent or Metastatic, UnresectableHead and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00054210 | Polyglutamate Paclitaxel Plus Carboplatin Compared With Paclitaxel Plus Carboplatin in Treating Patients With Advanced or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00189371 | Reinduction Chemotherapy Containing Carboplatin and Paclitaxel With or Without Epoetin Alpha in Recurrent Platinum Sensitive Ovarian Cancer, Cancer of the Fallopian Tube or Peritoneum | anemia (phenotype);ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT03604965 | GP Induction Chemotherapy us TPF Adjuvant Chemotherapy Combined With DDP Concurrent Chemoradiotherapy in the Treatment of Locally Advanced NPC | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT01702714 | A Study of RO5083945 in Combination With Cisplatin and Gemcitabine or Carboplatin and Paclitaxel in Patients With Advanced or Recurrent Non-Small Cell Lung Cancer of Squamous Histology Who Have Not Received Prior Chemotherapy for The Metastatic Disease | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02419495 | Selinexor With Multiple Standard Chemotherapy or Immunotherapy Regimens in Treating Patients With Advanced Malignancies | renal cell carcinoma;cutaneous melanoma;Fallopian Tube Carcinoma;metastatic melanoma;ovarian carcinoma;breast carcinoma;non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00848718 | A Phase I Study of MK-2206 in Combination With Standard Chemotherapy in Participants With Locally Advanced or Metastatic Solid Tumors (MK-2206-003) | neoplasm | Paclitaxel (NPC208553) | |
| NCT00753909 | Paclitaxel, Carboplatin Plus Bevacizumab in Pretreated, Advanced or Metastatic Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00434252 | A Study of Bevacizumab With Carboplatin and Paclitaxel Chemotherapy for the First-Line Treatment of Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00616031 | Paclitaxel and Carboplatin With or Without Nitroglycerin in Treating Patients With Previously Untreated Stage III or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02588443 | Study of Neo-adjuvant RO7009789 Alone or Neo-adjuvant RO7009789 Plus Nab-Paclitaxel and Gemcitabine Followed by Adjuvant RO7009789 Plus Nab-Paclitaxel and Gemcitabine for Patients With Newly Diagnosed Resectable Pancreatic Carcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00002969 | S9714: Paclitaxel in Treating Patients With Stage IIIB or Stage IV Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00016315 | Gemcitabine, Carboplatin or Paclitaxel Plus Radiation Therapy in Treating Patients With Stage IIIA or IIIB Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02735239 | Study of Anti-PD-L1 in Combination With Chemo(Radio)Therapy for Oesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01994031 | Dose Escalation and Pharmacokinetic Study of Paclitaxel Liposome Injection in Treating Patients With Advanced Solid Tumor After Failure From Conventional Treatments | neoplasm | Paclitaxel (NPC208553) | |
| NCT05007145 | PD-1 Inhibitor Combined With Neoadjuvant Chemotherapy in Subjects With Resectable Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00823186 | Dose-finding Study of Weekly Paclitaxel and Cisplatin in FIGO IB2 and Bulky IIA Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT05381909 | Study Investigating BGB-24714 as Monotherapy and in Combination With Chemotherapy in Participants With Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01372202 | CHFR Methylation Status Esophageal Cancer Study | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04077983 | Nab-Paclitaxel Combined With Gemcitabine Adjuvant Chemotherapy After Radical Resection of Intrahepatic Cholangiocarcinoma | cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT05201547 | Endometrial Cancer Patientes MMR Deficient Comparing Chimiotherapy vs Dostarlimab in First Line | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT05177796 | Panitumumab and Pembrolizumab in Combination With Neoadjuvant Chemotherapy for the Treatment of Stage III-IV Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03690739 | Evaluation of Symptom Benefit Rate of Trabectedin/PLD in Patients With Recurrent Ovarian Cancer | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT03496662 | BMS-813160 With Nivolumab and Gemcitabine and Nab-paclitaxel in Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma (PDAC) | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00596830 | Carboplatin And Paclitaxel With Or Without CP-751, 871 (An IGF-1R Inhibitor) For Advanced NSCLC Of Squamous, Large Cell And Adenosquamous Carcinoma Histology | large cell lung carcinoma;squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003394 | Combination Chemotherapy in Treating Patients With Advanced Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT02024009 | Systemic Therapy and Chemoradiation in Advanced Localised Pancreatic Cancer - 2 | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00088556 | Carboplatin, Paclitaxel and TLK286 (Telcyta) as First-Line Therapy in Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00736944 | Trial of 2 Cycles of Induction Chemo With Abraxane, Cetuximab, Cisplatin, & 5-FU for Advanced Head and Neck Cancer | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00062179 | Paclitaxel and Carboplatin With or Without Celecoxib Before Surgery in Treating Patients With Stage IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03029611 | Pembrolizumab, Paclitaxel, and Carboplatin in Patients With Advanced Stage Epithelial Ovarian Cancer (EOC). | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00042302 | A Double-Blind, Randomized, Placebo-Controlled, Multicenter, Phase III Study of Tariquidar + Paclitaxel/Carboplatin as First-Line Therapy in Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05168527 | The First Line Treatment of Fruquintinib Combined With Albumin Paclitaxel and Gemcitabine in Pancreatic Cancer Patients | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01158144 | Concurrent Endostar, Paclitaxel/Carboplatin and Radiotherapy for Locally Advanced Non-small Cell Lung (RT0902) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00499083 | Paclitaxel, Cyclophosphamide, and Doxorubicin Followed by Autologous Dendritic Cells and Surgery With or Without Radiation Therapy and/or Hormone Therapy in Treating Women With Stage II or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01644825 | A Phase IIclinical Trial of Carboplatin and Paclitaxel or Carboplatin and Gemcitabine in Platinum-sensitive, Recurrent Ovarian, Fallopian Tube, and Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01236716 | Nab-Paclitaxel Treatment in Advanced Squamous Cell Carcinoma of Lung | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02020707 | Abraxane/Bevacizumab | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02598687 | Testing TH-302, in Combination With Preoperative Chemoradiotherapy, in Esophageal Cancer. | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02762474 | Trial of Concurrent Chemoradiotherapy for Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04266249 | CompassHER2-pCR: Decreasing Chemotherapy for Breast Cancer Patients After Pre-surgery Chemo and Targeted Therapy | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00788931 | A Trial l of Panobinostat Given in Combination With Trastuzumab and Paclitaxel in Adult Female Patients With HER2 Positive Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01024062 | Phase II Study of Weekly Paclitaxel (BMS-181339) in Patient With Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00550784 | Combination Chemotherapy and Autologous Peripheral Stem Cell Transplant in Treating Patients With Stage III, Stage IV, or Recurrent Ovarian Epithelial Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT03393507 | ROCKIF Trial: Re-sensitization of Carboplatin-resistant Ovarian Cancer With Kinase Inhibition of FAK | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00455533 | Study to Assess Effectiveness of Giving Combination of Standard Chemotherapy Drugs Versus Combination of Standard Chemotherapy and New Drug Ixabepilone When Given Before Surgical Removal of Early Stage Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02039791 | Study of Nimotuzumab in Combination With Neoadjuvant Chemotherapy for Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04158635 | Gemcitabine, Nab-Paclitaxel, and Bosentan for the Treatment of Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05108870 | TheraT® Vectors (Vaccines) Combined With Chemotherapy to Treat HPV16 Head and Neck Cancers | oropharynx squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03866993 | A Study of Anti-PD-1 AK105 in Patients With Metastatic Squamous Non-small Cell Lung Cancer | squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04603846 | A Study of Anti-PD-L1 Antibody ZKAB001 Combined With Albumin-bound Paclitaxel in Advanced Urothelial Carcinoma | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT00016406 | S0012 Doxorubicin, Cyclophosphamide, and Paclitaxel With or Without Filgrastim in Treating Women With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04177108 | A Study Of Ipatasertib in Combination With Atezolizumab and Paclitaxel as a Treatment for Participants With Locally Advanced or Metastatic Triple-Negative Breast Cancer. | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT01750073 | Paclitaxel and Cyclophosphamide With or Without Trastuzumab Before Surgery in Treating Patients With Previously Untreated Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00006459 | Paclitaxel With or Without Gemcitabine in Treating Women With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003088 | Combination Chemotherapy in Treating Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003399 | Peripheral Stem Cell Transplantation Plus Combination Chemotherapy in Treating Patients With Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT00540514 | Albumin-bound Paclitaxel (ABI-007) for Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00462423 | Abraxane and Avastin as Therapy for Patients With Malignant Melanoma, a Phase II Study | melanoma | Paclitaxel (NPC208553) | |
| NCT00191672 | A Trial Of Gemcitabine Plus Paclitaxel And Gemcitabine Plus Docetaxel In Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02756013 | Weight-based Dosing for Dense Weekly Paclitaxel and Carboplatin in Overweight Patients w/ Body Surface Area (BSA) > 2.0 | urogenital neoplasm | Paclitaxel (NPC208553) | |
| NCT00602797 | Vinorelbine Tartrate and Paclitaxel in Treating Older Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03830385 | Paclitaxel (Albumin Bound),Bleomycin And Cisplatin Or Carboplatin for Recurrent Or Metastatic Squamous Cell Carcinoma Of The Head And Neck | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00006248 | S0007 - Combination Chemotherapy in Treating Patients With Metastatic or Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00004067 | Doxorubicin and Cyclophosphamide Plus Paclitaxel With or Without Trastuzumab in Treating Women With Node-Positive Breast Cancer That Overexpresses HER2 | breast cancer | Paclitaxel (NPC208553) | |
| NCT02251951 | Study of Afuresertib Combined With Paclitaxel in Gastric Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT03928314 | Study of ORIC-101 in Combination With Anticancer Therapy | neoplasm | Paclitaxel (NPC208553) | |
| NCT00147680 | Uterine Papillary Serous Cancer (UPSC) Trial | uterine cancer | Paclitaxel (NPC208553) | |
| NCT01704690 | Combination Treatment of S-1 With Paclitaxel in Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02514031 | ARQ-761 Treatment With Gemcitabine/Nab-Paclitaxel Chemotherapy In Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03136406 | QUILT-3.039: NANT Pancreatic Cancer Vaccine: Combination Immunotherapy in Subjects With Pancreatic Cancer Who Have Progressed on or After Standard-of-care Therapy | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00887575 | Neoadjuvant Sunitinib With Paclitaxel/Carboplatin in Patients With Triple-Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00301730 | Cellular Adoptive Immunotherapy in Treating a Patient Who Has Undergone a Donor Stem Cell Transplant for Breast Cancer That Has Spread to the Lung | breast cancer | Paclitaxel (NPC208553) | |
| NCT00005831 | Trastuzumab and Combination Chemotherapy in Treating Patients With Locally Recurrent or Metastatic Urinary Tract Cancer | urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT00284752 | Phase II Trial of Abraxane in Front Line Therapy of Hormone Refractory Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00467012 | Phase Ⅱ Clinical Study of R435 (Bevacizumab) in Combination With Paclitaxel in Patients With Inoperable Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01797900 | The Role of Induction Chemotherapy for High-risk Locally Advanced Nasopharyngeal Carcinoma in the Era of IMRT | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00084214 | STA-4783/Paclitaxel or Paclitaxel Alone in Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT03344172 | Pre-Operative Trial (PGHA vs. PGH) for Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03412799 | Study of SBP-101 Combined With Nab-Paclitaxel and Gemcitabine in Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00034541 | Study of Cetuximab in Combination With Carboplatin-Paclitaxel in Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00024414 | DHA-Paclitaxel in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03577704 | The Safety,Efficacy of Anti-EGFR Humanized Monoclonal Antibody Combined With Chemotherapy in Advanced Solid Tumors. | neoplasm | Paclitaxel (NPC208553) | |
| NCT05193604 | A Clinical Trial to Evaluate Tolerability and Security of TQB2858 Injection in Subjects With Advanced Pancreatic Carcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03964753 | Neoadjuvant Therapy With Nab-paclitaxel and Cisplatin for Locally Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT04843098 | Study of TL117 in Patients With Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01204996 | A Study of the Safety and Efficacy of CNTO 888 in Combination With SoC (Standard of Care) Chemotherapy in Patients With Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT00003242 | Bryostatin 1 Plus Paclitaxel and Cisplatin in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00020007 | Paclitaxel and Hyperthermic Perfusion in Treating Patients With Lung Cancer or Lung Metastases That Cannot Be Removed By Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00680940 | A Study of Mycobacterium w in Combination With Paclitaxel Plus Cisplatin in Advanced Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04888663 | Study of Ramucirumab, Trastuzumab and Paclitaxel in Patients With HER2-positive Recurrent/Metastatic Gastric Cancer (HER-RAM Study) | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00611962 | Phase II Study of Oxaliplatine-Paclitaxel in Patients With Metastatic Germ Cell Tumor Refractory to Cisplatin | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT00025389 | Bevacizumab, Paclitaxel, and Carboplatin Before Surgery in Treating Patients With Stage IB, Stage II, or Stage IIIA Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT02470585 | Dose Dense Paclitaxel With Pembrolizumab (MK-3475) in Platinum Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01730222 | A Phase I-II Study of PAXG in Stage III-IV Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00038168 | Intravenous Estramustine With Taxol in Hormone Refractory Prostate Adenocarcinoma | prostate cancer | Paclitaxel (NPC208553) | |
| NCT04679064 | Efficacy and Safety of Paclitaxel (Albumin-bound) Combination With Carboplatin in Ovarian Cancer. | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03492047 | N-acetyl Cysteine Effect in Peripheral Neuropathy in Cancer Patients | peripheral neuropathy | Paclitaxel (NPC208553) | |
| NCT01224652 | Efficacy Study of Paclitaxel Versus Irinotecan in Patients With Recurrent or Metastatic Gastric Cancer Who Progress Following First-line Therapy | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00003105 | Combination Chemotherapy in Treating Patients With Metastatic or Locally Advanced Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00119262 | Bevacizumab and Combination Chemotherapy in Patients With Lymph Node Positive Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02845908 | POF Versus FOLFOX Versus FOLFOX Plus ip Paclitaxel in AGC | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00129727 | Carboplatin Taxol Avastin in Ovarian Cancer (OVCA) | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02559674 | QUILT-2.001: ALT-803 in Patients With Advanced Pancreatic Cancer in Conjunction With Gemcitabine and Nab-Paclitaxel | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00639522 | Dose Escalation Study of Liposomal Paclitaxel With/Without Capecitabine in Patients With Advanced Gastric Carcinoma | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT05485766 | Novel Neoadjuvant and Adjuvant Strategy for Germline BRCA 1/2 Mutated Triple Negative Breast Cancer | Hereditary breast and ovarian cancer syndrome | Paclitaxel (NPC208553) | |
| NCT05044871 | Oregovomab in Combination With Bevacizumab Plus Chemo in BRCA Wild Type Platinum Sensitive Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03189446 | Vaginal Cuff Brachytherapy Followed by Chemotherapy in Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01604863 | A Study of HGS1036 in Combination With Chemotherapy in Subjects With Advanced Solid Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00795899 | Taxol Epirubicin Cyclophosphamide Herceptin Neoadjuvant (TECHNO) | breast cancer | Paclitaxel (NPC208553) | |
| NCT05371301 | Effect of Albumin-bound Paclitaxel Plus Carboplatin Compared With Paclitaxel Plus Carboplatin in Patients Receiving Neoadjuvant Chemotherapy for Advanced Ovarian, Fallopian Tube or Peritoneal Cancer: A Phase 2 Single Center Clinical Trial | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02367794 | A Study of Atezolizumab in Combination With Carboplatin + Paclitaxel or Carboplatin + Nab-Paclitaxel Compared With Carboplatin + Nab-Paclitaxel in Participants With Stage IV Squamous Non-Small Cell Lung Cancer (NSCLC) [IMpower131] | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00768469 | Study Evaluating Safety And Tolerability, Solid Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT03432598 | Anti-PD-1 in Combination With Chemotherapy as First-Line Treatment to Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00879359 | Trial of Vascular Endothelial Growth Factor (VEGF), Bevacizumab, in Combination With Cytotoxic Chemotherapy for Endometrial Cancer | endometrial carcinoma | Paclitaxel (NPC208553) | |
| NCT01968915 | Safety and Tolerability of Oral LCL161 in Japanese Adult Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01084083 | Induction Chemotherapy Followed By Cetuximab and Radiation in HPV-Associated Resectable Stage III/IV Oropharynx Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT03169764 | QUILT-3.047: NANT Head and Neck Squamous Cell Carcinoma (HNSCC) Vaccine: Combination Immunotherapy in Subjects With HNSCC Who Have Progressed on or After Chemotherapy and PD-1/PD-L1 Therapy | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT03941093 | Evaluation of Efficacy and Safety of Neoadjuvant Treatment With Pamrevlumab in Combination With Chemotherapy (Either Gemcitabine Plus Nab-paclitaxel or FOLFIRINOX) in Participants With Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01836432 | Immunotherapy Study in Borderline Resectable or Locally Advanced Unresectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03020329 | Induction Chemotherapy in Young Patients With Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT02934464 | Assessment of Ramucirumab Plus Paclitaxel as Switch MANteInance Versus Continuation of First-line Chemotherapy in Patients With Advanced HER-2 Negative Gastric or Gastroesophageal Junction Cancers | stomach neoplasm | Paclitaxel (NPC208553) | |
| NCT00473720 | Phase I Study of the Combination of Satraplatin and Abraxane in Advanced Cancers | cancer | Paclitaxel (NPC208553) | |
| NCT00986674 | Carboplatin and Paclitaxel Combined With Cetuximab and/or IMC-A12 in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02283489 | Recombinant Human Endostatin Adenovirus Combined With Chemotherapy for Advanced Head and Neck Malignant Tumors | upper aerodigestive tract neoplasm | Paclitaxel (NPC208553) | |
| NCT00021320 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02993731 | A Study of Napabucasin Plus Nab-Paclitaxel With Gemcitabine in Adult Patients With Metastatic Pancreatic Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05024773 | Study of ONCOFID-P-B (PACLITAXEL-HYALURONIC ACID) | urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT00151060 | Estramustine, Etoposide and Paclitaxel Treatment for Hormonally Responsive Adenocarcinoma of the Prostate | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00062062 | Gefitinib With or Without Carboplatin and Paclitaxel in Treating Older Patients With Unresectable or Metastatic Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00170664 | Taxol/ Carboplatin as First Line Therapy in Patients With Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03600623 | Folfirinox or Gemcitabine-Nab Paclitaxel Followed by Stereotactic Body Radiotherapy for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02486601 | NAB-PACLITAXEL Plus FOLFOX as Perioperative Chemotherapy in Patients With Operable Oesogastric Adenocarcinoma | stomach neoplasm | Paclitaxel (NPC208553) | |
| NCT00915018 | Study Evaluating Neratinib Plus Paclitaxel VS Trastuzumab Plus Paclitaxel In ErbB-2 Positive Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01834235 | QUILT-3.010: A Study of Gemcitabine and Nab-paclitaxel With or Without NPC-1C to Treat Patients With Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00154882 | Paclitaxel (Phyxol) and Cisplatin as First-line Chemotherapy for Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00323583 | Weekly Dosing of an Integrative Chemotherapy Combination to Treat Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03240016 | Abraxane With Anti-PD1/PDL1 in Patients With Advanced Urothelial Cancer | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT00004093 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00655850 | Lower Dose Chemotherapy Given More Frequent With Avastin to Treat Advanced Non-Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03435250 | Study of AG-270 in Participants With Advanced Solid Tumors or Lymphoma With MTAP Loss | lymphoma | Paclitaxel (NPC208553) | |
| NCT04151277 | PRIMUS 001: A Study Looking at Two Different Chemotherapy Regimens in Patients With Metastatic Pancreatic Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT03776812 | A Phase 1b Study of Sevacizumab in Combination With Chemotherapy in Chinese Patients With Platinum-Resistant Recurrent Ovarian Cancer. | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02794571 | Safety and Pharmacokinetics (PK) of Escalating Doses of Tiragolumab as a Single Agent and in Combination With Atezolizumab and/or Other Anti-Cancer Therapies in Locally Advanced or Metastatic Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00433420 | Combination Chemotherapy With or Without Fluorouracil and/or Pegfilgrastim in Treating Women With Node-Positive Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT05081609 | A Study Evaluating Targeted Therapies in Participants Who Have Advanced Solid Tumors With Genomic Alterations or Protein Expression Patterns Predictive of Response | cancer | Paclitaxel (NPC208553) | |
| NCT01042379 | I-SPY TRIAL: Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer | angiosarcoma;triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT00003342 | Combination Chemotherapy in Treating Patients With Advanced Bladder or Kidney Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01015222 | Dasatinib, Bevacizumab, Paclitaxel in Patients With Advanced Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT00245154 | Paclitaxel + Carboplatin With/Out Cediranib Maleate in Stage III or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00384826 | Therapeutic Strategy in Advanced Bronchioloalveolar Carcinoma | bronchoalveolar adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00717340 | A Phase 1b/2a, Open-Label, Multi-Center Study of Tivozanib (AV-951) in Combination With Paclitaxel in Subjects With Advanced or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04949256 | Efficacy and Safety of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) Plus Chemotherapy in Participants With Metastatic Esophageal Carcinoma (MK-7902-014/E7080-G000-320/LEAP-014) | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00795340 | Cediranib, Paclitaxel, and Carboplatin in Treating Patients With Stage IIIB or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04175912 | Testing the Combination of Pevonedistat With Chemotherapy for Bile Duct Cancer of the Liver | hepatocellular carcinoma;intrahepatic cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT00030654 | Hormone Therapy Plus Chemotherapy in Treating Patients With Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT04592861 | Carbon Ion RT for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00974584 | A Study of the Safety and Pharmacology Of PI3-Kinase Inhibitor GDC-0941 In Combination With Either Paclitaxel And Carboplatin (With or Without Bevacizumab) or Pemetrexed, Cisplatin, And Bevacizumab in Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03943043 | Gemcitabine + Oxaliplatin +Nab-paclitaxel in Subjects With Advanced Biliary Tract Cancer | cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT03908333 | High Dose Ascorbic Acid and Nanoparticle Paclitaxel Protein Bound and Cisplatin and Gemcitabine (AA NABPLAGEM) in Patients Who Have Metastatic Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02617849 | Pembrolizumab With Carboplatin/Paclitaxel in Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT04541108 | Phase 0 Master Protocol for CIVO Intratumoral Microdosing of Anti-Cancer Therapies | neoplasm | Paclitaxel (NPC208553) | |
| NCT01196234 | Paclitaxel/Carboplatin (PC) Followed by Gefitinib Versus PC in Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03989310 | An Open-label, Phase I/II Study of the Pan-immunotherapy in Patients With Local Advanced/Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01676649 | Ipilimumab With Carboplatin and Paclitaxel in Patients With Unresectable Stage III and Stage IV Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT03885219 | Nab-paclitaxel and S-1 in Patients With Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003592 | Methotrexate Compared With Paclitaxel in Treating Patients With Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02158520 | Nab-Paclitaxel and Bevacizumab or Ipilimumab as First-Line Therapy in Treating Patients With Stage IV Melanoma That Cannot Be Removed by Surgery | cutaneous melanoma;metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT03273595 | Study of Pembrolizumab (MK-3475) Plus Chemotherapy vs Placebo Plus Chemotherapy as Neoadjuvant Therapy and Pembrolizumab vs Placebo as Adjuvant Therapy in Participants With Triple Negative Breast Cancer (TNBC) (MK-3475-522/KEYNOTE-522) | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT04129996 | A Trial of Camrelizumab in Combination With Nab-paclitaxel and Famitinib as a First Line Treatment in Patients With Unresectable Locally Advanced or Metastatic Immunomodulatory Triple Negative Breast Cancer(FUTURE-C-PLUS) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT04016142 | Carboplatin-Paclitaxel Adjuvant Chemotherapy in the Treatment of Locally Advanced Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01376310 | GSK1120212 Rollover Study | cancer | Paclitaxel (NPC208553) | |
| NCT00401674 | MITO-5: Weekly Carboplatin and Paclitaxel as First Line Chemotherapy for Elderly Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04104672 | A Study to Evaluate the Safety and Tolerability of AB680 in Participants With Gastrointestinal Malignancies | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04581876 | The Safety and Efficacy of the Combination of Raltitrexed for Injection and Nab-Paclitaxel in Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03316599 | Study of Gemcitabine, Nab-paclitaxel, and Ficlatuzumab (AV-299) in Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT05174156 | Study on the Biomarker of First-line Immunotherapy Combined With Chemotherapy in Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01684878 | ICON8: Weekly Chemotherapy in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02436993 | Phase II Breast Ca Carboplatin + Paclitaxel With Pertuzumab + Trastuzumab or Bevacizumab | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00191646 | An Ovarian, Primary Peritoneal or Fallopian Tube Cancer Study for Patients That Have Not Received Prior Chemotherapy | Fallopian Tube Carcinoma;ovarian neoplasm;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00833261 | Nab-Paclitaxel, Cetuximab, Cisplatin, and Radiation Therapy in Treating Patients With Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00881816 | Dose Escalation Study of Liposomal Paclitaxel Plus Capecitabine in Chinese Patients With Advanced Gastric Carcinoma | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT00807612 | QUILT-2.017: Phase 1b/2 Study of AMG 479 in Combination With Paclitaxel and Carboplatin for 1st Line Treatment of Advanced Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00014118 | Chemotherapy and Radiation Therapy in Treating Patients With Stage III or Stage IV Larynx or Oropharynx Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02420314 | Pharmacological Ascorbate for Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00801736 | ERCC1 Targeted Trial | lung cancer | Paclitaxel (NPC208553) | |
| NCT00193141 | Chemotherapy With or Without Surgical Resection in Locally Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT04714190 | A Study of RC48-ADC in Local Advanced or Metastatic Gastric Cancer With the HER2-Overexpression | gastric carcinoma | Paclitaxel (NPC208553) | |
| NCT00686959 | Chemotherapy and Radiation in Treating Participants With Stage 3 Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00803556 | Clinical Trial of the Combination of Intravenous Alvespimycin (KOS-1022), Trastuzumab With or Without Paclitaxel in Patients With Advanced Solid Tumor Malignancies or Her2 Positive Metastatic Breast Cancer Who Have Previously Failed Trastuzumab Therapy | breast cancer | Paclitaxel (NPC208553) | |
| NCT00096226 | Paclitaxel, Carboplatin, and Radiation Therapy in Treating Patients Who Are Undergoing Surgery for Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00874848 | Efficacy/Safety of Imprime PGG With Cetuximab & Paclitaxel/Carboplatin Therapy in Pts With Untreated Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00625573 | Phase II Trial of Abraxane and Capecitabine in Metastatic Colon Cancer (COL 01) | colorectal carcinoma | Paclitaxel (NPC208553) | |
| NCT02834975 | Ruxolitinib Phosphate, Paclitaxel, and Carboplatin in Treating Patients With Stage III-IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01555853 | Phase I/II Study of Abraxane in Recurrent and Refractory Lymphoma | Hodgkins lymphoma;non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT00864318 | Dose Intensification Study in Refractory Germ Cell Tumors With Relapse and Bad Prognosis | germ cell tumor | Paclitaxel (NPC208553) | |
| NCT00079430 | Paclitaxel, Bevacizumab And Adjuvant Intraperitoneal Carboplatin in Treating Patients Who Had Initial Debulking Surgery for Stage II, Stage III, or Stage IV Ovarian Epithelial, Primary Peritoneal, or Fallopian Tube Cancer | ovarian serous cystadenocarcinoma;endometrioid carcinoma;fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02382406 | Carboplatin/Nab-Paclitaxel and Pembrolizumab in NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00479765 | A Phase 1 / 2 Dose Escalation Study of Locally-Administered OncoGel™ in Subjects With Recurrent Glioma | brain neoplasm;glioblastoma multiforme | Paclitaxel (NPC208553) | |
| NCT00128856 | Gemcitabine, Doxorubicin and Paclitaxel (GAT) as Neoadjuvant Treatment of Breast Cancer Patients | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003086 | Repeated Bone Marrow Transplantation in Treating Women With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02264990 | Study Comparing Veliparib Plus Carboplatin and Paclitaxel Versus Investigator's Choice of Standard Chemotherapy in Adults Receiving First Cytotoxic Chemotherapy for Metastatic or Advanced Non-Squamous Non-Small Cell Lung Cancer (NSCLC) and Who Are Current or Former Smokers | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00030368 | Bortezomib and Paclitaxel in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03907475 | Durvalumab in Combination With Chemotherapy in Treating Patients With Advanced Solid Tumors, (DURVA+ Study) | neoplasm | Paclitaxel (NPC208553) | |
| NCT01608009 | [18F]Fluciclatide-PET, Pazopanib and Paclitaxel in Ovarian Cancer | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00002937 | Paclitaxel With or Without PSC 833 in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003065 | Topotecan and Paclitaxel in Treating Patients With Recurrent or Metastatic Cancer of the Cervix | cervical cancer | Paclitaxel (NPC208553) | |
| NCT02855788 | Metronomic Chemotherapy in Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00003717 | Paclitaxel Plus Estramustine in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03810872 | An Explorative Study of Afatinib in the Treatment of Advanced Cancer Carrying an EGFR, a HER2 or a HER3 Mutation | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03740165 | A Study of the Efficacy and Safety of Bevacizumab in Chinese Women With Newly Diagnosed, Previously Untreated Stage III or Stage IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00002984 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Advanced or Metastatic Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01891357 | Phase II Trial to Validate Markers for a Response Evaluation of a Combined Therapy in Patients With HER2+ Breast Cancer | breast ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00513292 | Combination Chemotherapy and Paclitaxel Plus Trastuzumab in Treating Women With Palpable Breast Cancer That Can Be Removed by Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT04683939 | Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Efficacy Trial of BNT141 in Patients With Unresectable or Metastatic CLDN18.2-positive Gastric, Pancreatic, Ovarian and Biliary Tract Tumors | cholangiocarcinoma;gastric cancer;pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00064077 | Comparison of Four Combination Chemotherapy Regimens Using Cisplatin in Treating Patients With Stage IVB, Recurrent, or Persistent Cancer of the Cervix | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01667211 | Clinical Study of Albumin-bound Paclitaxel Plus Nedaplatin in Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00004174 | Combination Chemotherapy Followed By Peripheral Stem Cell Transplantation in Treating Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00256243 | Neoadjuvant Biweekly Treatment Followed by Weekly Treatment of Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00524303 | EndoTAG-1 in Triple Receptor Negative Breast Cancer Patients | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00581971 | Radiosensitization With Celecoxib and Chemoradiation for Head and Neck Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT02057380 | A Rollover Study for Subjects That Have Participated in an Astellas Sponsored Linsitinib Trial | neoplasm | Paclitaxel (NPC208553) | |
| NCT02241551 | Phase II Neoadjuvant Chemotheraphy (Gemcitabine and Nab-Paclitaxel vs. mFOLFIRINOX) and Sterotatic Body Radiation Therapy for Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02301143 | Phase 2 Nab® -Paclitaxel (Abraxane®) Plus Gemcitabine in Subjects With Locally Advanced Pancreatic Cancer (LAPC) | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT01930292 | Debio 1143 in Combination With Carboplatin and Paclitaxel in Patient With Advanced Solid Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT03393884 | Neo-adjuvant Pembrolizumab in Primary Stage IV Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT04921527 | A Phase III Study of BD0801 Combined With Chemotherapy in Recurrent, Platinum-resistant Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03989336 | Apatinib With Albumin-bound Paclitaxel in Patients With Platinum-resistant Recurrent Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00971945 | Rollover Study of Weekly Paclitaxel (BMS-181339) in Patients With Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01110603 | A Study of MK-4827 in Combination With Standard Chemotherapy in Participants With Advanced Solid Tumors (MK-4827-008 AM1) | cancer | Paclitaxel (NPC208553) | |
| NCT03932409 | Frontline Immunotherapy Combined With Radiation and Chemotherapy in High Risk Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02047500 | Phase I TH-302 Plus Gemcitabine Plus Nab-Paclitaxel in Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00002913 | Paclitaxel, Cisplatin, and Topotecan With or Without Filgrastim in Treating Patients With Newly Diagnosed Stage III or Stage IV Epithelial Ovarian Cancer | ovarian serous cystadenocarcinoma;endometrioid carcinoma | Paclitaxel (NPC208553) | |
| NCT02873598 | A Dose Escalation Trial of SBRT After Induction Chemotherapy for Locally Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01026116 | Treatment With Pazopanib for Neoadjuvant Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00964626 | Combination Chemotherapy With CS-1008 to Treat Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02429700 | TC or BEP in Treating Patients With Ovarian Malignant Sex Cord-Stromal Tumors | ovarian sex cord-stromal tumor | Paclitaxel (NPC208553) | |
| NCT00193531 | Paclitaxel, Carboplatin, and Oral Etoposide Followed by Weekly Paclitaxel in High Grade Neuroendocrine Carcinoma | neuroendocrine carcinoma | Paclitaxel (NPC208553) | |
| NCT03126812 | Olaparib +/- Cediranib or Chemotherapy in Patients With Platinum-resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02926183 | Study of NAC of GA Therapy for Patients With BRPC | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT05394415 | Chemoradiation Plus Tislelizumab for Conversion Therapy of Locally Nonresectable ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00281658 | ErbB2 Over-expressing Metastatic Breast Cancer Study Using Paclitaxel, Trastuzumab, and Lapatinib | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00702299 | Alimta® Plus Cisplatin & Paclitaxel Given Intraperitonelly | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00003160 | Weekly Infusions of Paclitaxel in Treating Women With Stage III or Stage IV Ovarian Cancer Refractory to Paclitaxel and Platinum | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00226590 | Induction Gemcitabine & Carboplatin Followed by Paclitaxel & Carboplatin +XRT in NSCLC | lung cancer | Paclitaxel (NPC208553) | |
| NCT00005059 | Carboplatin and Paclitaxel in Treating Older Patients With Metastatic or Recurrent Unresectable Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003881 | Trastuzumab Plus Chemotherapy in Treating Patients With Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01889420 | Phase I Trial of Everolimus, Pomalidomide and Dexamethasone in Patients With Relapsed/Refractory Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT00008047 | Combination Chemotherapy Combined With Radiation Therapy in Treating Patients Who Have Stage II or Stage III Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00003298 | Chemotherapy, Surgery, and Radiation Therapy in Treating Patients With Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00779714 | Comparative Study of Individualized Sensitivity-Directed Chemotherapy Versus DTIC | melanoma | Paclitaxel (NPC208553) | |
| NCT03430882 | Sapanisertib, Carboplatin, and Paclitaxel in Treating Patients With Recurrent or Refractory Malignant Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00512096 | Neoadjuvant Chemotherapy for Patients With Squamous Cell Carcinoma of the Penis | penile cancer | Paclitaxel (NPC208553) | |
| NCT03777462 | Borderline Resectable Pancreatic Cancer Neoadjuvant Chemoradiotherapy Clinicaltrial-1 | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03029611 | Two Therapeutic Strategies in First-line in Patients With Epithelial Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02730481 | A Study to Evaluate the Safety, Tolerability, MTD, PK, and Activity of Oraxol in Subjects w Adv. Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT00373620 | A Safety and Efficacy of CCRT With Paclitaxel as Adjuvant Therapy to Post-Operative Advanced Endometrial Cancer Patients | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT00661167 | Phase II Study of ABI-007 for Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00976573 | Carboplatin, Paclitaxel, and Bevacizumab With or Without Everolimus in Treating Patients With Metastatic Malignant Melanoma | cutaneous melanoma | Paclitaxel (NPC208553) | |
| NCT02135822 | Nab-paclitaxel Plus Gemcitabine in Chinese Patients With Advanced Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04282109 | Study to Assess the Efficacy and Safety of Nivolumab in Combination With Paclitaxel in Subjects With Head and Neck Cancer Unable for Cisplatin-based Chemotherapy (NIVOTAX) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00129922 | Fluorouracil, Epirubicin, and Cyclophosphamide Alone or Followed by Paclitaxel for Early Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01702844 | Single Arm on the Tolerability of Weekly Nab-paclitaxel | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00326456 | MITO-2: A Study Comparing 2 Chemotherapy Regimens (Carboplatin/Liposomal Doxorubicin vs Carboplatin/Paclitaxel) in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01362374 | Safety and Clinical Pharmacology of GDC-0068 in Combination With Docetaxel, Fluoropyrimidine Plus Oxaliplatin, Paclitaxel, or Enzalutamide in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04551950 | Bintrafusp Alfa Combination Therapy in Participants With Cervical Cancer (INTR@PID 046) | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00789581 | A Randomized Trial of Ixempra Versus Taxol in Adjuvant Therapy of Triple Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00723125 | Carboplatin, Abraxane, Avastin as Neoadjuvant Therapy for Her2-Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04196283 | Nab-paclitaxel in Combination With Gemcitabine for Pediatric Relapsed and Refractory Solid Tumors | cancer | Paclitaxel (NPC208553) | |
| NCT04643405 | APG-1387 Plus Chemotherapy in Advanced Pancreatic Adenocarcinoma | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT02446652 | Evaluation of TRANSKRIP ® Plus Chemotherapy in Recurrent-Persistent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT04225364 | Efficacy of Neoadjuvant PD-1 Blockade Plus Chemotherapy for Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01851174 | Study to Evaluate Bi-weekly Dosing of Gemcitabine Plus Nab-Paclitaxel to Treat Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT04675528 | Food Effect on Pharmacokinetics and SafEty of DHP107 (Liporaxel®) FEEL Study | neoplasm | Paclitaxel (NPC208553) | |
| NCT00971841 | Rollover Study of Weekly Paclitaxel (BMS-181339) in Patients With Advanced or Recurrent Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02207465 | A Phase I Dual Dose Escalation Study of Radiation and Nab-Paclitaxel in Patients With Unresectable and Borderline Resectable Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01253681 | A Study of the Addition of Avastin (Bevacizumab) to Carboplatin and Paclitaxel Therapy in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00610714 | AZD0530 Phase II Study in Patients With Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01712919 | Chemoradiation Plus Weekly c225 Against Locoregionally Advanced Nasopharyngeal Carcinoma (NPC) | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT00547443 | Sorafenib and High-Dose Carboplatin, Paclitaxel, and External-Beam Radiation Therapy in Treating Patients With Stage III Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00637897 | Paricalcitol and Chemotherapy in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02455141 | A Study Assessing the Safety and Efficacy of Adding Ipatasertib to Paclitaxel Treatment in Participants With Breast Cancer That Has Spread Beyond the Initial Site, and the Cancer Does Not Have Certain Hormonal Receptors | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT05254171 | Study of Nab-Paclitaxel and Gemcitabine With or Without SBP-101 in Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00021372 | Paclitaxel and Estramustine in Treating Patients With Relapsed or Refractory Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT00281788 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Esophageal Cancer or Gastroesophageal Junction Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00024401 | DHA-Paclitaxel in Treating Patients With Metastatic Colorectal Cancer | colorectal carcinoma | Paclitaxel (NPC208553) | |
| NCT03462212 | Study of GEN-1 With NACT for Treatment of Ovarian Cancer (OVATION 2) | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT00100139 | Comparison of Liposome Entrapped Paclitaxel Easy to Use (LEP-ETU) and Taxol® Pharmacokinetics in Patients With Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT01165216 | Japanese Study of Ipilimumab Administered in Combination With Paclitaxel/Carboplatin in Patients With Nonsmall-cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00730925 | Single-arm Trial of BIBW 2992 (Afatinib) in Demographically and Genotypically Selected NSCLC Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00886717 | Imatinib Mesylate (Gleevec) and Paclitaxel in Recurrent Patients of Ovarian and Other Cancers of Mullerian Origin | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04935359 | Study of Efficacy and Safety of NIS793 in Combination With Standard of Care (SOC) Chemotherapy in First-line Metastatic Pancreatic Ductal Adenocarcinoma (mPDAC) - daNIS-2 | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00702975 | Study of Combination Therapy of Carboplatin -Gemcitabine Plus Bevacizumab Beyond Progression in Patients With Locally Advanced and/or Metastatic Non-small Cell Lung Cancer (NSCLC) Who Have Not Received Prior Systemic Therapy | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03997123 | A Study Comparing Two Standard of Care Adjuvant Chemotherapy Regimens for Lower Risk HER-2 Positive Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00855764 | Late Phase II Study of Weekly Paclitaxel (BMS-181339) in Patients With Advanced or Recurrent Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00288041 | Bortezomib, Paclitaxel, and Carboplatin in Treating Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00520000 | Carboplatin and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00193193 | Weekly Paclitaxel, Low-Dose Estramustine, and Carboplatin in the Treatment of Hormone Refractory Prostate Carcinoma | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00313768 | Randomized Ph 2 Trial Of Paclitaxel/Carboplatin /Bevacizumab + PF-3512676 And P/C/B Alone In Advanced Nonsquamous NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00378313 | A Study of Gemcitabine, Epirubicin, and Paclitaxel Combination Chemotherapy Given Before Surgery to Patients With Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00490711 | Treatment of Paclitaxel Plus Carboplatin Followed by Gemcitabine Plus Carboplatin for Patients With Epithelial Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT01253525 | Study of Weekly Paclitaxel With Ramucirumab in Participants With Advanced Gastric Adenocarcinomas | adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00408070 | Bevacizumab Study With Carboplatin & Paclitaxel in Ovarian, Fallopian Tube or Primary Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT02467907 | Safety and Efficacy of Bevacizumab in Combination With Carboplatin and Paclitaxel for Metastatic, Recurrent or Persistent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT00003862 | Chemotherapy and Radiation Therapy in Treating Patients With Stomach Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00748553 | A Phase I/II Clinical Trial of Vidaza With Abraxane in Patients With Advanced/Metastatic Solid Tumors and Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00004173 | Oxaliplatin and Paclitaxel in Treating Patients With Metastatic or Unresectable Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00102622 | Intraperitoneal tgDCC-E1 and Intravenous Paclitaxel in Women With Platinum-Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02776917 | Adjuvant Treatment of EC Followed by Taxane +/- Carboplatin in Triple-Negative Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00003998 | Carboplatin Plus Paclitaxel or Docetaxel in Treating Patients With Ovarian Epithelial Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01486602 | Specialized Radiation Therapy and Chemotherapy in Treating Patients With Stage III Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00139633 | Selective Dose Escalation for Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01881230 | Evaluate Risk/Benefit of Nab Paclitaxel in Combination With Gemcitabine and Carboplatin Compared to Gemcitabine and Carboplatin in Triple Negative Metastatic Breast Cancer (or Metastatic Triple Negative Breast Cancer) | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT03136055 | Pembrolizumab-based Therapy in Previously Treated High Grade Neuroendocrine Carcinomas | neuroendocrine carcinoma | Paclitaxel (NPC208553) | |
| NCT00031577 | Paclitaxel Plus Radiation Therapy in Treating Children With Newly Diagnosed Brain Stem Glioma | Central Nervous System Neoplasm | Paclitaxel (NPC208553) | |
| NCT02924909 | Phase II Trial of Neoadjuvant Treatment and Minimal Invasive Surgery for Esophageal and GEJ Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT02125513 | Dose-Escalation Study of Intraperitoneal (IP) Cisplatin, IV/IP Paclitaxel, IV Bevacizumab, and Oral Olaparib for Newly Diagnosed Ovarian, Primary Peritoneal, and Fallopian Tube Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04336098 | Study of SRF617 in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00542191 | Phase II Trial of Neoadjuvant Metronomic Chemotherapy in Triple-Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00006245 | Flavopiridol and Paclitaxel in Treating Patients With Locally Advanced or Metastatic Esophageal Cancer That Has Not Responded to Previous Paclitaxel | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01924533 | Efficacy and Safety Study of Olaparib in Combination With Paclitaxel to Treat Advanced Gastric Cancer. | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00004160 | Chemotherapy Plus Radiation Therapy in Treating Patients With Stage III Non-small Cell Lung Cancer That Cannot Be Surgically Removed | lung cancer | Paclitaxel (NPC208553) | |
| NCT00028769 | S0032, Combination Chemotherapy Plus Hormone Therapy in Treating Patients With Metastatic Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00002928 | Paclitaxel in Treating Patients With Recurrent or Progressive Advanced Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT05204173 | Efficacy and Safety of Sintilimab Combined Intraperitoneal and Intravenous Paclitaxel Plus Oral S-1 in Gastric Cancer Patients With Peritoneal Metastasis | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00063999 | Doxorubicin Hydrochloride, Cisplatin, and Paclitaxel or Carboplatin and Paclitaxel in Treating Patients With Stage III-IV or Recurrent Endometrial Cancer | uterine neoplasm | Paclitaxel (NPC208553) | |
| NCT04005170 | Combination of Toripalimab and Chemoradiotherapy in Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00559845 | A Study of Avastin (Bevacizumab) in Patients With Inflammatory or Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03731442 | Salvage Chemoradiation Therapy for Recurrence After Radical Surgery or Palliative Surgery in Esophageal Cancer Patients | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT01770171 | Carboplatin-Paclitaxel ± Bevacizumab in Advanced (Stage III-IV) or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT01992341 | AMG 386 Drug-Drug Interaction Study With Paclitaxel | neoplasm | Paclitaxel (NPC208553) | |
| NCT03721744 | A Study of Napabucasin in Combination With Weekly Paclitaxel and Low-dose Gemcitabine in Patients With Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00085501 | S0342: Paclitaxel, Carboplatin, and Cetuximab in Treating Patients With Stage IIIB or Stage IV Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01493505 | EP-100 Plus Paclitaxel Versus Paclitaxel Alone in Patients With Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04251533 | A Study of Novel Anti-cancer Agents in Patients With Metastatic Triple Negative Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02044601 | Biomarker-Integrated Approach of Targeted Therapy for Lung Cancer Elimination Plus External Beam Radiation Therapy (BATTLE-XRT) | lung cancer | Paclitaxel (NPC208553) | |
| NCT00003397 | Peripheral Stem Cell Transplantation Plus Combination Chemotherapy and Monoclonal Antibody Therapy in Treating Patients With Non-Hodgkin's Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT03126812 | Ribociclib (Ribociclib (LEE-011)) With Platinum-based Chemotherapy in Recurrent Platinum Sensitive Ovarian Cancer | fallopian tube cancer | Paclitaxel (NPC208553) | |
| NCT01137994 | A Randomized Trial Comparing EC-wPversus EP-wP as Adjuvant Therapy for Operable Breast Cancer Patients Less Than 40 Years Old | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02083679 | Sym004 in Subjects With Stage IV Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03887442 | Paclitaxel vs Paclitaxel + Cetuximab in Recurrent - Metastatic Head & Neck Carcinoma After Failure of a 1º Chemotherapy | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT05222204 | DDP ip Combined With AG in PDAC With Peritoneal Metastasis | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00004887 | Paclitaxel and Carboplatin Chemotherapy Compared With Standard Chemotherapy in Treating Patients With Stage III or Stage IV Non-small Cell Lung Cancer That Cannot Be Removed During Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT02307227 | A Study to Evaluate the Potential Benefit of the Addition of BYL719 to Paclitaxel in the Treatment of Breast Cancer and Head-and-neck Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT03169790 | QUILT-3.052: NANT Non-Hodgkin Lymphoma (NHL) Vaccine: Combination Immunotherapy in Subjects With Relapsed CD20-positive NHL | non-Hodgkins lymphoma | Paclitaxel (NPC208553) | |
| NCT03940001 | A Study of Sintilimab Plus Chemoradiation Before Surgery for Esophageal Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00811993 | A Study of R1507 in Combination With Multiple Standard Chemotherapy Treatments in Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00006018 | Paclitaxel and BMS-214662 in Treating Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00983424 | Cyclosporine A in Combination With Nab-Paclitaxel in Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01081951 | Intraperitoneal vs Intravenous Chemotherapy Following Neoadjuvant Chemotherapy in Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00003622 | Vinorelbine Plus Paclitaxel in Treating Patients With Metastatic Prostate Cancer That Is Refractory to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT01730833 | Pertuzumab, Trastuzumab, and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With HER2-Positive Advanced Breast Cancer | inflammatory breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00009932 | Combination Chemotherapy in Treating Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01560104 | A Clinical Study Conducted in Multiple Centers Comparing Veliparib in Combination With Carboplatin and Paclitaxel Versus a Placebo in Combination With Carboplatin and Paclitaxel in Patients With Advanced Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02881125 | Paclitaxel and Nortriptyline Hydrochloride in Treating Patients With Relapsed Small Cell Carcinoma | small cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00097227 | Trial of Carboplatin/Paclitaxel/Cetuximab in Stage IIIB/IV Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03894540 | Dose Escalation and Dose Expansion Study of IPN60090 in Patients With Advanced Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT00756470 | Phase II Neoadjuvant in Inflammatory Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00081302 | Cetuximab, Paclitaxel, Carboplatin, and Radiation Therapy in Treating Patients With Unresectable Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00189566 | Taxol® in Monotherapy or in Combination With Topotecan or Carboplatin in Patients With Epithelial Ovarian Cancer in Early Relapse | fallopian tube cancer;ovarian cancer;peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT00732836 | Hepatic Arterial Infusion (HAI) of Abraxane | liver cancer | Paclitaxel (NPC208553) | |
| NCT00728845 | Hydroxychloroquine, Carboplatin, Paclitaxel, and Bevacizumab in Recurrent Advanced Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01325441 | A Study of BBI608 Administered With Paclitaxel in Adult Patients With Advanced Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT04902261 | Tislelizumab Combined With Nab-paclitaxel and Gemcitabine for Recurrent Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00570531 | Phase II Trial for Patients With Loco-Regional Esophageal Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03038100 | IGFBP-2 Vaccine and Combination Chemotherapy in Treating Patients With Stage III-IV Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Undergoing Surgery | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02985333 | Paclitaxel,Cyclophosphamide and Dexamethasone for Relapsed or Refractory Multiple Myeloma | multiple myeloma | Paclitaxel (NPC208553) | |
| NCT03691090 | Study of SHR-1210 in Combination With Chemotherapy in Advanced Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00318136 | A Study of Bevacizumab Plus Carboplatin and Paclitaxel in Subjects With Advanced, Previously Untreated, Squamous Non-Small Cell Lung Cancer (BRIDGE) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00022620 | Paclitaxel in Treating Patients With Refractory or Recurrent Endometrial Cancer | endometrial cancer | Paclitaxel (NPC208553) | |
| NCT02389751 | Ganetespib in Combination With Paclitaxel, Carboplatin, and Radiation Therapy in Treating Patients With Stage II-III Esophageal Cancer | esophageal carcinoma | Paclitaxel (NPC208553) | |
| NCT00093119 | Trial of ABI-007 in Previously Treated Patients With Metastatic Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00003206 | Carboplatin and Paclitaxel in Treating Patients With Locally Recurrent or Metastatic Nasopharyngeal Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00273507 | Neoadjuvant Chemotherapy in Non Small Cell Lung Cancer (NSCLC) Stages IB, IIA, IIB, and IIIA/T3 | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00024310 | Paclitaxel, Folic Acid, and Lometrexol in Treating Patients With Locally Advanced or Metastatic Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00522834 | Elesclomol (STA-4783) With Paclitaxel Versus Paclitaxel Alone in Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT02476955 | Veliparib With Carboplatin and Paclitaxel and as Continuation Maintenance Therapy in Adults With Newly Diagnosed Stage III or IV, High-grade Serous, Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02392637 | Gemcitabine Hydrochloride, Cisplatin, and Nab-Paclitaxel in Treating Patients With Advanced or Metastatic Biliary Cancers | gallbladder cancer;intrahepatic cholangiocarcinoma | Paclitaxel (NPC208553) | |
| NCT03944304 | Paclitaxel in Patients With GIST With Low P-glycoprotein Expression After Failure of at Least Imatinib and Sunitinib, and Regorafenib. | gastrointestinal stromal tumor | Paclitaxel (NPC208553) | |
| NCT02204345 | A Study Evaluating RO5479599 in Combination With Carboplatin and Paclitaxel in Participants With Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) of Squamous Histology | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004125 | Combination Chemotherapy in Treating Women With Stage II or Stage IIIA Breast Cancer That Has Spread to the Lymph Nodes | breast cancer | Paclitaxel (NPC208553) | |
| NCT00181701 | Intraperitoneal Paclitaxel and Carboplatin in the Treatment of Women With Carcinoma of Mullerian Origin | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00004924 | Paclitaxel and Irinotecan in Treating Patients With Advanced Non-small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01240655 | A Study of LCL161 in Combination With Weekly Paclitaxel in Adult Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02117024 | A Phase 2 Study of Viagenpumatucel-L (HS-110) in Patients With Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01174238 | A Two Arm Trial of Axitinib and Carboplatin/Paclitaxel in Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT00107094 | Study of Adriamycin Plus Cyclophosphamide Followed by Abraxane as Adjuvant Therapy for Patients With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003084 | Combination Chemotherapy With Ketoconazole in Treating Patients With Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT01739218 | A Phase I/II Trial of VB-111 and Paclitaxel for Recurrent Platinum-Resistant Müllerian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04035473 | A Study to Determine the Bioequivalence of Oraxol in Cancer Patients Treated With Intravenous Paclitaxel | neoplasm | Paclitaxel (NPC208553) | |
| NCT00136175 | Paclitaxel, Carboplatin and Gemcitabine in the Treatment of Patients With Advanced Transitional Cell Cancer of the Bladder | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT03278015 | Phase 2 Trial of Gemcitabine vs S-1 vs Gemcitabine Plus Nab-paclitaxel as Adjuvant Chemotherapy of Post-operative Pancreatic Cancer Patients | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00003555 | Paclitaxel Plus Chemoprotection With Amifostine in Treating Patients With Recurrent or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00768859 | Trastuzumab in a Neo-adjuvant Regimen for HER2+ Breast Cancer - the TRAIN Study | breast cancer | Paclitaxel (NPC208553) | |
| NCT01491217 | A Study of Oraxol® in Gastric Cancer Patients | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00948675 | Study of Participants With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02273713 | The Addition of Nab-paclitaxel (Abraxane) to First Line Treatment of Metastasized Oesophagogastric Carcinoma (ACTION) | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00194753 | Adjuvant Therapy for High-Risk Breast Cancer With Wkly Adriamycin & Oral Cytoxan With G-CSF for 12 Wks | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01383148 | Phase IIB/III Of TG4010 Immunotherapy In Patients With Stage IV Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00032032 | Combination Chemotherapy Plus Radiation Therapy in Treating Patients With Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00274456 | Phase II Trial Comparing ABI-007 (Abraxane®, Nab®-Paclitaxel) to Taxotere in First Line Therapy of Patients With Stage IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03556839 | Platinum Chemotherapy Plus Paclitaxel With Bevacizumab and Atezolizumab in Metastatic Carcinoma of the Cervix | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT00073723 | Trial of ABI-007 Administered Weekly in Chemotherapy Naive Patients With Advanced Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02492503 | Effectiveness of Chemotherapy in Metastatic or Recurrent Carcinoma Cervix | cervical carcinoma | Paclitaxel (NPC208553) | |
| NCT03475615 | A Study of Intraperitoneal Paclitaxel in Combination With SOX Compared With SOX Alone in Gastric Cancer With Malignant Ascites | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00002734 | Radiolabeled Monoclonal Antibody, Paclitaxel, and Interferon Alfa in Treating Patients With Recurrent Ovarian Cancer | ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01419548 | ABT-888, Carboplatin, and Paclitaxel for Cancer With Liver or Kidney Problems | neoplasm | Paclitaxel (NPC208553) | |
| NCT00603538 | Study of CP-751,871 in Combination With Carboplatin and Paclitaxel in Advanced Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00482391 | Doxorubicin and Cyclophosphamide Followed by Paclitaxel, Trastuzumab, and Lapatinib in Treating Patients With HER2/Neu-Overexpressed Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04644250 | Combination of Toripalimab and Neoadjuvant Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01264328 | Study of the Combination of Panitumumab With Paclitaxel as First-line Treatment of Subjects With Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00194779 | Combination Chemotherapy and Filgrastim Before Surgery in Treating Patients With HER2-Positive Breast Cancer That Can Be Removed By Surgery | breast cancer | Paclitaxel (NPC208553) | |
| NCT00003701 | Combination Chemotherapy in Treating Patients With Bladder Cancer | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT02182245 | Dose Escalation Study of BIBF 1120 in Patients With Advanced Gynaecological Malignancies | Genital neoplasm, female | Paclitaxel (NPC208553) | |
| NCT00344552 | Phase II Study of Weekly Paclitaxel (BMS-181339)in Patients With Advanced or Recurrent Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT05017012 | A Study to Evaluate the Bioavailability of Pembrolizumab (MK-3475) Via Subcutaneous (SC) Injection of MK-3475A (Pembrolizumab Formulated With MK-5180) In Advanced Solid Tumors (MK-3475A-C18) | neoplasm | Paclitaxel (NPC208553) | |
| NCT00558636 | A Trial Comparing Safety and Efficacy of Carboplatin and Paclitaxel Plus or Minus Sorafenib (BAY43-9006) in Chemonaive Patients With Stage IIIB-IV Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00024388 | Chemotherapy in Treating Patients With Metastatic Kidney Cancer | kidney cancer | Paclitaxel (NPC208553) | |
| NCT00054028 | Suramin and Paclitaxel in Treating Women With Stage IIIB-IV Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00473889 | A Clinical Trial of Vorinostat (MK0683, SAHA) in Combination With FDA Approved Cancer Drugs in Patients With Advanced Non-Small Cell Lung Cancer (NSCLC)(0683-056) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00002953 | Epirubicin and Cyclophosphamide Compared With Epirubicin and Paclitaxel in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04929041 | Testing the Addition of Radiation Therapy to the Usual Treatment (Immunotherapy With or Without Chemotherapy) for Stage IV Non-Small Cell Lung Cancer Patients Who Are PD-L1 Negative | lung adenocarcinoma;squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00061646 | Safety and Effectiveness of ABT-510 Plus Combination Chemotherapy in Subjects With Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02937272 | A Study of LY3200882 in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT03718624 | A Study of Intraperitoneal and Intravenous Paclitaxel Plus Apatinib and S-1 Conversion Therapy for Gastric Cancer With Positive Exfoliative Cancer Cells | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00199758 | Study of an Early Change of a Chemotherapeutic Doublet Versus Four Cycles of Chemotherapy in Advanced Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05446870 | Pembrolizumab With Chemotherapy and MK-4830 for Treating Participants With Ovarian Cancer (MK-4830-002) | ovarian carcinoma | Paclitaxel (NPC208553) | |
| NCT00900627 | Phase I/II AZD8931/Paclitaxel in Treatment of Advanced Solid Tumours (Phase I) and Advanced Breast Cancer (Phase II) | breast cancer | Paclitaxel (NPC208553) | |
| NCT00110695 | Therapy With Abraxane and 5-Fluorouracil, Epirubicin, Cyclophosphamide (FEC) for Patients With Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT02220894 | Study of Pembrolizumab (MK-3475) Versus Platinum-Based Chemotherapy for Participants With Programmed Cell Death-Ligand 1 (PD-L1)-Positive Advanced or Metastatic Non-Small Cell Lung Cancer (MK-3475-042/KEYNOTE-042) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT04483076 | Oxaliplatin Combined With S-1(SOX) Neoadjuvant Chemotherapy for Different Cycles in Patients With Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT01340131 | CKD-828 (40/5mg) Pharmacokinetic Study | hypertension | Paclitaxel (NPC208553) | |
| NCT00933426 | Lenalidomide and Paclitaxel in Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT03193918 | Study of Crenolanib With Ramucirumab and Paclitaxel for Advanced Esophagogastric Adenocarcinoma | gastric adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02050009 | Metformin Hydrochloride, Carboplatin, and Paclitaxel in Treating Patients With Recurrent Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | ovarian serous cystadenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01200342 | Genasense, Carboplatin, Paclitaxel (GCP) Combination in Uveal Melanoma | melanoma | Paclitaxel (NPC208553) | |
| NCT02607202 | A Study of Nab-paclitaxel-based Doublet as First Line Therapy in Patients With Cancer of Unknown Primary (CUP) | neoplasm | Paclitaxel (NPC208553) | |
| NCT02059967 | Phase I IGART Study Using Active Breathing Control and Simultaneous Boost for Patients With NSCLC | lung adenocarcinoma;squamous cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00009828 | Paclitaxel Combined With Fluorouracil-Uracil and Leucovorin in Treating Patients With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01494688 | A Study of RO5509554 as Monotherapy and in Combination With Paclitaxel in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04825938 | Neoadjuvant Toripalimab in Combination With Carboplatin and Nab-paclitaxel in Untreated Salivary Gland Malignant Neoplasms | salivary gland neoplasm | Paclitaxel (NPC208553) | |
| NCT05185869 | SHR6390 Plus Nab-paclitaxel and Gemcitabine in Advanced/Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00849472 | Phase II Lapatinib Plus Nab-Paclitaxel As First And Second Line Therapy In her2+ MBC | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01581476 | Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial | type 1 diabetes mellitus | Paclitaxel (NPC208553) | |
| NCT04027764 | Toripalimab Combined With S1 and Albumin Paclitaxel in Patients With Advanced Biliary Tract Cancer | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT04390958 | Neoadjuvant Chemotherapy With Nab-paclitaxel Plus Cisplatin and Capecitabine for Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02302807 | A Study of Atezolizumab Compared With Chemotherapy in Participants With Locally Advanced or Metastatic Urothelial Bladder Cancer [IMvigor211] | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT01411410 | Phase I Study of PI3(Phosphoinositol 3)-Kinase Inhibitor BAY80-6946 With Paclitaxel in Patients With Advanced Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT04563975 | Efficacy and Safety of Toripalimab Combined With Docetaxel or Nab-paclitaxel in Patients With Advanced Gastric Cancer | gastric cancer | Paclitaxel (NPC208553) | |
| NCT03026881 | A Study of Fluzoparib Given in Combination With Apatinib and Paclitaxel in Gastric Cancer Patients | gastric cancer | Paclitaxel (NPC208553) | |
| NCT05203913 | Cisplatin, Nab-paclitaxel, Nivolumab With Radiotherapy After Resection of Non-Metastatic Muscle Invasive Bladder Cancer | urinary bladder carcinoma | Paclitaxel (NPC208553) | |
| NCT01447706 | Pazopanib Hydrochloride, Paclitaxel, and Carboplatin in Treating Patients With Refractory or Resistant Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Peritoneal Cancer | fallopian tube cancer;ovarian cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT01704287 | Study of Pembrolizumab (MK-3475) Versus Chemotherapy in Participants With Advanced Melanoma (MK-3475-002/P08719/KEYNOTE-002) | melanoma | Paclitaxel (NPC208553) | |
| NCT01003158 | Study Assessing Safety and Tolerability of AZD8931 Alone or in Combination With Paclitaxel in Japanese Patients. | breast cancer | Paclitaxel (NPC208553) | |
| NCT01507428 | Study of Positron Emission Tomography and Computed Tomography in Guiding Radiation Therapy in Patients With Stage III Non-small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00850577 | Ph II of a Novel Anti-angiogenic Agent in Combination With Chemotherapy for the Treatment of Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT01297452 | BKM120 + Carboplatin + Paclitaxel for Patients With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT02551432 | Pembrolizumab and Paclitaxel in Refractory Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03946969 | Sintilimab in Combination With Chemotherapy in Neoadjuvant Treatment of Potentially Resectable Esophageal Cancer | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00005021 | Combination Chemotherapy With or Without Biological Therapy in Treating Patients With Refractory Solid Tumor or Lymphoma | lymphoma | Paclitaxel (NPC208553) | |
| NCT01402271 | Alisertib (MLN8237) in Participants With Ovarian, Fallopian Tube or Peritoneal Cancer Preceded by Phase 1 Study of MLN8237 Plus Paclitaxel Treatment of Ovary or Breast Cancer | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00818090 | Paclitaxel and Cisplatin for Thymic Neoplasm | Thymic Carcinoma;Thymoma | Paclitaxel (NPC208553) | |
| NCT03852979 | Neo-Adjuvant Chemotherapy and Conservative Surgery in Cervical Cancer to Preserve Fertility | cervical cancer | Paclitaxel (NPC208553) | |
| NCT03490292 | Avelumab With Chemoradiation for Stage II/III Resectable Esophageal and Gastroesophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00750386 | An Efficacy and Safety Study of MORAb-003 in Platinum-Resistant or Refractory Relapsed Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00075270 | Paclitaxel With / Without GW572016 (Lapatinib) As First Line Therapy For Women With Advanced Or Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00146549 | Trastuzumab in Combination With Vinorelbine or Taxane-Based Chemotherapy in Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02159820 | Intraperitoneal tgDCC-E1 and Intravenous Paclitaxel in Women With Platinum-Resistant Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00298415 | Chemotherapy of Elderly Patients With Non-Small-Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02050178 | Dose Escalation Study of OMP-54F28 in Combination With Nab-Paclitaxel and Gemcitabine in Patients With Previously Untreated Stage IV Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00226746 | Efficacy Study of Gemcitabine, Paclitaxel, and Irradiation in the Treatment of Pancreatic Cancer | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00002627 | Addition of Paclitaxel to High-Dose Combination Chemotherapy in Treating Patients With High-Risk Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04176952 | PRIMUS002: Looking at 2 Neo-adjuvant Treatment Regimens for Resectable and Borderline Resectable Pancreatic Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00014573 | Chemotherapy and Vaccine Therapy Followed by Bone Marrow or Peripheral Stem Cell Transplantation and Interleukin-2 in Treating Patients With Recurrent or Refractory Brain Cancer | Central Nervous System Neoplasm | Paclitaxel (NPC208553) | |
| NCT00617409 | To Immunize Patients With Extensive Stage SCLC Combined With Chemo With or Without All Trans Retinoic Acid | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003644 | Carboplatin Plus Paclitaxel With or Without Continued Low-Dose Paclitaxel in Treating Patients With Early-Stage Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00933374 | Trial to Evaluate Paclitaxel Plus RAD001 in Urothelial Carcinoma | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT00096343 | Paclitaxel and Carboplatin in Treating Women Who Are Undergoing Surgery for Newly Diagnosed, Locally Advanced Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00903942 | Abraxane and RT for Non-Small Cell Lung Cancer (NSCLC) | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT05322720 | HR Positive, HER2 Negative Advanced Breast Cancer With Progression After Endocrine Therapy | neoplasm | Paclitaxel (NPC208553) | |
| NCT01705483 | A Study of MK-8109 (Vintafolide) Given Alone or With Chemotherapy in Participants With Advanced Cancers (MK-8109-001) | cancer | Paclitaxel (NPC208553) | |
| NCT04698941 | Combined Simvastatin and Albumin Paclitaxel in Treating ES-SCLC Patients Relapsed From 1st Chemotherapy | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00544648 | Ph I/II Nab-Paclitaxel & Carboplatin w/Concurrent Radiation Therapy for Unresectable Stg III NSCLC | lung cancer | Paclitaxel (NPC208553) | |
| NCT02358161 | Phase I/II Study of LDE225 With Gemcitabine and Nab-paclitaxel in Patients With Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00004159 | Gemcitabine Plus Paclitaxel in Treating Patients With Advanced Non-small Cell Lung Cancer or Other Solid Tumor | lung cancer | Paclitaxel (NPC208553) | |
| NCT00079287 | Vinorelbine, Gemcitabine, and Docetaxel Compared With Paclitaxel and Carboplatin in Treating Patients With Advanced Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT05281003 | Pembrolizumab Plus Chemo in Neoadjuvant Treatment of Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00034151 | Safety and Efficacy of S-8184 in Second Line Treatment of Stage III or IV Ovarian Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00475670 | A Study of Herceptin (Trastuzumab) in Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT01091428 | Trial of Best Supportive Care and Either Cisplatin or Paclitaxel to Treat Patients With Primary Ovarian Cancer, Primary Peritoneal Cancer or Fallopian Tube Cancer and Inoperable Malignant Bowel Obstruction | fallopian tube cancer;peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00367471 | Lapatinib Combined With Paclitaxel For Patients With First-Line ErbB2-Amplified Metastatic Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT01593306 | Concurrent Chemoradiotherapy With Weekly Cisplatin Versus Concurrent Chemoradiotherapy With Weekly Cisplatin and Paclitaxel in Locally Advanced Carcinoma Cervix | carcinoma | Paclitaxel (NPC208553) | |
| NCT01978184 | Randomized Phase II Trial of Pre-Operative Gemcitabine and Nab Paclitacel With or With Out Hydroxychloroquine | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03834948 | AO-176 in Multiple Solid Tumor Malignancies | neoplasm | Paclitaxel (NPC208553) | |
| NCT00458315 | Cisplatin/Paclitaxel/Gemcitabine +/- Avastin in Patients With Unknown Primary Tumor | neoplasm | Paclitaxel (NPC208553) | |
| NCT00460317 | MONET1-MOtesanib NSCLC Efficacy and Tolerability Study | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00329641 | Sorafenib, Carboplatin, and Paclitaxel in Treating Patients With Stage IV Melanoma of the Eye | melanoma | Paclitaxel (NPC208553) | |
| NCT00002628 | Addition of Paclitaxel to High-Dose Combination Chemotherapy in Treating Women With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00033696 | Combination Chemotherapy and Radiation Therapy in Treating Patients With Limited-Stage Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT04568200 | Study of Anti-PD-L1 in Combination With Chemo(Radio)Therapy for Resectable Esophageal Squamous Cell Carcinoma | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT02317419 | Nab-Paclitaxel as Salvage Treatment in Locally Advanced or Metastatic Gastric Cancer | cancer | Paclitaxel (NPC208553) | |
| NCT05441254 | Camrelizumab Combined With Intraperitoneal Infusion of Nab-paclitaxel, Intravenous Chemotherapy and S-1 in the Treatment of Advanced Gastric Cancer With Peritoneal Metastasis:Single-arm, Prospective Clinical Study | gastric cancer | Paclitaxel (NPC208553) | |
| NCT02810418 | Mesothelin-Targeted Immunotoxin LMB-100 Alone or in Combination With Nab-Paclitaxel in People With Previously Treated Metastatic and/or Locally Advanced Pancreatic Ductal Adenocarcinoma and Mesothelin Expressing Solid Tumors | pancreatic neoplasm | Paclitaxel (NPC208553) | |
| NCT00268450 | Cisplatin, Bevacizumab, and Gemcitabine Followed by Surgery, Bevacizumab, and Paclitaxel in Treating Patients With Locally Advanced Nonmetastatic Bladder Cancer That Can Be Removed By Surgery | urinary bladder cancer | Paclitaxel (NPC208553) | |
| NCT05235542 | A Study to Investigate Safety and Tolerability of TransCon IL-2 β/γ Alone or in Combination With Pembrolizumab and/or Chemotherapy or TransCon TLR7/8 Agonist in Adult Participants With Locally Advanced or Metastatic Solid Tumor Malignancies | cancer | Paclitaxel (NPC208553) | |
| NCT04814485 | Efficacy and Safety of SHR-1020 Combined With Albumin-bound Paclitaxel in the Second-line Treatment of Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00005806 | Combination Chemotherapy in Treating Patients With Advanced Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00551733 | Carboplatin With Either Paclitaxel Poliglumex or Paclitaxel in Treating Women With Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT00295789 | Trial of TP Versus TC Regimens for Stage IVb, Persistent, or Recurrent Cervical Cancer (JCOG0505, CC-TPTC-P3) | cervical adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT01196741 | Neoadjuvant Therapy for Ovarian Cancer | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT03884101 | Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) Versus Chemotherapy for Endometrial Carcinoma (ENGOT-en9 / MK-7902-001) | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT04548440 | Efficacy and Safety of Preoperative Sintilimab Plus Nab-paclitaxel and Cisplatin in BR-ESCC Patients | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05491694 | To Evaluate the Efficacy of Toripalimab Combined With Chemotherapy After HIFU Induction in the Treatment of Early Triple-negative Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT02051751 | Paclitaxel and BKM120 Before Surgery in Treating Patients With Stage II or III Estrogen Receptor-Positive and HER2-Negative Breast Cancer | breast neoplasm | Paclitaxel (NPC208553) | |
| NCT00369551 | Bevacizumab, Paclitaxel, Carboplatin, and Radiation Therapy to the Chest in Treating Patients With Locally Advanced Non-Small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02186847 | Chemotherapy and Radiation Therapy With or Without Metformin Hydrochloride in Treating Patients With Stage III Non-small Cell Lung Cancer | bronchoalveolar adenocarcinoma;large cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT02279732 | Phase 3 Trial in Squamous Non Small Cell Lung Cancer Subjects Comparing Ipilimumab Plus Paclitaxel and Carboplatin Versus Placebo Plus Paclitaxel and Carboplatin | lung cancer | Paclitaxel (NPC208553) | |
| NCT01220154 | Neoadjuvant Chemotherapy IV Carboplatin With Weekly Paclitaxel Bevacizumab for Primary Ovarian | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT00276796 | Paclitaxel, Topotecan, and Cisplatin in Treating Patients With Advanced, Persistent, or Recurrent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT05042128 | The ASCEND Study: Gemcitabine and Nab-Paclitaxel With CEND-1 or Placebo in Patients With Untreated Metastatic Pancreatic Ductal Adenocarcinoma | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04540211 | A Study of Atezolizumab Plus Tiragolumab in Combination With Paclitaxel and Cisplatin Compared With Paclitaxel and Cisplatin as First-Line Treatment in Participants With Unresectable Locally Advanced, Unresectable Recurrent, or Metastatic Esophageal Carcinoma | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00201734 | Capecitabine, Carboplatin and Weekly Paclitaxel for Patients With Solid Tumors and Adenocarcinoma of Unknown Primary | adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT00191256 | Efficacy Trial of Gemcitabine Containing Regimens As Preoperative Chemotherapy in Non Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03085914 | A Study of Epacadostat in Combination With Pembrolizumab and Chemotherapy in Participants With Advanced or Metastatic Solid Tumors (ECHO-207/KEYNOTE-723) | neoplasm | Paclitaxel (NPC208553) | |
| NCT04475016 | TIP (Paclitaxel + Ifosfamide + Cisplatin) Combined With Nimotuzumab & Triprilimab as Neoadjuvant Treatment in Locally Advanced Penile Cancer | penile cancer | Paclitaxel (NPC208553) | |
| NCT00009750 | Monoclonal Antibody Therapy Plus Chemotherapy Followed by Peripheral Stem Cell Transplantation in Treating Patients With Metastatic Prostate Cancer That Has Not Responded to Hormone Therapy | prostate cancer | Paclitaxel (NPC208553) | |
| NCT00533949 | High-Dose or Standard-Dose Radiation Therapy and Chemotherapy With or Without Cetuximab in Treating Patients With Newly Diagnosed Stage III Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | lung cancer | Paclitaxel (NPC208553) | |
| NCT00997009 | Study of Adding Cetuximab to Chemotherapy for the Treatment of Advanced and/or Recurrent Cervical Cancer | cervical cancer | Paclitaxel (NPC208553) | |
| NCT01636622 | Study of Vemurafenib, Carboplatin, and Paclitaxel | cancer | Paclitaxel (NPC208553) | |
| NCT04428151 | Lenvatinib (E7080/MK-7902) in Combination With Pembrolizumab (MK-3475) vs. Standard Chemotherapy and Lenvatinib Monotherapy in Participants With Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma That Progressed After Platinum Therapy and Immunotherapy (MK-7902-009/E7080-G000-228/LEAP-009) | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05173987 | Study of Pembrolizumab (MK-3475) Versus Chemotherapy in Mismatch Repair Deficient (dMMR) Advanced or Recurrent Endometrial Carcinoma (MK-3475-C93/KEYNOTE-C93/GOG-3064/ENGOT-en15) | endometrial neoplasm | Paclitaxel (NPC208553) | |
| NCT00003013 | Chemotherapy Plus Surgery in Treating Women With Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00618826 | A Study of Biweekly Gemcitabine, Paclitaxel, and Avastin in Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00546156 | Preoperative Dose-dense Doxorubicin and Cyclophosphamide Followed by Paclitaxel With Bevacizumab in Operable Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT03853707 | Ipatasertib in Combination With Carboplatin, Carboplatin/Paclitaxel, or Capecitabine/Atezolizumab in Treating Patients With Metastatic Triple Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT00148681 | Preoperative Herceptin and Navelbine for Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00002852 | Surgery With or Without Chemotherapy in Treating Patients With Stage I Non-small Cell Lung Cancer | lung adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT04210037 | Study of APG-1252 Plus Paclitaxel in Patients With Relapsed/Refractory Small Cell Lung Cancer | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00580333 | Preoperative Cisplatin and Bevacizumab in ER-, PR-, HER2 Negative Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02226757 | Paclitaxel-trastuzumab in EGFR-mutated NSCLC Patients | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00004863 | Paclitaxel and GEM 231 in Treating Patients With Recurrent or Refractory Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT01493310 | Nab-paclitaxel (Abraxane) With or Without Mifepristone in Patients With Advanced Breast Cancer | male breast carcinoma | Paclitaxel (NPC208553) | |
| NCT03116152 | Study of IBI308 With Advanced/Metastatic Esophageal Squamous Cell Carcinoma After Failure of First-line Treatment | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT05312372 | S095033 in Combination With Paclitaxel as 2nd- or 3rd-line Treatment in Participants With Advanced or Metastatic ESCC | esophageal squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT01862328 | Dose Escalation, Multi-arm Study of MLN4924 Plus Docetaxel, Gemcitabine, or Combination of Carboplatin and Paclitaxel in Participants With Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT04247126 | A Study of SY 5609, a Selective CDK7 Inhibitor, in Advanced Solid Tumors | small cell lung carcinoma;pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT01014351 | Study of Everolimus With Paclitaxel and Carboplatin in Patients With Metastatic Melanoma | metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT00031876 | Capecitabine and Paclitaxel in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02272738 | A Study of Chemoradiotherapy Using Gem Plus Nab-paclitaxel for Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT00189553 | Caelyx Plus Carboplatin Versus Paclitaxel Plus Carboplatin in Patients With Epithelial Ovarian Cancer in Late Relapse | fallopian tube cancer;ovarian cancer | Paclitaxel (NPC208553) | |
| NCT02723331 | Perioperative Therapy for Resectable and Borderline-Resectable Pancreatic Adenocarcinoma With Molecular Correlates | pancreatic ductal adenocarcinoma | Paclitaxel (NPC208553) | |
| NCT02124707 | Weekly Carboplatin, Paclitaxel and Cetuximab Treatment for Patients With Recurrent or Metastatic SCCHN | head and neck squamous cell carcinoma | Paclitaxel (NPC208553) | |
| NCT00003326 | Paclitaxel in Treating Patients With Metastatic, Recurrent, or Unresectable Cancer of the Esophagus | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT00404404 | ABI-007 and Bevacizumab in Treating Women With Recurrent or Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT04711161 | First-in-Human Evaluation of GRN-300 in Subjects With Recurrent Ovarian, Primary Peritoneal, and Fallopian Tube Cancers. | ovarian neoplasm | Paclitaxel (NPC208553) | |
| NCT00527735 | Phase II Study for Previously Untreated Subjects With Non Small Cell Lung Cancer (NSCLC) or Small Cell Lung Cancer (SCLC) | small cell lung carcinoma;non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00662129 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation, Gemcitabine, and Bevacizumab in Treating Patients With Metastatic Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT00733408 | Nab-Paclitaxel and Bevacizumab Followed By Bevacizumab and Erlotinib in Metastatic Breast Cancer | triple-negative breast cancer | Paclitaxel (NPC208553) | |
| NCT02761694 | Absorption, Metabolism, Excretion and Pharmacokinetics of a Single Dose [14C]AZD2014 Followed by a Multiple Dose Phase | cancer | Paclitaxel (NPC208553) | |
| NCT01126216 | Reduced Radiotherapy With Pac/Cis vs Standard Radiotherapy With 5-FU/Cis in Locally Advanced Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT01519804 | A Study of Onartuzumab (MetMAb) Versus Placebo in Combination With Paclitaxel Plus Platinum in Patients With Squamous Non-Small Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00003402 | Peripheral Stem Cell Transplantation Plus Combination Chemotherapy in Treating Patients With Low-Grade Non-Hodgkin's Lymphoma or Chronic Lymphocytic Leukemia | leukemia;lymphoma | Paclitaxel (NPC208553) | |
| NCT00063258 | Tarceva Surgery for Resectable Stage IIIA(N2) and IIIB (T4 N2) Non-Small-Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT01435018 | Three Chemo Regimens as an Adjunct to ART for Treatment of Advanced AIDS-KS | HIV-1 infection | Paclitaxel (NPC208553) | |
| NCT04148885 | A Trial of Paclitaxel (Albumin-binding) for Castration-resistant Prostate Cancer | prostate cancer | Paclitaxel (NPC208553) | |
| NCT02038647 | Phase 2 Study of Alisertib (MLN8237) in Combination With Paclitaxel Versus Placebo in Combination With Paclitaxel as Second Line Therapy for Small Cell Lung Cancer (SCLC) | small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT00002568 | Combination Chemotherapy With or Without Surgery in Treating Patients With Stage III Ovarian Epithelial Cancer | ovarian cancer | Paclitaxel (NPC208553) | |
| NCT04155671 | POF Versus FOLFOX Plus IP Paclitaxel in AGC | gastric cancer | Paclitaxel (NPC208553) | |
| NCT00100789 | S0329, Gemcitabine and Paclitaxel in Treating Patients With Persistent, Recurrent, or Metastatic Head and Neck Cancer | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT02739529 | Study to Evaluate the Efficacy and Safety of Genexol-PM Once a Week for Gynecologic Cancer | female reproductive system neoplasm | Paclitaxel (NPC208553) | |
| NCT03464734 | Pembrolizumab and Nab Paclitaxel in Patients With Metastatic Urothelial Carcinoma | urothelial carcinoma | Paclitaxel (NPC208553) | |
| NCT03330106 | A Study to Evaluate the Effects of Pevonedistat on the Corrected QT (QTc) Interval in Participants With Advanced Solid Tumors | neoplasm | Paclitaxel (NPC208553) | |
| NCT00049790 | Safety and Efficacy Study of rhAngiostatin Administered in Combination With Paclitaxel and Carboplatin to Patients With Non-Small-Cell Lung Cancer | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03649321 | Clinical Trial of Chemotherapy and Bemcentinib for Metastatic Pancreatic Cancer | pancreatic carcinoma | Paclitaxel (NPC208553) | |
| NCT03008512 | A Phase II Study of Weekly Genexol-PM in Patients With Hepatocelluar Carcinoma After Failure of Sorafenib | hepatocellular carcinoma | Paclitaxel (NPC208553) | |
| NCT01463072 | Nab-Paclitaxel in Treating Older Patients With Locally Advanced or Metastatic Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT02689427 | Enzalutamide and Paclitaxel Before Surgery in Treating Patients With Stage I-III Androgen Receptor-Positive Triple-Negative Breast Cancer | breast carcinoma | Paclitaxel (NPC208553) | |
| NCT01933061 | Study to Determine Efficacy and Safety of CC-486 With Nab-Paclitaxel Versus Nab-Paclitaxel in Patients With Chemotherapy naïve Metastatic Melanoma | metastatic melanoma | Paclitaxel (NPC208553) | |
| NCT03328494 | Trial of ZW25 (Zanidatamab) in Patients With Advanced HER2-expressing Cancers | cancer | Paclitaxel (NPC208553) | |
| NCT03544567 | A Study of Oraxol in Subjects With Cutaneous Angiosarcoma | angiosarcoma | Paclitaxel (NPC208553) | |
| NCT03740165 | Study of Chemotherapy With Pembrolizumab (MK-3475) Followed by Maintenance With Olaparib (MK-7339) for the First-Line Treatment of Women With BRCA Non-mutated Advanced Epithelial Ovarian Cancer (EOC) (MK-7339-001/KEYLYNK-001/ENGOT-ov43/GOG-3036) | peritoneal neoplasm | Paclitaxel (NPC208553) | |
| NCT03830606 | The Efficacy and Safety of Nab-paclitaxel Plus S-1 in First-line Treatment of Advanced Biliary Tract Adenocarcinoma | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT04492033 | A Study of CTX-009 (ABL001) in Combination With Irinotecan or Paclitaxel in Advanced or Metastatic Solid Tumor Patients | biliary tract cancer | Paclitaxel (NPC208553) | |
| NCT00496028 | Phase I Study in Patients With Solid Tumours | neoplasm | Paclitaxel (NPC208553) | |
| NCT01107626 | Bevacizumab or Pemetrexed Disodium Alone or In Combination After Induction Therapy in Treating Patients With Advanced Non-Squamous Non-Small Cell Lung Cancer | lung cancer | Paclitaxel (NPC208553) | |
| NCT03126812 | Pembrolizumab, Paclitaxel, and Carboplatin in Patients With Advanced Stage Epithelial Ovarian Cancer (EOC). | peritoneum cancer | Paclitaxel (NPC208553) | |
| NCT00154700 | Twice Weekly TP-HDFL for Recurrent or Metastatic Esophageal Cancer | esophageal cancer | Paclitaxel (NPC208553) | |
| NCT03047265 | Concurrent Chemoradiotherapy in Patients With Locoregionally Advanced Nasopharyngeal Carcinoma | nasopharyngeal neoplasm | Paclitaxel (NPC208553) | |
| NCT02064491 | Erlotinib Treatment Beyond Progression in EGFR Mutant NSCLC | non-small cell lung carcinoma | Paclitaxel (NPC208553) | |
| NCT03143491 | Study of SOR007 Ointment for Cervical Intraepithelial Neoplasia (CIN) | cervical intraepithelial neoplasia | Paclitaxel (NPC208553) | |
| NCT00016874 | 3-AP Plus Cisplatin and Paclitaxel in Treating Patients With Advanced or Metastatic Cancer | neoplasm | Paclitaxel (NPC208553) | |
| NCT00997906 | Cisplatin and RT With or Without Gemcitabine, Carboplatin, and Paclitaxel in Treating Patients With Locally Advanced NPC | head and neck malignant neoplasia | Paclitaxel (NPC208553) | |
| NCT00072319 | Neoadjuvant or Adjuvant Epirubicin, Cyclophosphamide, and Paclitaxel in Treating Women With Stage I, Stage II, or Stage III Breast Cancer | breast cancer | Paclitaxel (NPC208553) | |
| NCT02448329 | Study of AZD1775 in Combination With Paclitaxel, in Advanced Gastric Adenocarcinoma Patients Harboring TP53 Mutation as a Second-line Chemotherapy | gastric adenocarcinoma | Paclitaxel (NPC208553) |
❱❱❱ Associated Human Diseases and Detailed Association Evidence
How do we define the Plant-Targeted Human Disease Association?
Associated human diseases of an individual plant are summurized based on FOUR types of association evidence, these include:
❶ Association by Therapeutic Target: Bioactive protein targets of the plant were defined in "Molecular Targets" section, target-disease associations collected from TTD database were subsequently used to build the associations between the plant and its targeted human diseases.
❷ Association by Disease Gene Reversion: Plant and a specific disease will be associated when >= 1 plant target gene overlaped with disease's DEGs.
❸ Association by Clinical Trials of Plant: Plant and a specific disease will be associated when >= 1 clinical trial (the plant is the intervetion) can be matched in ClinicalTrials.gov database.
❹ Association by Clinical Trials of Plant Ingredients: Plant and a specific disease will be associated when >= 1 clinical trial (the plant ingredient is the intervetion) can be matched in ClinicalTrials.gov database.
Associated Disease of the Plant |
Association Type & Detailed Evidence |
|---|---|
Pleomorphic sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B54 |
MTOR
NCT03678883,NCT00020449,NCT00003624,NCT00003128,NCT00003054,NCT00003008,NCT00217607,NCT00112489 |
Endometrioid adenocarcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0SD2 |
NCT00079430,NCT00002913,NCT00262847,NCT00466960,NCT00989651,NCT00085358,NCT00108745,NCT02713386,NCT01167712
|
Malignant neoplasms of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72 |
NCT04342910,NCT01063517,NCT03751761,NCT01746771,NCT02229058,NCT03322969,NCT01224652,NCT01980810,NCT00003298,NCT02038621,NCT04286711,NCT03977220,NCT05025033,NCT05319639,NCT01457846,NCT04155671,NCT03704077,NCT05052931,NCT04149015,NCT03718624,NCT01283204,NCT03801668,NCT05002686,NCT02574663,NCT03475615,NCT05171530,NCT02931890,NCT04781413,NCT04258657,NCT04483076,NCT01839773,NCT04602117,NCT02072317,NCT03026881,NCT04308837,NCT05190445,NCT04694183,NCT04135781,NCT05410847,NCT05441254,NCT04888663,NCT02486601,NCT01170663,NCT04001569,NCT02934464,NCT02855788,NCT02845908,NCT01471132,NCT01739894,NCT05095467,NCT02442362,NCT05204173,NCT03766607,NCT04258644,NCT01136031,NCT04683939,NCT04267549,NCT04195828,NCT01491217,NCT03263741,NCT02890511,NCT04563975,NCT00209612,NCT00003862,NCT01183559,NCT03579784,NCT03428425,NCT05400915,NCT05070598,NCT04592211,NCT02451956,NCT01249443,NCT04943653,NCT05427383,NCT04835896,NCT00154726,NCT01641939,NCT01924533,NCT04182724,NCT01248403,NCT01015339,NCT03286244,NCT00661167,NCT04526470,NCT02697838
|
Esophageal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B70 |
NCT04879368,NCT03691090,NCT02389751,NCT02569242,NCT04426955,NCT00139633,NCT03281369,NCT00570531,NCT02861690,NCT02858206,NCT02598687,NCT04460066,NCT03087864,NCT03279237,NCT02279134,NCT01183559,NCT02891083,NCT03308552,NCT03490292,NCT05013697,NCT00021320,NCT04821765,NCT02273713,NCT00971841,NCT02429622,NCT02574663,NCT04540211,NCT00016900,NCT00281788,NCT02644863,NCT02735239,NCT03731442,NCT03165994,NCT04046575,NCT02446574,NCT01704690,NCT05357846,NCT00655876,NCT01843829,NCT00107341,NCT02924909,NCT00154804,NCT02317991,NCT04005170,NCT01372202,NCT02133612,NCT02461043,NCT00815308,NCT00344552,NCT02741856,NCT00003087,NCT00002711,NCT00230451,NCT00066716,NCT02297217,NCT00453323,NCT00006472,NCT00493025,NCT05174156,NCT00003326,NCT01444547,NCT02570893,NCT04006041,NCT00069953,NCT00730353,NCT01249443,NCT00686114,NCT00006245,NCT02607982,NCT00193141,NCT01034189,NCT04278287,NCT01998347,NCT03126708,NCT00002984,NCT02762474,NCT00154700,NCT03328234,NCT00393068,NCT00008047
|
Squamous cell carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B60-2D01 |
NCT01249443,NCT03748134,NCT01822613,NCT04568200,NCT01591135,NCT04943445,NCT05322499,NCT02033538,NCT03292822,NCT04084158,NCT01258192,NCT03278626,NCT03964753,NCT00596830,NCT04844385,NCT00816634,NCT04804696,NCT04138212,NCT03719924,NCT00042835,NCT03603756,NCT03957590,NCT02027428,NCT04764227,NCT03116152,NCT01688700,NCT04225364,NCT04644250,NCT04354961,NCT03783442,NCT04460352,NCT05007145,NCT03762564,NCT04767295,NCT02784288,NCT01166542,NCT02272699,NCT04937673,NCT05125055,NCT05394415,NCT04548440,NCT00001442,NCT05189730,NCT04949256,NCT00998192,NCT02677597,NCT05342636,NCT02059967,NCT05312372,NCT02350517,NCT03946969,NCT01225523,NCT03940001,NCT01386385,NCT04888403,NCT00176267,NCT02916511,NCT04390958,NCT05319730,NCT03430843,NCT04063683,NCT01752205,NCT02459457,NCT03866993,NCT00176254,NCT04807673,NCT02976909,NCT01969955,NCT02611700,NCT00753038,NCT04506138,NCT03975270,NCT03387111,NCT05449483,NCT05281003,NCT04718415,NCT04929041,NCT05213312,NCT04625543,NCT04472429
|
AngiosarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B56 |
NCT02212015,NCT01303497,NCT01042379,NCT04339738,NCT01055028,NCT03512834,NCT03544567,NCT04859465
|
Multiple myelomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A83 |
MTOR
NCT01646762,NCT02985333,NCT00003401,NCT00003399,NCT01889420,NCT02075021 |
Colorectal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B91 |
ITGAV
NCT00625573,NCT03169777,NCT00024401,NCT02103062,NCT02574663,NCT01730586 |
Malignant neoplasms of cervix uteri, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C77.Z |
NCT05290935,NCT02312804,NCT02595554,NCT02492503,NCT03556839,NCT04652076,NCT04188860
|
Urethral cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C93 |
NCT00022633,NCT00003376,NCT04579224,NCT00478361,NCT00005644,NCT00022191,NCT00045630
|
Biliary tract cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C17 |
NCT05170438,NCT04027764,NCT03830606,NCT04492033,NCT04692051,NCT02632305,NCT04004234
|
Adenocarcinoma of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.0 |
LMNA,PMP22,F2,NPC1,TUBB6,SLCO1B3,ITGAV
|
Fallopian tube cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C74 |
NCT00226915,NCT01402271,NCT03462212,NCT00028743,NCT03029611,NCT00003120,NCT00517621,NCT01091428,NCT00520013,NCT01146795,NCT00004934,NCT03038100,NCT00008138,NCT00003624,NCT00091377,NCT01570582,NCT02050009,NCT00189566,NCT00373217,NCT00075712,NCT00652119,NCT03740165,NCT02713386,NCT00052468,NCT00993655,NCT00672295,NCT01219777,NCT00408070,NCT02121990,NCT01220154,NCT03393884,NCT02520154,NCT00003064,NCT00003944,NCT00331422,NCT00003385,NCT00550784,NCT00466960,NCT02012192,NCT03056833,NCT00003896,NCT00479817,NCT00006454,NCT01253681,NCT00838656,NCT01204749,NCT03635489,NCT00466986,NCT00702299,NCT00085358,NCT01083537,NCT01447706,NCT01196741,NCT01649336,NCT00079430,NCT01493505,NCT00003160,NCT03126812,NCT00031954,NCT02834975,NCT00189553,NCT00483782
|
Adenocarcinoma, endocervical typeDisease Category: X.Extension CodesDisease ICD-11 Code: XH0GS9 |
NCT02020707,NCT01755897,NCT04723875,NCT00064077,NCT04516616,NCT00295789
|
GerminomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH1E13 |
NCT01873326,NCT02414685,NCT04581265,NCT00864318,NCT00611962,NCT05455918
|
Neoplasms of unknown behaviour of urinary organsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F98 |
NCT05203913,NCT02560038,NCT00478361,NCT05024773,NCT04876313,NCT00005831
|
Thyroid cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D10 |
NCT03085056,NCT00507429,NCT02152137,NCT01701349,NCT00603941,NCT00786552
|
Uterine ligament/parametrium/uterine adnexa neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C72 |
NCT00231868,NCT00584909,NCT00584857,NCT01650376,NCT00147680,NCT00506779
|
Urogenital cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C95-2C9Z |
NCT02256436,NCT02033993,NCT04527991,NCT00034177,NCT02756013,NCT00151034
|
Transitional cell carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH8EH1 |
NCT03197571,NCT04603846,NCT03464734,NCT03871036,NCT03240016,NCT04211012
|
Diffuse large B-cell lymphomasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A81 |
LMNA,GMNN,THRB,PMP22,TUBB6,ITGAV
|
AdenocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D40 |
NCT05257993,NCT04935359,NCT03915444,NCT03517176,NCT02723331,NCT02186847,NCT03345810,NCT03908333,NCT05254171,NCT04257448,NCT01578551,NCT03496662,NCT00368992,NCT04481204,NCT00040794,NCT02993731,NCT00384826,NCT01253525,NCT02468557,NCT03697239,NCT01676259,NCT04390763,NCT03410030,NCT01218516,NCT04046887,NCT02754726,NCT04821284,NCT02101021,NCT04581343,NCT04134468,NCT02926183,NCT02715804,NCT00005838,NCT02243007,NCT00369551,NCT02583477,NCT02487277,NCT02048943,NCT04827953,NCT01506973,NCT04929041,NCT00201734,NCT03361319,NCT02047513,NCT04634539,NCT02501902,NCT02426281,NCT00226746,NCT00002852,NCT04469556,NCT04329949,NCT02059967,NCT02303977,NCT00042835,NCT00955305,NCT01431794,NCT02732938,NCT00021060,NCT05042128
|
Neoplasms of unknown behaviour of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F95 |
NCT03705429,NCT03997123,NCT02622074,NCT02776917,NCT00046514,NCT01026116,NCT02051751,NCT00201435,NCT01271725,NCT00075270,NCT02455141,NCT00194753,NCT00174434,NCT03396445,NCT00709761,NCT03036488,NCT01220128,NCT00388076,NCT00373256,NCT00281658,NCT00048893,NCT00272987,NCT00251095,NCT01779050,NCT00550771,NCT01953536,NCT00448305,NCT00524303,NCT01137994,NCT00356811,NCT04251533,NCT04895358,NCT00110695,NCT01205217,NCT05067530,NCT01138046,NCT02162719,NCT03742102,NCT00191672,NCT00353717,NCT00111787,NCT00367471,NCT00553358,NCT00849472,NCT00046527,NCT01572038,NCT00429299,NCT05244993,NCT03678883,NCT01953445,NCT03272477,NCT03273595,NCT02307227,NCT00246571
|
Unspecified malignant neoplasms of unspecified sitesDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D4Z |
NCT01113476,NCT01376310,NCT01325441,NCT01497470,NCT01688791,NCT02640755,NCT01204996,NCT01604863,NCT00002854,NCT01351350,NCT01110603,NCT00337376,NCT01192165,NCT02251951,NCT00881166,NCT02366949,NCT03498521,NCT02240212,NCT02412722,NCT05235542,NCT00581971,NCT01705483,NCT01057264,NCT05081609,NCT01653470,NCT00508326,NCT01301716,NCT02327169,NCT01636622,NCT02761694,NCT02193633,NCT01633970,NCT00473720,NCT00798252,NCT04196283,NCT00954642,NCT03507491,NCT01579578,NCT00358163,NCT04632992,NCT02630199,NCT01235897,NCT01374620,NCT02317419,NCT03328494,NCT02383212,NCT02483247,NCT02892123,NCT01187199,NCT02138812,NCT00113438,NCT01015222,NCT01075464
|
Malignant neoplasms of retroperitoneumDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C50 |
NCT00028743,NCT02020707,NCT00003896,NCT00003998,NCT02834975,NCT00075712,NCT00003880,NCT00003160,NCT00838656,NCT00005026,NCT00002894,NCT01091428,NCT00008138,NCT00672295,NCT00003624,NCT00003322,NCT00031954,NCT00091377,NCT01821859,NCT00079430,NCT00005046,NCT00003064,NCT00702299,NCT00408070,NCT00085358,NCT01493505,NCT00017303,NCT00004934,NCT00003385,NCT00002734,NCT00825201,NCT00466960,NCT03126812,NCT00003944,NCT00002717,NCT00023907,NCT00084448,NCT00483782,NCT00652119,NCT00373217,NCT00003378,NCT00993655,NCT01402271,NCT01447706,NCT02050009,NCT01649336,NCT00491855,NCT00331422,NCT00550784,NCT02272790,NCT00003120,NCT01083537
|
Cervical cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C77 |
NCT04864782,NCT02036164,NCT00003065,NCT01487226,NCT04799639,NCT00003379,NCT00002949,NCT05247619,NCT01405235,NCT03852979,NCT04341883,NCT03635567,NCT00276796,NCT00003624,NCT00003377,NCT00980954,NCT00138151,NCT01755845,NCT02432365,NCT01756170,NCT00997009,NCT04016142,NCT05179239,NCT00626561,NCT01566240,NCT04150575,NCT05367206,NCT04974944,NCT04884906,NCT01594099,NCT02039791,NCT04868708,NCT00823186,NCT02467907,NCT04238988,NCT00340184,NCT00462397,NCT04551950,NCT02422563,NCT02446652,NCT01229930,NCT04731038,NCT03367871,NCT01667211,NCT03912415,NCT04806945,NCT04982237,NCT03912402,NCT00057928,NCT04973904,NCT03534713
|
Head and neck cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D42 |
MTOR
NCT00003251,NCT00176241,NCT00833261,NCT00002922,NCT00006248,NCT00003592,NCT00301028,NCT04338399,NCT00855764,NCT00002888,NCT00003193,NCT00003327,NCT01126216,NCT01264328,NCT00337532,NCT00392704,NCT00002632,NCT00083057,NCT00319839,NCT00089297,NCT00025298,NCT00100789,NCT00004089,NCT00971867,NCT04883281,NCT00003206,NCT00004097,NCT00959387,NCT01024101,NCT00005087,NCT00011999,NCT00113399,NCT00004865,NCT00014118,NCT02013453,NCT00851877,NCT00022217,NCT00731380,NCT00343083,NCT00566540,NCT00005647,NCT00004094,NCT00270790,NCT01333085,NCT01911598,NCT00997906,NCT03887442,NCT01412229,NCT01084083 |
Malignant neoplasms of corpus uteriDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76 |
NCT01970722,NCT02112552,NCT02684227,NCT00505492,NCT00063999
|
Malignant lymphoma, not elsewhere classifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B33.5 |
NCT00038545,NCT03013218,NCT00088530,NCT03169790,NCT01555853
|
Testicular cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C80 |
NCT00231582,NCT00072215,NCT00004077,NCT00104676,NCT00531687
|
CholangiocarcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH7M15 |
NCT01963325,NCT03943043,NCT02181634,NCT04077983,NCT04683939
|
Malignant neoplasms of stomach, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.Z |
NCT02163291,NCT03081143,NCT00881816,NCT00639522,NCT04714190
|
ovarian cystadenocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.Y |
NCT00079430,NCT02050009,NCT00002913,NCT00466960,NCT00085358
|
Serous cystadenoma,borderline malignancy of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.4 |
GMNN,TUBB3,THRB,SLCO1B3,CYP1A2
|
Malignant neoplasms of biliary tract, distal bile ductDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C15 |
LMNA,GMNN,PMP22,TUBB6,ITGAV
|
Superficial ovarian endometriosisDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA10.B4 |
LMNA,TUBB3,RAB9A,PMP22,TUBB6
|
Glioblastoma of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00.00 |
LMNA,GMNN,RAB9A,TUBB6,ITGAV
|
Germ cell tumour of testisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C80.2 |
GMNN,THRB,RAB9A,F2,SLCO1B1
|
Pulmonary hypertensionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BB01 |
MTOR
NCT01246193,NCT01352689,NCT01340131 |
Renal cell carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90 |
MTOR
NCT00001383,NCT05092373,NCT02419495 |
Large cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.3 |
NCT03345810,NCT00596830,NCT02186847,NCT00955305
|
Squamous cell carcinoma of oropharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6A.0 |
NCT03829722,NCT05108870,NCT04718415,NCT04572100
|
Hepatocellular carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH4W48 |
NCT05092373,NCT04175912,NCT03008512,NCT03563170
|
Adenocarcinoma of prostateDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C82.0 |
SLCO1B3,SLCO1B1
NCT00521781,NCT04221828 |
Squamous cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.2 |
GMNN,F2,SLCO1B3,SLCO1B1
|
Adenocarcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.0 |
GMNN,F2,SLCO1B3,SLCO1B1
|
Breast in situ carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E65 |
NCT01935492,NCT01091428,NCT02603679,NCT00785291,NCT03201861,NCT02593175,NCT02654119,NCT00520975,NCT03853707,NCT01463072,NCT02474173,NCT05233696,NCT01750073,NCT02419495,NCT03179904,NCT04081389,NCT05092373,NCT03554044,NCT01238133,NCT02632071,NCT01263145,NCT02883062,NCT05422794,NCT02187991,NCT03101748,NCT03606967,NCT02689427,NCT02672475,NCT02436993,NCT00542451,NCT03725436,NCT04216472,NCT04001829,NCT04249167,NCT04862585,NCT01275677,NCT04266249,NCT05177796
|
Endometrial cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76 |
MTOR
NCT02630823,NCT04652076,NCT03503786,NCT00515073,NCT05489848,NCT03935256,NCT02730416,NCT00016341,NCT01367002,NCT00006027,NCT01244789,NCT00513786,NCT05092373,NCT00003691,NCT00003127,NCT03603184,NCT00002949,NCT05201547,NCT00052312,NCT00334321,NCT00411138,NCT05481645,NCT02476955,NCT00003624,NCT00373620,NCT00006377,NCT00022620,NCT04159155,NCT00879359,NCT03189446,NCT00883116,NCT04527900,NCT03932409,NCT02744898,NCT01770171 |
Malignant neoplasm metastasis in lymph nodes of head, face or neckDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D60.0 |
SLCO1B3,F2,SLCO1B1
NCT04882462,NCT02573493,NCT03830385,NCT04282109,NCT01612351,NCT04428151,NCT05272696,NCT04826679,NCT01852292,NCT05294900,NCT02270814,NCT04807140,NCT00736944,NCT04831320,NCT04278092,NCT05459129,NCT01154920,NCT04164238,NCT05189184,NCT00513383,NCT05054439,NCT05283226,NCT01732640,NCT03723967,NCT04843098,NCT03169764,NCT02124707 |
Gastrointestinal stromal tumourDisease Category: X.Extension CodesDisease ICD-11 Code: XH9HQ1 |
NCT02607332,NCT03944304,NCT02574663
|
Central nervous system diseaseDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A04-8D87 |
NCT00002814,NCT00031577,NCT00014573
|
LeukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A60-2B33 |
NCT00003402,NCT00003294,NCT00003230
|
Malignant neoplasms of oesophagusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B70 |
SLCO1B3,SLCO1B1
NCT02395705 |
Malignant neoplasms of kidney, except renal pelvisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90 |
NCT00024388,NCT00077129,NCT03678883
|
Carcinosarcoma of uterusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.43 |
GMNN
NCT00687687,NCT00954174 |
Mixed tumour, malignant, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0V86 |
NCT05000892,NCT04825938,NCT01969578
|
Thymic carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH6AK2 |
NCT00010257,NCT00818090,NCT03694002
|
Intrahepatic cholangiocarcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.10 |
NCT02392637,NCT03579771,NCT04175912
|
Malignant mixed epithelial mesenchymal tumour of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B5D.0 |
NCT02822157,NCT02107937,NCT01249443
|
Hereditary breast and ovarian cancer syndromeDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C65 |
NCT05485766,NCT00535119,NCT02978495
|
HepatoblastomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.01 |
PMP22,GMNN,TUBB3
|
Mesothelioma of pleuraDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C26.0 |
LMNA,GMNN,NPC1
|
Other specified malignant neoplasms ofcolonDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B90.Y |
F2,SLCO1B3,SLCO1B1
|
Urothelial carcinoma of bladderDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94.2 |
CYP1A2,SLCO1B3,SLCO1B1
|
MelanomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30 |
NCT01200342,NCT00779714,NCT04979585,NCT01039844,NCT00093119,NCT00255762,NCT00462423,NCT00522834,NCT01676649,NCT00084214,NCT00970996,NCT00288041,NCT03167177,NCT01704287,NCT01174238,NCT00483301,NCT01827111,NCT02300935,NCT00329641,NCT01666418,NCT01009515,NCT00796991,NCT02023710,NCT00111007,NCT00434252,NCT02617849,NCT00864253,NCT01006252,NCT01107665
|
Prostate cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C82 |
MTOR
NCT01558492,NCT00016913,NCT00193193,NCT00151060,NCT00030654,NCT00009750,NCT00284752,NCT00933426,NCT00003394,NCT00003622,NCT00028769,NCT00038168,NCT00024414,NCT00038246,NCT02911350,NCT00003084,NCT04148885,NCT00003717,NCT00005028,NCT00005048,NCT00004054,NCT00084565,NCT00049257,NCT00003614,NCT00005847 |
Malignant neoplasms of bladder, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94.Z |
NCT00003930,NCT00585689,NCT00268450,NCT00045630,NCT00003342,NCT05328336,NCT00006118,NCT00583349,NCT00003105,NCT04060459,NCT00136175,NCT00022191,NCT00022633,NCT00310011,NCT00002949,NCT00003701,NCT00003133,NCT00933374,NCT00002917,NCT04602117,NCT00055601,NCT00028860,NCT02302807,NCT00005644,NCT00003376
|
Breast cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C60-2C6Y |
MTOR
NCT00003877,NCT00003440,NCT00002937,NCT00934895,NCT00003086,NCT00002772,NCT00014222,NCT00001384,NCT00052351,NCT00003539,NCT00003050,NCT03872791,NCT00003972,NCT00031876,NCT00003035,NCT00003392,NCT00002679,NCT00479856,NCT04138719,NCT00003992,NCT00050167,NCT00004092,NCT00006120,NCT00533936,NCT00680758,NCT00007904,NCT02413320,NCT00016406,NCT00004067,NCT00028990,NCT04177108,NCT00002662,NCT00096343,NCT00475670,NCT00316199,NCT00004174,NCT00691912,NCT00254592,NCT00003013,NCT00561119,NCT00431080,NCT00433420,NCT00004013,NCT00072319,NCT00002627,NCT00944047,NCT00068757,NCT05227664,NCT00756470,NCT05491694,NCT00511459,NCT00544232,NCT00005581,NCT00006256,NCT00070564,NCT00001498,NCT00616967,NCT00006459,NCT00054028,NCT01009983,NCT00724386,NCT00004125,NCT00003088,NCT00039546,NCT05192798,NCT00559845,NCT00005635,NCT00140140,NCT00236899,NCT00407888,NCT00110084,NCT00002826,NCT00009997,NCT00001383,NCT00022230,NCT00629499,NCT00513292,NCT00600340,NCT01818063,NCT00915018,NCT00146549,NCT00622466,NCT00919880,NCT00274456,NCT00256243,NCT00971945,NCT00499083,NCT00006110,NCT01008150,NCT00455533,NCT00356681,NCT00503750,NCT00006108,NCT00038402,NCT00846027,NCT00070278,NCT00336791,NCT00009763,NCT00028873,NCT00499603,NCT00591851,NCT00635050,NCT00019812,NCT00093795,NCT00930930,NCT02315196,NCT00002628,NCT00003042,NCT00148681,NCT00442260,NCT00654836,NCT00887575,NCT00768859,NCT00668616,NCT00467012,NCT00107094,NCT00803556,NCT00789581,NCT00392392,NCT00002953,NCT00003612,NCT00295893,NCT00041119,NCT00093145,NCT00096668,NCT00707707,NCT00426556,NCT00301730,NCT00456846,NCT00912639,NCT00191854,NCT00900627,NCT00609791,NCT00532285,NCT00041470,NCT00951665,NCT00320541,NCT00003927,NCT00960960,NCT04770272,NCT03057600,NCT00194792,NCT00002837,NCT04148911,NCT00807859,NCT00129922,NCT00723125,NCT00005649,NCT00645177,NCT00788931,NCT00542191,NCT00194779,NCT00331630,NCT03197935,NCT00777673,NCT00667251,NCT04213898,NCT00370552,NCT00322400,NCT00580333,NCT04129996,NCT00607438,NCT00679029,NCT00404404,NCT00773695,NCT00445458,NCT00618657,NCT00311636,NCT00334802,NCT00795899,NCT00574587,NCT00129389,NCT01881230,NCT00289263,NCT03805399,NCT03125902,NCT00637897,NCT00691379,NCT00394082,NCT00196872,NCT00717340,NCT00499525,NCT00136539,NCT00662129,NCT00193206,NCT00820170,NCT00532857,NCT00675259,NCT00378313,NCT05390710,NCT00486668,NCT00856492,NCT00025688,NCT04584112,NCT00983424,NCT00589238,NCT00251472,NCT00629278,NCT04296175,NCT00434356,NCT01042379,NCT00876395,NCT00476827,NCT00618826,NCT00154882,NCT00748553,NCT01003158,NCT00503906,NCT00436566,NCT00397761,NCT00394251,NCT00479674,NCT00146562,NCT00448591,NCT00915369,NCT00915603,NCT00403130,NCT00128856,NCT00482391,NCT04958785,NCT00546156,NCT00733408,NCT03002103,NCT00567554,NCT04085276,NCT00536939 |
Non-small cell lung cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25 |
NCT01179269,NCT00933803,NCT01225302,NCT01090830,NCT02182232,NCT00866528,NCT01234038,NCT01160601,NCT01820754,NCT00832819,NCT01507428,NCT00986674,NCT01268059,NCT01560104,NCT00966914,NCT00991796,NCT02064491,NCT01496742,NCT01836679,NCT00903942,NCT01366131,NCT01165216,NCT01310244,NCT02117024,NCT02252796,NCT00730925,NCT00602797,NCT02322281,NCT01527864,NCT00614484,NCT00001450,NCT01966003,NCT00191256,NCT00948675,NCT01171170,NCT02016209,NCT00768131,NCT00093756,NCT00752115,NCT01649947,NCT01763671,NCT02041533,NCT02220894,NCT02477826,NCT02319889,NCT01158144,NCT00708812,NCT00832494,NCT01769391,NCT00874848,NCT00280787,NCT01620190,NCT00254891,NCT00294762,NCT02226757,NCT00976677,NCT01413750,NCT01711697,NCT01980472,NCT00174356,NCT01454102,NCT01993810,NCT01011075,NCT00787852,NCT00918203,NCT00807612,NCT01100931,NCT01364012,NCT00753909,NCT02172846,NCT02250326,NCT00762034,NCT01196234,NCT01872403,NCT01702714,NCT01822496,NCT02083679,NCT02392507,NCT01783197,NCT00259675,NCT01326767,NCT02106546,NCT02367794,NCT00583830,NCT01763645,NCT00087802,NCT00199758,NCT00686322,NCT01069328,NCT01519804,NCT00168883,NCT01249443,NCT01493843,NCT01912625,NCT02142738,NCT02264990,NCT00702975,NCT00654758,NCT01702844,NCT00198354,NCT00006929,NCT00050960,NCT00313768,NCT02289456,NCT01757288,NCT00603538,NCT00434135,NCT00042302,NCT00112294,NCT02419495,NCT01236716,NCT00861627,NCT00655850,NCT00806286,NCT02073968,NCT00974584,NCT00049790,NCT00527735,NCT02204345,NCT01383148,NCT00070629,NCT02366143,NCT01024062,NCT02453282,NCT00322452,NCT02405910,NCT00686959,NCT02403895,NCT00970580,NCT00735696,NCT00558636,NCT00035152,NCT01303926,NCT00723957,NCT02364999,NCT00481078,NCT02259621,NCT00273507,NCT00662311,NCT00680940,NCT02039674,NCT00318136,NCT01199055,NCT00666692,NCT00480831,NCT02276560,NCT00152477,NCT01820325,NCT00960297,NCT00300885,NCT00460317,NCT02412371,NCT00269828,NCT00178256,NCT00343291,NCT00347412,NCT00539331,NCT00578149,NCT00291850,NCT00094835,NCT00088556,NCT00540514,NCT00034541,NCT00702572,NCT00198367,NCT00005065,NCT00716534,NCT00449657,NCT00147537,NCT00437749,NCT00252798,NCT00369070,NCT00071188,NCT00800202,NCT00085839,NCT00874107,NCT00198432,NCT00828841,NCT00268970,NCT00915005,NCT00563784,NCT00298415,NCT00642759,NCT00073723,NCT00850577,NCT00034164,NCT00508625,NCT00061646,NCT00473889,NCT00576225,NCT00687817,NCT00001499,NCT02382406,NCT02420314,NCT00097227,NCT00662597,NCT00113516,NCT00088088,NCT00434226,NCT00161278
|
Pain, unspecifiedDisease Category: 21.Symptoms, signs or clinical findings, not elsewhere classifiedDisease ICD-11 Code: MG3Z |
MTOR
NCT02311907 |
Liver cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12 |
MTOR
NCT00732836 |
Solid tumour/cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00-2F9Z |
MTOR,ITGAV
|
Multiple sclerosisDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8A40 |
F2,ITGAV
|
Pleural mesotheliomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C26 |
MTOR
NCT02798536 |
Skin cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30-2C37 |
NCT02054884,NCT03167164
|
Carcinoma, undifferentiated, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH1YY4 |
NCT00989651,NCT00108745
|
Chronic arterial occlusive disease, unspecifiedDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD4Z |
NCT00518284,NCT03064126
|
Soft tissue disorderDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FB56 |
NCT03524898,NCT05189483
|
Penile cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C81 |
NCT04475016,NCT00512096
|
strictureDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BB80.Z-LB15.1 |
NCT01868984,NCT02040454
|
Polyneuropathy, unspecifiedDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C0Z |
NCT03492047,NCT00979082
|
Hodgkin lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B30 |
NCT01555853,NCT01929941
|
ThymomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C27 |
NCT00818090,NCT00010257
|
Hepatocellular carcinoma of liverDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C12.02 |
GMNN,GMNN
|
Other specified malignant neoplasms of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.Y |
SLCO1B3,TUBB3
|
Renal cell carcinoma, chromophobe typeDisease Category: X.Extension CodesDisease ICD-11 Code: XH6153 |
CYP1A2,NPC1
|
Malignant neoplasms of adrenal glandDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D11 |
GMNN,TUBB3
|
Ovarian cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73 |
NCT03410784,NCT03635489,NCT02545010,NCT03776812,NCT02121990,NCT04729387,NCT03740165,NCT03737643,NCT05145218,NCT05255471,NCT04931342,NCT04938583,NCT04815408,NCT03246074,NCT05145569,NCT04261465,NCT03901118,NCT04729608,NCT04679064,NCT03117933,NCT02834975,NCT03989336,NCT03806049,NCT00772798,NCT04072263,NCT00652119,NCT00003998,NCT05044871,NCT00052468,NCT03763123,NCT03639246,NCT00002894,NCT02476955,NCT00672295,NCT00331422,NCT00003378,NCT00005051,NCT00005026,NCT04590625,NCT05371301,NCT02001272,NCT02520154,NCT03393884,NCT03539328,NCT02157870,NCT00005612,NCT03056833,NCT04921527,NCT03197584,NCT02159820,NCT00490711,NCT03398655,NCT03942068,NCT00889382,NCT00028743,NCT04000295,NCT00945191,NCT01684878,NCT00084448,NCT02948075,NCT01402271,NCT04908787,NCT00391118,NCT00326456,NCT01204749,NCT00231075,NCT00483782,NCT00002734,NCT01954355,NCT00003322,NCT02383251,NCT02766582,NCT00466986,NCT00193297,NCT00660842,NCT00937560,NCT00886717,NCT01649336,NCT00003624,NCT00550784,NCT00189371,NCT00003064,NCT00003120,NCT00520013,NCT00929162,NCT00002928,NCT00003644,NCT03393507,NCT01778803,NCT00002717,NCT00034151,NCT02092363,NCT04337632,NCT03940196,NCT01219777,NCT00750386,NCT00003413,NCT01654146,NCT03287271,NCT03029611,NCT00003733,NCT00063401,NCT04608409,NCT01357161,NCT01739218,NCT00838656,NCT00170664,NCT04661696,NCT00003160,NCT02470585,NCT03699449,NCT03025477,NCT03818282,NCT01493505,NCT03394885,NCT00005046,NCT00011986,NCT00129727,NCT01696032,NCT05316376,NCT01253681,NCT00003896,NCT00993655,NCT00102622,NCT01146795,NCT01220154,NCT04374630,NCT00738699,NCT01083537,NCT00031954,NCT00004921,NCT00002819,NCT00825201,NCT01711970,NCT00585052,NCT00001383,NCT02012192,NCT01570582,NCT01644825,NCT00003944,NCT00003880,NCT00401674,NCT01802749,NCT00228319,NCT00610714,NCT01821859,NCT00003214,NCT00840450,NCT00964626,NCT01447706,NCT00408070,NCT00976911,NCT03467178,NCT02440425,NCT00003386,NCT00003449,NCT00006454,NCT02389985,NCT00226915,NCT00002568,NCT03038100,NCT02004093,NCT00158379,NCT01485848,NCT01196741,NCT03126812,NCT02125513,NCT01332656,NCT02483104,NCT01650376,NCT00004934,NCT01239732,NCT01081951,NCT00003385,NCT00517621,NCT01653912,NCT02718417,NCT00023907,NCT00075712,NCT00017303,NCT02312661,NCT01838538,NCT00390611,NCT00702299,NCT00189566,NCT00314678,NCT00008138,NCT00003732,NCT00479817,NCT00091377,NCT00189553,NCT00373217,NCT00657878
|
Small cell carcinoma of bronchus or lungDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25.1 |
NCT04056949,NCT04210037,NCT02038647,NCT03810872,NCT04247126,NCT01441349,NCT04314895,NCT04213937,NCT00317200,NCT03219762,NCT04672928,NCT02262897,NCT00617409,NCT05420636,NCT00527735,NCT00454324,NCT02551432,NCT04698941,NCT02769832
|
Carcinomas of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.0 |
NCT05092373,NCT03690739,NCT02437812,NCT02244502,NCT02272790,NCT04602117,NCT05446870,NCT01970722,NCT01847677,NCT02419495,NCT03030287,NCT03598270,NCT02107378,NCT01091428,NCT00181701,NCT03756818,NCT03400306,NCT05158062
|
Adenocarcinoma of stomachDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B72.0 |
SLCO1B3,F2,SLCO1B1
NCT02449655,NCT04572542,NCT01336062,NCT03193918,NCT04510064,NCT03990103,NCT02625623,NCT04190745,NCT04604132,NCT04077255,NCT04209686,NCT01641783,NCT02615730,NCT03281369,NCT02448329 |
Malignant neoplasms of nasopharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6B |
NCT02250599,NCT03306121,NCT01694576,NCT04766359,NCT04282070,NCT04223024,NCT03047265,NCT04850235,NCT01797900,NCT03604965,NCT03668730,NCT03574324,NCT01534585,NCT03020329,NCT04015661,NCT01712919,NCT04004871
|
Mature T-cell lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A90 |
MTOR
NCT00004916,NCT00008021,NCT00003397,NCT00027937,NCT00003294,NCT04856137,NCT00004057,NCT00003402,NCT00003957,NCT00009776,NCT00005021,NCT03435250,NCT00021372,NCT03884556,NCT00001383 |
NeoplasmsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 02 |
NCT03503604,NCT01994031,NCT02597036,NCT04047862,NCT02317874,NCT03330106,NCT03421353,NCT03577704,NCT03892018,NCT03907475,NCT02134067,NCT01938846,NCT02379416,NCT02979392,NCT04336098,NCT01525602,NCT01293630,NCT01411410,NCT02762981,NCT02937272,NCT01930292,NCT04594005,NCT04794699,NCT00307255,NCT03537690,NCT02341456,NCT03600090,NCT02057380,NCT02430311,NCT02730481,NCT04046016,NCT02715531,NCT05322720,NCT00881101,NCT01946074,NCT00028587,NCT02711137,NCT03894540,NCT03486314,NCT04035473,NCT00003092,NCT04151277,NCT00454649,NCT01515306,NCT00607048,NCT03918278,NCT03588039,NCT01362374,NCT00486954,NCT00003899,NCT03601897,NCT02723955,NCT01031212,NCT03928314,NCT04449549,NCT04675528,NCT03693612,NCT03430882,NCT00809133,NCT04143711,NCT04180384,NCT05262335,NCT01967043,NCT04541108,NCT03085914,NCT04742036,NCT05017012,NCT00848718,NCT01617928,NCT03154294,NCT02794571,NCT04999969,NCT04176952,NCT01548924,NCT00080418,NCT00001387,NCT05344742,NCT01285466,NCT02607202,NCT00954512,NCT02122770,NCT04761601,NCT04481009,NCT03834948,NCT04802980,NCT00287937,NCT00399126,NCT01862328,NCT00360360,NCT00003242,NCT03057366,NCT03309150,NCT01248949,NCT00042809,NCT02593708,NCT01968915,NCT01419548,NCT00793897,NCT00002939,NCT00095914,NCT00458315,NCT05381909,NCT01593228,NCT01868022,NCT00002587,NCT03981796,NCT00100139,NCT02051751,NCT00003004,NCT01491204,NCT03564691,NCT00193596,NCT00006018,NCT00009828,NCT00556088,NCT00024310,NCT00653939,NCT00511849,NCT01455532,NCT00004979,NCT00005078,NCT02630264,NCT03595059,NCT01240655,NCT00811993,NCT02283489,NCT01992341,NCT00350025,NCT00520000,NCT00009932,NCT01297452,NCT00006257,NCT00891605,NCT00003130,NCT04778839,NCT00768469,NCT00046423,NCT00087217,NCT00004173,NCT00003742,NCT00005819,NCT00054548,NCT01159418,NCT00003555,NCT00001442,NCT00016874,NCT00009802,NCT00030368,NCT05214976,NCT00088114,NCT01281176,NCT01494688,NCT00496028,NCT00422682,NCT01566435,NCT00004863
|
Malignant neoplasms of ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73 |
NCT01121406,NCT00191646,NCT03574779,NCT00001272,NCT04711161,NCT01279291,NCT01608009,NCT01616303,NCT00919984,NCT00069901,NCT00001426,NCT03314740,NCT00653952,NCT04561817,NCT02903004
|
Neuroendocrine carcinomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C34 |
NCT00731861,NCT00894569,NCT01593306,NCT00936702,NCT01253681,NCT00001569,NCT00873119,NCT04705519,NCT00737243,NCT03136055,NCT00003943,NCT00193531,NCT00003558,NCT02215447
|
Peritoneal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C51 |
NCT00189371,NCT00191646,NCT00666991,NCT03740165,NCT03038100,NCT03030287,NCT02125513,NCT00001569,NCT00063401,NCT00391118,NCT03056833,NCT00189566,NCT01279291,NCT00517621
|
Malignant neoplasm of pancreas, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10.Z |
NCT04674956,NCT05193604,NCT03815461,NCT04707118,NCT03257033,NCT04390399,NCT04617067,NCT03721744,NCT03329248,NCT02608229,NCT03777462,NCT02047500,NCT03586869,NCT03885219,NCT03585062,NCT03649321,NCT04158635,NCT02574663,NCT01488552,NCT03797443,NCT04589234,NCT04592861,NCT05241249,NCT05497778,NCT02399137,NCT01632306,NCT03825328,NCT03693677,NCT05047991,NCT04858009,NCT05346146,NCT04672005,NCT04054362,NCT05185869,NCT01934634,NCT03563144,NCT03415802,NCT04902261,NCT03520790,NCT04581876,NCT01730222,NCT00024375,NCT03487016,NCT03252808,NCT05168527,NCT02717091,NCT04683939,NCT02827201,NCT02358161,NCT03989310,NCT02391662,NCT04683315,NCT04498689,NCT03435289,NCT04643405,NCT04753879,NCT00111904,NCT03417921,NCT04089150,NCT03600623,NCT03316599,NCT03714555,NCT02382263,NCT04447092,NCT03502343,NCT04723030,NCT05218889,NCT00882973,NCT02506803,NCT05251038,NCT05298020,NCT02570711,NCT01858883,NCT02155088,NCT01834235,NCT01836432,NCT02705196,NCT03519308,NCT04241276,NCT02124317,NCT03641183,NCT02207465,NCT01010945,NCT02588443,NCT00691054,NCT02241551,NCT02106884,NCT02340117,NCT03850769,NCT02559674,NCT04814485,NCT01839487,NCT04104672,NCT02975141,NCT03387098,NCT03336216,NCT03941093,NCT03086369,NCT04247126,NCT02651727,NCT02481635,NCT03636308,NCT02210559,NCT01470417,NCT02767557,NCT01461915,NCT01693276,NCT01978184,NCT01893801,NCT00003591,NCT00323583,NCT01621243,NCT01964534,NCT03344172,NCT02043730,NCT02283372,NCT02514031,NCT02506842,NCT02135822,NCT02109445,NCT00844649,NCT02930902,NCT02109341,NCT01851174,NCT02873598,NCT01088815,NCT01822756,NCT03412799,NCT01647828,NCT02272738,NCT00398086,NCT02050178,NCT03278015,NCT02005315,NCT02581501,NCT02138383,NCT03136406
|
Malignant neoplasm metastasis, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E2Z |
NCT02419495,NCT01721746,NCT00781612,NCT00984464,NCT01933061,NCT00731861,NCT00046423,NCT00046514,NCT01014351,NCT02158520,NCT00046527,NCT00048893,NCT03678883
|
Carcinoma of male breastDisease Category: X.Extension CodesDisease ICD-11 Code: XH5KW8 |
NCT01493310,NCT01288261,NCT00368875,NCT01281150,NCT00770809,NCT02060253,NCT00470301,NCT00513695,NCT01938833,NCT03018080,NCT00861705,NCT00119262,NCT01276496
|
Malignant neoplasms of fallopian tube, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C74.Z |
NCT00191646,NCT01167712,NCT01279291,NCT02125513,NCT01970722,NCT03030287,NCT02419495,NCT05092373,NCT02272790,NCT03776812,NCT00063401,NCT00954174,NCT00391118
|
Inflammatory carcinoma of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C62 |
NCT03515798,NCT02125344,NCT01583426,NCT00513695,NCT01730833,NCT02199418,NCT02876302,NCT02879513,NCT02221999,NCT00016276,NCT01938833,NCT02682693,NCT05383196
|
Lung cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C25 |
MTOR
NCT01057342,NCT00795340,NCT00728845,NCT01486602,NCT02328105,NCT00077246,NCT02513563,NCT00828009,NCT03432598,NCT00006229,NCT03348904,NCT04647344,NCT00003943,NCT00003696,NCT00661193,NCT00079287,NCT00005849,NCT00533585,NCT01024712,NCT01107626,NCT00544648,NCT02525653,NCT00547443,NCT00002972,NCT00748163,NCT00002969,NCT00004887,NCT02279732,NCT00081302,NCT00526890,NCT00801736,NCT00006378,NCT00005806,NCT00004253,NCT00011921,NCT00193310,NCT00278148,NCT00003117,NCT00553462,NCT00459121,NCT00043108,NCT00334763,NCT00062062,NCT00003235,NCT00032032,NCT00004202,NCT00003284,NCT00004859,NCT00465907,NCT00144053,NCT00039039,NCT00003299,NCT00077220,NCT00054392,NCT00006469,NCT01285609,NCT00004093,NCT00003881,NCT00452803,NCT00005059,NCT00020007,NCT00245154,NCT00807573,NCT00006049,NCT02733250,NCT00017407,NCT01862081,NCT00003158,NCT00006012,NCT03322566,NCT00290537,NCT00004159,NCT00280150,NCT00004126,NCT00276588,NCT00025480,NCT00006374,NCT00533949,NCT00004137,NCT00003159,NCT00551733,NCT00004011,NCT00226590,NCT00028938,NCT00287989,NCT00033410,NCT00004265,NCT00004924,NCT02044601,NCT00003589,NCT00054210,NCT00063258,NCT00025389,NCT00006004,NCT00055887,NCT00453167,NCT00096226,NCT00550537,NCT00085501,NCT00023673,NCT00062179,NCT00006484,NCT00495170,NCT00003111,NCT00062010,NCT00005032,NCT00005646,NCT00616031,NCT00003281,NCT00016315,NCT00729612,NCT00002519,NCT00003317,NCT00003089,NCT00003387,NCT00193362,NCT00033696,NCT00033553,NCT00003812,NCT00004055,NCT00003587,NCT00979212,NCT00004160,NCT00003313 |
Melanoma of skin, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C30.Z |
NCT00081042,NCT00976573,NCT02419495,NCT02158520,NCT00626405,NCT00404235,NCT00278122,NCT02020707,NCT02308553,NCT00568451,NCT00110019
|
Malignant neoplasms of corpus uteri, unspecifiedDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C76.Z |
F2,SLCO1B3
NCT02725268,NCT04602117,NCT04634877,NCT04269200,NCT03884101,NCT05173987,NCT03517449,NCT04865289,NCT01820858 |
Malignant neoplasm of pancreasDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10 |
NCT02810418,NCT04808687,NCT02017015,NCT05493995,NCT05083247,NCT03807999,NCT04289792,NCT05222204,NCT02301143,NCT02024009
|
Ischaemic/haemorrhagic strokeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B20 |
F2
|
Malignant mesenchymal neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B5D-2B5Y |
MTOR
|
Myocardial infarctionDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA41-BA43 |
F2
|
Hypo-thyroidismDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A00 |
THRB
|
ThrombosisDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DB61-GB90 |
F2
|
Angina pectorisDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA40 |
F2
|
AsthmaDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CA23 |
F2
|
Coagulation defectDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3B10 |
F2
|
Inborn lipid metabolism errorDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5C52 |
THRB
|
Bleeding disorderDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA20-GA21 |
F2
|
Cardiovascular diseaseDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA00-BE2Z |
F2
|
Fungal infectionDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1F29-1F2F |
RAB9A
|
Chronic myelomonocytic leukaemiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A40 |
MTOR
|
Pancreatic cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C10 |
MTOR
|
Transplant rejectionDisease Category: 22.Injury, poisoning or certain other consequences of external causesDisease ICD-11 Code: NE84 |
MTOR
|
ThrombocytopeniaDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3B64 |
F2
|
Metastatic lymph node neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D60 |
MTOR
|
Arteries/arterioles disorderDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BD52 |
MTOR
|
Type 2 diabetes mellitusDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A11 |
F2
|
LymphangioleiomyomatosisDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB07 |
MTOR
|
Bladder cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C94 |
MTOR
|
HydrocephalusDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8D64 |
MTOR
|
Malignant haematopoietic neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B33 |
MTOR
|
Cerebral ischaemic strokeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B11 |
F2
|
Nutritional deficiencyDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5B50-5B71 |
F2
|
Hyper-lipoproteinaemiaDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5C80 |
THRB
|
Coronary thrombosisDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA43 |
F2
|
Glioblastoma, primary, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0MB1 |
NCT00479765
|
Malignant neoplasms of other or unspecified parts of mouthDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B66 |
NCT00933387
|
IschemiaDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8B10-8B11 |
NCT02835586
|
Malignant neoplasm metastasis in bone or bone marrowDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E03 |
NCT03678883
|
Mesotheliomas of peritoneumDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C51.2 |
NCT05449366
|
Allergic/hypersensitivity disorderDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4A80-4A8Z |
NCT00277043
|
Other specified malignant neoplasms of colonDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B90.Y |
NCT00034190
|
AnemiaDisease Category: 03.Diseases of the blood or blood-forming organsDisease ICD-11 Code: 3A00-3A9Z |
NCT00189371
|
Sex cord-gonadal stromal tumour, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH9G57 |
NCT01770301
|
Gallbladder/bile ducts/liver structural developmental anomalyDisease Category: 20.Developmental anomaliesDisease ICD-11 Code: LB20 |
NCT02392637
|
Malignant neoplasms of oropharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6A |
NCT03416153
|
NeuropathyDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C0Z |
NCT02311907
|
Pleomorphic adenomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH2KC1 |
NCT00502203
|
Kaposi sarcomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B57 |
NCT00003350
|
Prostate hyperplasiaDisease Category: 16.Diseases of the genitourinary systemDisease ICD-11 Code: GA90 |
NCT03423979
|
Other specified malignant neoplasms of the ovaryDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C73.Y |
NCT02429700
|
Malignant trophoblastic neoplasms of placentaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C75.0 |
NCT02639650
|
Malignant neoplasms of anus or anal canalDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C00 |
NCT01249443
|
Teratoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH83G5 |
NCT00104676
|
Human immunodeficiency virus disease without mention of associated disease or condition, clinical stage unspecifiedDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1C62.Z |
NCT01249443
|
Type1diabetesmellitusDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A10 |
NCT01581476
|
Glioma, malignantDisease Category: X.Extension CodesDisease ICD-11 Code: XH4RQ3 |
NCT03678883
|
PsoriasisDisease Category: 14.Diseases of the skinDisease ICD-11 Code: EA90 |
NCT00006276
|
Rheumatoid arthritisDisease Category: 15.Diseases of the musculoskeletal system or connective tissueDisease ICD-11 Code: FA20 |
NCT00055133
|
Small cell carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH0YB0 |
NCT02881125
|
Actinic keratosisDisease Category: X.Extension CodesDisease ICD-11 Code: XH36H6 |
NCT03083470
|
Adrenal cortical carcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH4KH2 |
NCT00786110
|
Ductal carcinoma in situ of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E65.2 |
NCT02779855
|
Cervical intraepithelial neoplasiaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E66 |
NCT03143491
|
Other specified malignant neoplasms of oropharynxDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6A.Y |
NCT01249443
|
Rectum cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B92 |
NCT02574663
|
Chronic lymphocytic leukaemia or small lymphocytic lymphomaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A82.0 |
NCT03003546
|
Diseases of coronary artery, unspecifiedDisease Category: 11.Diseases of the circulatory systemDisease ICD-11 Code: BA8Z |
NCT00124943
|
Malignant neoplasm metastasis in female reproductive systemDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E05 |
NCT02739529
|
Neuroendocrine tumour, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH8DS0 |
NCT04602117
|
Primary neoplasms of brainDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2A00 |
NCT00479765
|
Diseases of the digestive system, unspecifiedDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DE2Z |
NCT00208936
|
Malignant neoplasms of vaginaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C71 |
NCT00002949
|
Neoplasms of unknown behaviour of female genital organsDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2F96 |
NCT02182245
|
Hypopharyngeal cancerDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2B6D |
NCT01249443
|
Metastatic lung neoplasmDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2D70 |
NCT03678883
|
GliosarcomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH9RC8 |
NCT04528680
|
Other specified carcinoma in situ of breastDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2E65.Y |
NCT01891357
|
Malignant neoplasms of vulvaDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C70 |
NCT00014599
|
Verrucous carcinoma, NOSDisease Category: X.Extension CodesDisease ICD-11 Code: XH5PM0 |
NCT01249443
|
Parathyroid carcinomaDisease Category: X.Extension CodesDisease ICD-11 Code: XH7JQ0 |
NCT03181100
|
Human immunodeficiency virus type 1Disease Category: X.Extension CodesDisease ICD-11 Code: XN8LD |
NCT01435018
|
Crohn diseaseDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DD70 |
SLCO1B3
|
Inflammatory bowel diseases, unspecifiedDisease Category: 13.Diseases of the digestive systemDisease ICD-11 Code: DD7Z |
SLCO1B3
|
Idiopathic pulmonary fibrosisDisease Category: 12.Diseases of the respiratory systemDisease ICD-11 Code: CB03.4 |
SLCO1B3
|
Tetralogy of FallotDisease Category: 20.Developmental anomaliesDisease ICD-11 Code: LA88.2 |
F2
|
Other specified malignant neoplasms of kidney, except renal pelvisDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C90.Y |
F2
|
Cytomegaloviral diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D82 |
THRB
|
Systemic lupus erythematosusDisease Category: 04.Diseases of the immune systemDisease ICD-11 Code: 4A40.0 |
TUBB3
|
Chikungunya virus diseaseDisease Category: 01.Certain infectious or parasitic diseasesDisease ICD-11 Code: 1D40 |
LMNA
|
Polycystic ovary syndromeDisease Category: 05.Endocrine, nutritional or metabolic diseasesDisease ICD-11 Code: 5A80.1 |
ITGAV
|
Malignant neoplasms of thymusDisease Category: 02.NeoplasmsDisease ICD-11 Code: 2C27 |
GMNN
|
Carpal tunnel syndromeDisease Category: 08.Diseases of the nervous systemDisease ICD-11 Code: 8C10.0 |
TUBB6
|

